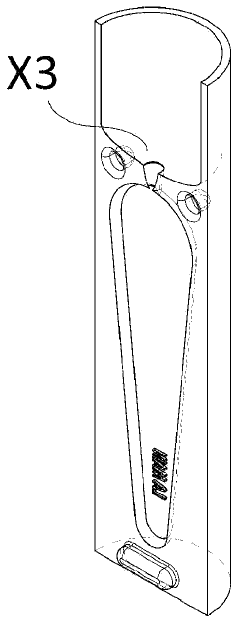
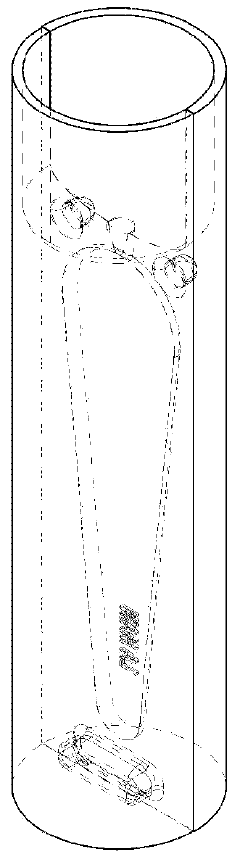
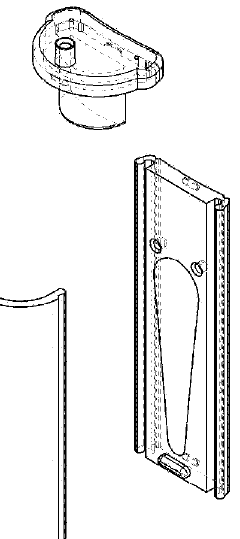
Qualifying Standards for Microinjection Molding Applications
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microinjection Molding Technology Background and Objectives
Microinjection molding technology emerged in the late 1980s as a specialized adaptation of conventional injection molding, designed specifically to produce microscale components with high precision. This technology has evolved significantly over the past three decades, driven by increasing demands in medical devices, electronics, and microfluidics industries. The fundamental principle involves injecting polymer melts into micro-cavities under controlled conditions to produce parts with features in the micrometer range, often weighing less than 1 mg.
The evolution of microinjection molding has been characterized by continuous improvements in machine design, material development, and process control. Early systems were essentially scaled-down versions of conventional molding equipment, but modern dedicated microinjection molding machines incorporate specialized features such as precise dosing systems, advanced temperature control, and vacuum-assisted mold filling to address the unique challenges of micro-scale production.
Material science advancements have played a crucial role in this technology's development, with the introduction of specialized polymers and polymer composites designed specifically for micro-molding applications. These materials offer enhanced flow properties, reduced viscosity at processing temperatures, and improved replication fidelity at the micro-scale.
The current technological trajectory points toward further miniaturization, increased complexity of geometries, and enhanced surface quality of molded components. Industry experts anticipate that feature sizes will continue to decrease, potentially reaching sub-micrometer dimensions in commercial applications within the next decade.
The primary objective of qualifying standards for microinjection molding applications is to establish robust, reproducible criteria for evaluating process capability and product quality in this highly specialized field. These standards aim to address the unique challenges associated with micro-scale manufacturing, including dimensional stability, surface finish, mechanical properties, and process repeatability.
Additionally, standardization efforts seek to create a common language and methodology for specifying microinjection molded components across different industries and applications. This includes developing appropriate testing protocols, measurement techniques, and acceptance criteria that account for the specific requirements of micro-scale parts.
The development of qualifying standards also aims to facilitate technology transfer between research institutions and industry, accelerating the adoption of innovative microinjection molding techniques in commercial applications. By establishing clear benchmarks for performance and quality, these standards will support the continued growth and maturation of the microinjection molding industry.
The evolution of microinjection molding has been characterized by continuous improvements in machine design, material development, and process control. Early systems were essentially scaled-down versions of conventional molding equipment, but modern dedicated microinjection molding machines incorporate specialized features such as precise dosing systems, advanced temperature control, and vacuum-assisted mold filling to address the unique challenges of micro-scale production.
Material science advancements have played a crucial role in this technology's development, with the introduction of specialized polymers and polymer composites designed specifically for micro-molding applications. These materials offer enhanced flow properties, reduced viscosity at processing temperatures, and improved replication fidelity at the micro-scale.
