Ethyl Acetate for Eco-Friendly Industrial Solutions
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background and Objectives
Ethyl acetate, a versatile organic compound with the formula CH3COOC2H5, has been a staple in various industries for decades. Its journey from a laboratory curiosity to an industrial powerhouse began in the early 20th century, with its production and applications expanding significantly post-World War II. As environmental concerns have grown, the focus on ethyl acetate has shifted towards its potential as an eco-friendly alternative to more harmful solvents.
The evolution of ethyl acetate technology has been driven by the need for more efficient and sustainable production methods. Initially produced through the esterification of ethanol and acetic acid, newer processes have emerged, including the Tishchenko reaction and the dehydrogenation of ethanol. These advancements have not only improved yield and purity but also reduced energy consumption and waste generation, aligning with global sustainability goals.
In recent years, the push for green chemistry has placed ethyl acetate at the forefront of eco-friendly industrial solutions. Its low toxicity, biodegradability, and relatively low volatility make it an attractive option for replacing chlorinated and aromatic solvents in various applications. From coatings and adhesives to pharmaceutical processes and food additives, ethyl acetate's versatility continues to expand its market reach.
The primary objective of current research on ethyl acetate is to further enhance its eco-friendly profile while maintaining or improving its performance in industrial applications. This includes developing more sustainable production methods, exploring novel applications in green technologies, and optimizing its use to reduce overall environmental impact. Researchers are also investigating ways to integrate ethyl acetate into circular economy models, where it can be efficiently recycled or biodegraded after use.
Another key goal is to address the challenges associated with ethyl acetate's flammability and volatility. While these properties are less problematic than those of many alternative solvents, there is still room for improvement in safety and handling. Ongoing research aims to develop formulations or handling methods that mitigate these risks without compromising the compound's effectiveness or eco-friendly characteristics.
As industries worldwide face increasing pressure to adopt more sustainable practices, the demand for green solvents like ethyl acetate is expected to grow significantly. This trend is driving research into scaling up production, improving cost-effectiveness, and expanding the range of applications where ethyl acetate can replace less environmentally friendly alternatives. The ultimate aim is to position ethyl acetate as a cornerstone of eco-friendly industrial solutions, contributing to a more sustainable and environmentally responsible global manufacturing landscape.
The evolution of ethyl acetate technology has been driven by the need for more efficient and sustainable production methods. Initially produced through the esterification of ethanol and acetic acid, newer processes have emerged, including the Tishchenko reaction and the dehydrogenation of ethanol. These advancements have not only improved yield and purity but also reduced energy consumption and waste generation, aligning with global sustainability goals.
In recent years, the push for green chemistry has placed ethyl acetate at the forefront of eco-friendly industrial solutions. Its low toxicity, biodegradability, and relatively low volatility make it an attractive option for replacing chlorinated and aromatic solvents in various applications. From coatings and adhesives to pharmaceutical processes and food additives, ethyl acetate's versatility continues to expand its market reach.
The primary objective of current research on ethyl acetate is to further enhance its eco-friendly profile while maintaining or improving its performance in industrial applications. This includes developing more sustainable production methods, exploring novel applications in green technologies, and optimizing its use to reduce overall environmental impact. Researchers are also investigating ways to integrate ethyl acetate into circular economy models, where it can be efficiently recycled or biodegraded after use.
Another key goal is to address the challenges associated with ethyl acetate's flammability and volatility. While these properties are less problematic than those of many alternative solvents, there is still room for improvement in safety and handling. Ongoing research aims to develop formulations or handling methods that mitigate these risks without compromising the compound's effectiveness or eco-friendly characteristics.
As industries worldwide face increasing pressure to adopt more sustainable practices, the demand for green solvents like ethyl acetate is expected to grow significantly. This trend is driving research into scaling up production, improving cost-effectiveness, and expanding the range of applications where ethyl acetate can replace less environmentally friendly alternatives. The ultimate aim is to position ethyl acetate as a cornerstone of eco-friendly industrial solutions, contributing to a more sustainable and environmentally responsible global manufacturing landscape.