The current technological trajectory points toward further miniaturization, increased complexity of geometries, and enhanced surface quality of molded components. Industry experts anticipate that feature sizes will continue to decrease, potentially reaching sub-micrometer dimensions in commercial applications within the next decade.
The primary objective of qualifying standards for microinjection molding applications is to establish robust, reproducible criteria for evaluating process capability and product quality in this highly specialized field. These standards aim to address the unique challenges associated with micro-scale manufacturing, including dimensional stability, surface finish, mechanical properties, and process repeatability.
Additionally, standardization efforts seek to create a common language and methodology for specifying microinjection molded components across different industries and applications. This includes developing appropriate testing protocols, measurement techniques, and acceptance criteria that account for the specific requirements of micro-scale parts.
The development of qualifying standards also aims to facilitate technology transfer between research institutions and industry, accelerating the adoption of innovative microinjection molding techniques in commercial applications. By establishing clear benchmarks for performance and quality, these standards will support the continued growth and maturation of the microinjection molding industry.
Market Demand Analysis for Microinjection Molded Components
The microinjection molding market has experienced significant growth in recent years, driven primarily by increasing demand for miniaturized components across multiple industries. The global market for microinjection molded components was valued at approximately $1.5 billion in 2022 and is projected to reach $2.8 billion by 2028, representing a compound annual growth rate of 11.2% during the forecast period.
Healthcare and medical devices represent the largest application segment, accounting for nearly 40% of the total market share. This dominance stems from the growing need for precise, biocompatible components in medical implants, drug delivery systems, and diagnostic equipment. The trend toward minimally invasive surgical procedures has further accelerated demand for micro-scale medical components with complex geometries and tight tolerances.
Electronics and telecommunications form the second-largest market segment, with approximately 30% market share. The continuous miniaturization of electronic devices, coupled with increasing functionality requirements, has created substantial demand for microinjection molded connectors, switches, and housings. The rollout of 5G infrastructure and IoT devices is expected to further boost this segment over the next five years.
Automotive applications represent a rapidly growing segment, currently at 15% of the market but expanding at a faster rate than the overall market. Modern vehicles incorporate an increasing number of sensors, microelectronic components, and fluid control systems that require precision microinjection molded parts. The transition to electric vehicles is accelerating this trend, as these vehicles typically contain more electronic components than traditional combustion engine vehicles.
Consumer electronics and wearable technology applications account for approximately 10% of the market. The miniaturization of consumer devices and the emergence of smart wearables have created new opportunities for microinjection molded components in areas such as hearables, smartwatches, and augmented reality devices.
Regional analysis indicates that North America and Europe currently lead the market with combined market share of 55%, primarily due to their advanced healthcare and automotive sectors. However, the Asia-Pacific region is experiencing the fastest growth, driven by expanding electronics manufacturing capabilities and increasing healthcare infrastructure development in countries like China, Japan, and South Korea.
Key market drivers include the growing demand for miniaturized components with complex geometries, increasing adoption of microfluidic devices in diagnostics and research, and the trend toward lightweight components in automotive and aerospace applications. Additionally, sustainability concerns are pushing manufacturers to develop bio-based and recyclable materials suitable for microinjection molding processes.
Healthcare and medical devices represent the largest application segment, accounting for nearly 40% of the total market share. This dominance stems from the growing need for precise, biocompatible components in medical implants, drug delivery systems, and diagnostic equipment. The trend toward minimally invasive surgical procedures has further accelerated demand for micro-scale medical components with complex geometries and tight tolerances.
Electronics and telecommunications form the second-largest market segment, with approximately 30% market share. The continuous miniaturization of electronic devices, coupled with increasing functionality requirements, has created substantial demand for microinjection molded connectors, switches, and housings. The rollout of 5G infrastructure and IoT devices is expected to further boost this segment over the next five years.
Automotive applications represent a rapidly growing segment, currently at 15% of the market but expanding at a faster rate than the overall market. Modern vehicles incorporate an increasing number of sensors, microelectronic components, and fluid control systems that require precision microinjection molded parts. The transition to electric vehicles is accelerating this trend, as these vehicles typically contain more electronic components than traditional combustion engine vehicles.
Consumer electronics and wearable technology applications account for approximately 10% of the market. The miniaturization of consumer devices and the emergence of smart wearables have created new opportunities for microinjection molded components in areas such as hearables, smartwatches, and augmented reality devices.
Regional analysis indicates that North America and Europe currently lead the market with combined market share of 55%, primarily due to their advanced healthcare and automotive sectors. However, the Asia-Pacific region is experiencing the fastest growth, driven by expanding electronics manufacturing capabilities and increasing healthcare infrastructure development in countries like China, Japan, and South Korea.
Key market drivers include the growing demand for miniaturized components with complex geometries, increasing adoption of microfluidic devices in diagnostics and research, and the trend toward lightweight components in automotive and aerospace applications. Additionally, sustainability concerns are pushing manufacturers to develop bio-based and recyclable materials suitable for microinjection molding processes.
Current Technical Challenges in Microinjection Molding Standards
Despite significant advancements in microinjection molding technology, the industry faces substantial challenges regarding standardization. Current standards for conventional injection molding prove inadequate when applied to micro-scale operations, creating a significant gap in quality assurance frameworks. The miniaturization of components, often featuring dimensions below 1mm and tolerances in the micrometer range, demands precision beyond traditional measurement capabilities.
A primary challenge lies in the absence of universally accepted dimensional tolerance standards specific to microinjection molded parts. While ISO 20753 addresses some aspects of test specimen measurement, it fails to account for the unique challenges presented by micro-features, such as high aspect ratios and complex geometries that characterize microinjection molded components.
Material qualification standards represent another critical gap. Conventional material testing protocols often require sample sizes incompatible with micro-scale production. Additionally, the behavior of polymers at the micro-scale differs significantly from macro-scale applications due to increased surface-to-volume ratios and altered crystallization kinetics, rendering traditional material property databases insufficient for microinjection applications.
Process validation standards face similar limitations. Current methodologies like Scientific Molding and Design of Experiments (DOE) require adaptation for micro-scale processes where traditional process parameters exhibit non-linear relationships. The industry lacks standardized approaches for validating critical micro-molding parameters such as micro-feature replication fidelity and internal stress distribution in microscale components.
Quality control presents unique challenges, with conventional statistical process control (SPC) methods struggling to accommodate the extreme precision requirements of microinjection molding. The industry lacks standardized non-destructive testing protocols suitable for micro-components, where traditional coordinate measuring machines (CMMs) often lack sufficient resolution.
Surface quality assessment standards remain underdeveloped for micro-molded parts. While ISO 4287 addresses surface roughness parameters, it doesn't adequately address the impact of surface irregularities at the micro-scale, where even nanometer-level variations can significantly affect functionality.
Environmental testing standards also require refinement. Micro-components often operate in specialized environments (medical implants, microfluidic devices) where conventional environmental testing protocols prove insufficient to predict long-term performance and reliability.
Cross-industry standardization represents perhaps the most significant challenge. Microinjection molding serves diverse sectors including medical, electronics, and automotive industries, each with distinct regulatory frameworks. The development of harmonized standards that satisfy requirements across these sectors while addressing the unique challenges of micro-scale manufacturing remains a formidable obstacle for the industry.
A primary challenge lies in the absence of universally accepted dimensional tolerance standards specific to microinjection molded parts. While ISO 20753 addresses some aspects of test specimen measurement, it fails to account for the unique challenges presented by micro-features, such as high aspect ratios and complex geometries that characterize microinjection molded components.
Material qualification standards represent another critical gap. Conventional material testing protocols often require sample sizes incompatible with micro-scale production. Additionally, the behavior of polymers at the micro-scale differs significantly from macro-scale applications due to increased surface-to-volume ratios and altered crystallization kinetics, rendering traditional material property databases insufficient for microinjection applications.
Process validation standards face similar limitations. Current methodologies like Scientific Molding and Design of Experiments (DOE) require adaptation for micro-scale processes where traditional process parameters exhibit non-linear relationships. The industry lacks standardized approaches for validating critical micro-molding parameters such as micro-feature replication fidelity and internal stress distribution in microscale components.
Quality control presents unique challenges, with conventional statistical process control (SPC) methods struggling to accommodate the extreme precision requirements of microinjection molding. The industry lacks standardized non-destructive testing protocols suitable for micro-components, where traditional coordinate measuring machines (CMMs) often lack sufficient resolution.