Market Demand Analysis for Green Solvents
The global market for green solvents has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations on volatile organic compounds (VOCs) emissions. Ethyl acetate, as a biodegradable and low-toxicity solvent, has emerged as a promising alternative to traditional petroleum-based solvents in various industrial applications.
The demand for ethyl acetate in eco-friendly industrial solutions is primarily fueled by its versatility and favorable environmental profile. Industries such as paints and coatings, adhesives, pharmaceuticals, and food processing are increasingly adopting ethyl acetate as a sustainable solvent option. This shift is particularly evident in regions with strict environmental regulations, such as Europe and North America.
In the paints and coatings sector, the market for ethyl acetate is expanding due to its excellent solvency properties and rapid evaporation rate. Manufacturers are reformulating their products to meet low-VOC requirements, driving the adoption of ethyl acetate as a key ingredient. The adhesives industry is another major consumer of ethyl acetate, with growing demand in packaging applications and consumer goods manufacturing.
The pharmaceutical industry represents a significant growth opportunity for ethyl acetate. Its use in drug formulation and as an extraction solvent in the production of active pharmaceutical ingredients is increasing. The food industry also contributes to the rising demand, utilizing ethyl acetate in the extraction of caffeine from coffee and tea, as well as in the production of food flavorings.
Market analysis indicates that the Asia-Pacific region is expected to witness the highest growth rate in ethyl acetate consumption for eco-friendly applications. This is attributed to rapid industrialization, increasing environmental awareness, and the implementation of stricter regulations in countries like China and India. North America and Europe continue to be mature markets with steady growth, driven by ongoing sustainability initiatives and consumer preferences for green products.
The market dynamics are further influenced by the growing trend of circular economy practices. Manufacturers are exploring bio-based sources for ethyl acetate production, such as fermentation of agricultural waste, to enhance its sustainability profile and meet the increasing demand for renewable chemicals. This trend is likely to create new opportunities and reshape the competitive landscape of the green solvents market.
Despite the positive outlook, challenges remain in the widespread adoption of ethyl acetate as a green solvent. These include the need for cost-effective production methods to compete with traditional solvents and the development of application-specific formulations to match the performance of conventional alternatives. Addressing these challenges will be crucial for the continued expansion of ethyl acetate in eco-friendly industrial solutions.
The demand for ethyl acetate in eco-friendly industrial solutions is primarily fueled by its versatility and favorable environmental profile. Industries such as paints and coatings, adhesives, pharmaceuticals, and food processing are increasingly adopting ethyl acetate as a sustainable solvent option. This shift is particularly evident in regions with strict environmental regulations, such as Europe and North America.
In the paints and coatings sector, the market for ethyl acetate is expanding due to its excellent solvency properties and rapid evaporation rate. Manufacturers are reformulating their products to meet low-VOC requirements, driving the adoption of ethyl acetate as a key ingredient. The adhesives industry is another major consumer of ethyl acetate, with growing demand in packaging applications and consumer goods manufacturing.
The pharmaceutical industry represents a significant growth opportunity for ethyl acetate. Its use in drug formulation and as an extraction solvent in the production of active pharmaceutical ingredients is increasing. The food industry also contributes to the rising demand, utilizing ethyl acetate in the extraction of caffeine from coffee and tea, as well as in the production of food flavorings.
Market analysis indicates that the Asia-Pacific region is expected to witness the highest growth rate in ethyl acetate consumption for eco-friendly applications. This is attributed to rapid industrialization, increasing environmental awareness, and the implementation of stricter regulations in countries like China and India. North America and Europe continue to be mature markets with steady growth, driven by ongoing sustainability initiatives and consumer preferences for green products.
The market dynamics are further influenced by the growing trend of circular economy practices. Manufacturers are exploring bio-based sources for ethyl acetate production, such as fermentation of agricultural waste, to enhance its sustainability profile and meet the increasing demand for renewable chemicals. This trend is likely to create new opportunities and reshape the competitive landscape of the green solvents market.