Surface quality assessment standards remain underdeveloped for micro-molded parts. While ISO 4287 addresses surface roughness parameters, it doesn't adequately address the impact of surface irregularities at the micro-scale, where even nanometer-level variations can significantly affect functionality.
Environmental testing standards also require refinement. Micro-components often operate in specialized environments (medical implants, microfluidic devices) where conventional environmental testing protocols prove insufficient to predict long-term performance and reliability.
Cross-industry standardization represents perhaps the most significant challenge. Microinjection molding serves diverse sectors including medical, electronics, and automotive industries, each with distinct regulatory frameworks. The development of harmonized standards that satisfy requirements across these sectors while addressing the unique challenges of micro-scale manufacturing remains a formidable obstacle for the industry.
Current Qualification Methodologies and Testing Protocols
01 Microinjection molding equipment and machinery
Specialized equipment and machinery designed specifically for microinjection molding processes. These include micro-molding machines with precise control systems, miniaturized injection units, and specialized tooling that can handle small shot sizes and deliver high precision. The equipment often features advanced control systems for temperature, pressure, and injection speed to ensure consistent quality in micro-scale parts.- Equipment and apparatus for microinjection molding: Specialized equipment and apparatus are essential for microinjection molding processes. These include micro-molds with precise cavity designs, advanced injection units capable of delivering small material volumes with high accuracy, and specialized clamping systems. The equipment often features enhanced temperature control systems, high-precision positioning mechanisms, and specialized ejection systems designed to handle delicate micro-components without damage.
- Materials and formulations for microinjection molding: Various materials and formulations are specifically developed for microinjection molding applications. These include specialized polymer blends with optimized flow properties, biocompatible materials for medical applications, and high-performance engineering plastics. Material selection considers factors such as melt viscosity, shrinkage characteristics, and mechanical properties at micro-scale. Some formulations incorporate additives to enhance flow behavior, reduce cycle times, or improve surface finish of micro-molded components.
- Biomedical and medical applications of microinjection molding: Microinjection molding has significant applications in the biomedical and medical fields. The technology enables production of miniaturized medical devices, microfluidic components for diagnostic systems, drug delivery devices, and implantable medical components. The process allows for the creation of complex microstructures with high precision, which is crucial for applications such as micro-needles, lab-on-chip devices, and miniaturized surgical tools. The ability to use biocompatible materials makes this technology particularly valuable for medical applications.
- Process optimization and control in microinjection molding: Process optimization and control are critical aspects of microinjection molding. This includes precise control of injection parameters such as pressure, temperature, and speed, as well as advanced monitoring systems to ensure consistency. Various techniques are employed to optimize the process, including simulation-based approaches, statistical process control methods, and real-time monitoring systems. Specialized cooling strategies, vacuum-assisted molding, and precise gate design are also important for achieving high-quality micro-molded components with minimal defects.
- Innovative microstructure designs and fabrication techniques: Advanced microstructure designs and fabrication techniques are being developed to enhance microinjection molding capabilities. These include multi-material microinjection molding, insert molding at micro-scale, and the integration of functional elements into micro-components. Novel approaches to mold design, such as using additive manufacturing for creating complex micro-cavities, are also being explored. Additionally, techniques for creating hierarchical structures, surface texturing, and high-aspect-ratio features are advancing the capabilities of microinjection molding for various applications.
02 Materials for microinjection molding
Various materials specifically formulated or selected for microinjection molding applications. These include specialized polymers, biocompatible materials, and composite materials that offer improved flow properties at micro-scale. The materials are often engineered to have specific characteristics such as low viscosity, rapid solidification, and minimal shrinkage to facilitate the production of micro-components with high precision and detail.Expand Specific Solutions03 Microinjection molding for biomedical applications
Applications of microinjection molding in the biomedical field, including the production of medical devices, drug delivery systems, and diagnostic tools. This technology enables the manufacturing of miniaturized components for implantable devices, lab-on-a-chip systems, and microfluidic devices. The process allows for the production of complex geometries with biocompatible materials at the micro-scale required for modern medical applications.Expand Specific Solutions04 Process optimization for microinjection molding
Methods and techniques for optimizing the microinjection molding process to improve quality, consistency, and efficiency. This includes parameter optimization such as injection speed, pressure profiles, cooling strategies, and cycle time reduction. Advanced process monitoring and control systems are employed to maintain tight tolerances and ensure repeatability in the production of micro-scale components.Expand Specific Solutions05 Mold design for microinjection molding
Specialized approaches to mold design for microinjection molding applications. This includes micro-feature creation techniques, advanced cooling channel designs, and innovative gating systems optimized for micro-scale parts. The mold designs incorporate high-precision machining, specialized surface treatments, and advanced venting systems to facilitate the flow of material into microscopic cavities while maintaining dimensional accuracy and surface quality.Expand Specific Solutions
Key Industry Players in Microinjection Molding Technology
The microinjection molding applications market is currently in a growth phase, characterized by increasing demand for miniaturized components across medical, electronics, and automotive sectors. The global market size is estimated to be expanding at a CAGR of 10-12%, driven by technological advancements and miniaturization trends. From a technical maturity perspective, the landscape shows varying degrees of sophistication. Industry leaders like Procter & Gamble (through iMFLUX), 3M Innovative Properties, and Fujitsu have established advanced qualifying standards and proprietary technologies. Academic institutions including Zhejiang University and Beijing University of Chemical Technology are contributing significant research to standardization efforts. Meanwhile, specialized players such as Mold-Masters, Taiwan Powder Technologies, and TOYO INNOVEX are developing niche expertise in precision molding technologies, creating a competitive ecosystem balancing innovation with practical implementation.
iMFLUX, Inc.
Technical Solution: iMFLUX has developed a revolutionary low-constant-pressure injection molding process specifically optimized for microinjection molding applications. Their technology utilizes adaptive pressure control algorithms that continuously monitor and adjust the pressure profile during the injection process, allowing for real-time compensation of material variations. The system employs proprietary sensor technology to detect microscopic flow front positions with precision down to 0.001mm, enabling unprecedented control over micro-feature formation. For qualifying standards, iMFLUX has established a comprehensive validation protocol that includes dimensional accuracy verification using high-resolution optical measurement systems, material characterization tests specific to micro-scale applications, and statistical process control methodologies tailored for high-volume micro-molding operations. Their platform integrates with Industry 4.0 systems to provide complete traceability of process parameters and part quality metrics, essential for medical and electronics applications where microinjection molding is critical.
Strengths: Superior control over material flow in micro-cavities, reduced internal stress in molded parts, and significantly lower rejection rates compared to conventional processes. Their adaptive control system excels in handling the high cooling rates characteristic of microinjection molding. Weaknesses: Requires specialized equipment upgrades, higher initial investment costs, and more extensive operator training compared to conventional injection molding systems.
Basell Polyolefine GmbH
Technical Solution: Basell Polyolefine has developed specialized polymer formulations specifically engineered for microinjection molding applications. Their technology centers on advanced metallocene catalysts that enable the production of ultra-homogeneous polyolefin grades with exceptionally narrow molecular weight distributions (Mw/Mn < 2.0) and controlled rheological properties optimized for micro-feature replication. For qualifying standards in microinjection molding, Basell has established the "MicroGrade" certification protocol that includes comprehensive material characterization focusing on micro-rheology, thermal stability during high-shear processing, and dimensional stability assessment at the micro-scale. Their materials undergo rigorous testing using specialized micro-rheometers capable of measuring viscosity behavior under the extreme shear rates (>100,000 s⁻¹) encountered in microinjection molding. Additionally, Basell has developed proprietary nucleating agents and processing stabilizers that enhance crystallization kinetics and prevent degradation during the rapid cooling cycles typical in microinjection molding, ensuring consistent part quality and dimensional stability for micro-components with wall thicknesses below 100 μm.
Strengths: Superior flow properties at micro-scale dimensions, exceptional lot-to-lot consistency critical for validated manufacturing processes, and enhanced surface finish quality for optical and precision components. Weaknesses: Premium pricing compared to standard polyolefin grades, more stringent processing window requirements, and limited color options due to specialized additive packages optimized for micro-molding.
Critical Patents and Technical Literature in Qualification Standards
Mould insert for an injection-moulding system, carrier substrate for such a mould insert, injection-moulding system with such a mould insert, and method for injection moulding
PatentWO2024183918A1
Innovation
- A deformable mold insert with a carrier substrate and structural element, designed for elastic deformation during demolding, which allows for improved demolding accuracy and throughput by enabling more than 50% volume deformation, and the use of microheating elements for localized temperature control to facilitate the injection molding process.
Precision sintering process for injecting low viscosity masses containing glass or metal powder into modular permanent injection molds made of mineral slip
PatentPendingFR3132656A1
Innovation
- Development of modular permanent injection molds made from mineral slip that can withstand high temperatures for precision sintering applications.
- Use of low viscosity masses containing glass or metal powder that can be injected at ambient temperature, eliminating the need for heating during the injection phase.
- Synchronous manufacturing process that combines primary molding and injection molding techniques to produce high-precision finished parts in glass or metal.
Material Compatibility and Selection Criteria
Material selection represents a critical factor in the success of microinjection molding applications, requiring careful consideration of both polymer properties and process compatibility. When evaluating materials for microinjection molding, manufacturers must first assess fundamental polymer characteristics including melt flow index (MFI), molecular weight distribution, and crystallinity structure. These properties directly influence the material's ability to fill microscale cavities with high precision and maintain dimensional stability during cooling.
Thermal properties constitute another essential consideration, particularly glass transition temperature (Tg) and melting point (Tm), which determine processing windows and cycle times. Materials with lower viscosity at processing temperatures typically demonstrate superior performance in microinjection applications, as they can more effectively fill intricate micro-features without excessive injection pressures that might damage delicate mold components.
Mechanical performance requirements must align with the intended application, balancing strength, flexibility, and durability. For medical microdevices, biocompatibility standards such as ISO 10993 compliance become mandatory, while electronic applications may prioritize electrical insulation properties or electromagnetic interference (EMI) shielding capabilities.
Environmental resistance factors, including chemical compatibility, moisture absorption tendencies, and UV stability, significantly impact long-term performance reliability. Materials exhibiting minimal shrinkage and warpage during processing are particularly valuable for maintaining tight dimensional tolerances essential in microcomponents, where deviations of even a few microns can render parts unusable.
The material selection process should incorporate standardized testing protocols specifically adapted for microinjection applications. These include modified spiral flow tests at microscale dimensions, micro-tensile testing for mechanical property verification, and specialized rheological assessments at high shear rates typical of microinjection processes. Such testing provides quantifiable data for comparing material performance under application-specific conditions.
Cost considerations must balance material price against processing efficiency and yield rates. Premium materials commanding higher prices may ultimately prove more economical when factoring reduced cycle times, lower reject rates, and enhanced part functionality. A systematic material selection matrix incorporating weighted criteria for technical requirements, processing compatibility, and economic factors offers the most comprehensive approach to material qualification for microinjection molding applications.
Thermal properties constitute another essential consideration, particularly glass transition temperature (Tg) and melting point (Tm), which determine processing windows and cycle times. Materials with lower viscosity at processing temperatures typically demonstrate superior performance in microinjection applications, as they can more effectively fill intricate micro-features without excessive injection pressures that might damage delicate mold components.
Mechanical performance requirements must align with the intended application, balancing strength, flexibility, and durability. For medical microdevices, biocompatibility standards such as ISO 10993 compliance become mandatory, while electronic applications may prioritize electrical insulation properties or electromagnetic interference (EMI) shielding capabilities.
Environmental resistance factors, including chemical compatibility, moisture absorption tendencies, and UV stability, significantly impact long-term performance reliability. Materials exhibiting minimal shrinkage and warpage during processing are particularly valuable for maintaining tight dimensional tolerances essential in microcomponents, where deviations of even a few microns can render parts unusable.
The material selection process should incorporate standardized testing protocols specifically adapted for microinjection applications. These include modified spiral flow tests at microscale dimensions, micro-tensile testing for mechanical property verification, and specialized rheological assessments at high shear rates typical of microinjection processes. Such testing provides quantifiable data for comparing material performance under application-specific conditions.
Cost considerations must balance material price against processing efficiency and yield rates. Premium materials commanding higher prices may ultimately prove more economical when factoring reduced cycle times, lower reject rates, and enhanced part functionality. A systematic material selection matrix incorporating weighted criteria for technical requirements, processing compatibility, and economic factors offers the most comprehensive approach to material qualification for microinjection molding applications.
Quality Assurance and Process Validation Frameworks
Quality assurance and process validation frameworks for microinjection molding applications require rigorous standards due to the precision-critical nature of this manufacturing process. Established frameworks typically incorporate a multi-tiered approach beginning with statistical process control (SPC) methodologies that enable real-time monitoring of critical process parameters such as injection pressure, melt temperature, and cooling time. These parameters must be continuously tracked against predetermined control limits to ensure process stability and repeatability.
Process validation in microinjection molding follows a three-phase methodology as recommended by regulatory bodies like FDA and ISO. The initial Installation Qualification (IQ) verifies that equipment specifications meet design requirements and are properly installed. Operational Qualification (OQ) confirms that the equipment operates within established parameters across its intended operating range. Performance Qualification (PQ) demonstrates consistent production of acceptable parts under normal operating conditions over extended production runs.
For medical and high-precision applications, validation protocols often incorporate Design of Experiments (DoE) techniques to establish robust process windows. This scientific approach identifies critical process parameters and their interactions, establishing acceptable ranges that consistently produce parts meeting dimensional and functional specifications. The resulting process capability indices (Cpk and Ppk) should typically exceed 1.33 for critical dimensions in validated processes.
Documentation requirements form a cornerstone of quality assurance frameworks, with comprehensive master validation plans, protocols, and reports being essential. These documents must detail acceptance criteria, sampling plans, and test methods with appropriate statistical justification. For regulated industries, these validation documents become part of the Device History File or Technical File submissions.
In-process quality control measures for microinjection molding include automated vision systems for dimensional verification, weight checks, and functional testing. Modern frameworks increasingly incorporate Industry 4.0 concepts with integrated sensors providing real-time feedback loops that can detect process deviations before defective parts are produced. Machine learning algorithms are being deployed to predict quality issues based on subtle parameter shifts.
Risk management methodologies such as Failure Mode and Effects Analysis (FMEA) are integrated into validation frameworks to identify potential failure modes, their causes, and appropriate mitigation strategies. This proactive approach ensures that validation activities focus on the most critical aspects of the process that could impact product quality or patient safety.
Revalidation requirements must be clearly defined within the quality system, typically triggered by significant changes to equipment, materials, processes, or after predetermined time intervals. This ensures that the validated state is maintained throughout the product lifecycle despite normal wear, environmental changes, or component replacements.
Process validation in microinjection molding follows a three-phase methodology as recommended by regulatory bodies like FDA and ISO. The initial Installation Qualification (IQ) verifies that equipment specifications meet design requirements and are properly installed. Operational Qualification (OQ) confirms that the equipment operates within established parameters across its intended operating range. Performance Qualification (PQ) demonstrates consistent production of acceptable parts under normal operating conditions over extended production runs.
For medical and high-precision applications, validation protocols often incorporate Design of Experiments (DoE) techniques to establish robust process windows. This scientific approach identifies critical process parameters and their interactions, establishing acceptable ranges that consistently produce parts meeting dimensional and functional specifications. The resulting process capability indices (Cpk and Ppk) should typically exceed 1.33 for critical dimensions in validated processes.
Documentation requirements form a cornerstone of quality assurance frameworks, with comprehensive master validation plans, protocols, and reports being essential. These documents must detail acceptance criteria, sampling plans, and test methods with appropriate statistical justification. For regulated industries, these validation documents become part of the Device History File or Technical File submissions.
In-process quality control measures for microinjection molding include automated vision systems for dimensional verification, weight checks, and functional testing. Modern frameworks increasingly incorporate Industry 4.0 concepts with integrated sensors providing real-time feedback loops that can detect process deviations before defective parts are produced. Machine learning algorithms are being deployed to predict quality issues based on subtle parameter shifts.
Risk management methodologies such as Failure Mode and Effects Analysis (FMEA) are integrated into validation frameworks to identify potential failure modes, their causes, and appropriate mitigation strategies. This proactive approach ensures that validation activities focus on the most critical aspects of the process that could impact product quality or patient safety.
Revalidation requirements must be clearly defined within the quality system, typically triggered by significant changes to equipment, materials, processes, or after predetermined time intervals. This ensures that the validated state is maintained throughout the product lifecycle despite normal wear, environmental changes, or component replacements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!