Despite the positive outlook, challenges remain in the widespread adoption of ethyl acetate as a green solvent. These include the need for cost-effective production methods to compete with traditional solvents and the development of application-specific formulations to match the performance of conventional alternatives. Addressing these challenges will be crucial for the continued expansion of ethyl acetate in eco-friendly industrial solutions.
Current Status and Challenges in Ethyl Acetate Production
The current status of ethyl acetate production is characterized by a mix of traditional and innovative approaches, with a growing emphasis on eco-friendly solutions. Conventional methods, such as the esterification of ethanol with acetic acid, remain widely used due to their established efficiency and cost-effectiveness. However, these processes often rely on petrochemical feedstocks and energy-intensive operations, raising environmental concerns.
In recent years, there has been a significant shift towards more sustainable production methods. Biotechnological approaches, utilizing enzymes or microorganisms for ethyl acetate synthesis, have gained traction. These bio-based processes offer the advantage of using renewable resources and operating under milder conditions, potentially reducing energy consumption and environmental impact.
The global ethyl acetate market has been experiencing steady growth, driven by increasing demand from various industries such as packaging, coatings, and pharmaceuticals. This growth has spurred research into more efficient and sustainable production methods. However, the industry faces several challenges in transitioning to greener alternatives.
One of the primary challenges is the economic viability of eco-friendly production methods compared to traditional processes. While bio-based approaches show promise, they often struggle to compete with the established petrochemical routes in terms of cost and scale. Overcoming this economic barrier requires further technological advancements and process optimizations.
Another significant challenge is the variability and availability of sustainable raw materials. Bio-based production methods often rely on agricultural feedstocks, which can be subject to price fluctuations and supply chain disruptions. Ensuring a stable and cost-effective supply of these renewable resources is crucial for the widespread adoption of green ethyl acetate production.
Technical challenges also persist in scaling up innovative production methods. Many promising eco-friendly techniques have been demonstrated at laboratory or pilot scales but face hurdles in industrial implementation. Issues such as product purity, reaction efficiency, and process stability need to be addressed to make these methods commercially viable.
Regulatory frameworks and environmental policies play a crucial role in shaping the industry's direction. While there is growing pressure to adopt more sustainable practices, the lack of uniform global standards and incentives can hinder the transition to greener production methods. Harmonizing regulations and providing appropriate incentives could accelerate the adoption of eco-friendly ethyl acetate production technologies.
In conclusion, the ethyl acetate industry is at a crossroads, balancing traditional production methods with the need for more sustainable alternatives. While progress has been made in developing eco-friendly solutions, significant challenges remain in terms of economic competitiveness, raw material sourcing, technical scalability, and regulatory support. Addressing these challenges will be crucial for the industry's transition towards more sustainable and environmentally responsible ethyl acetate production.
In recent years, there has been a significant shift towards more sustainable production methods. Biotechnological approaches, utilizing enzymes or microorganisms for ethyl acetate synthesis, have gained traction. These bio-based processes offer the advantage of using renewable resources and operating under milder conditions, potentially reducing energy consumption and environmental impact.
The global ethyl acetate market has been experiencing steady growth, driven by increasing demand from various industries such as packaging, coatings, and pharmaceuticals. This growth has spurred research into more efficient and sustainable production methods. However, the industry faces several challenges in transitioning to greener alternatives.
One of the primary challenges is the economic viability of eco-friendly production methods compared to traditional processes. While bio-based approaches show promise, they often struggle to compete with the established petrochemical routes in terms of cost and scale. Overcoming this economic barrier requires further technological advancements and process optimizations.
Another significant challenge is the variability and availability of sustainable raw materials. Bio-based production methods often rely on agricultural feedstocks, which can be subject to price fluctuations and supply chain disruptions. Ensuring a stable and cost-effective supply of these renewable resources is crucial for the widespread adoption of green ethyl acetate production.
Technical challenges also persist in scaling up innovative production methods. Many promising eco-friendly techniques have been demonstrated at laboratory or pilot scales but face hurdles in industrial implementation. Issues such as product purity, reaction efficiency, and process stability need to be addressed to make these methods commercially viable.
Regulatory frameworks and environmental policies play a crucial role in shaping the industry's direction. While there is growing pressure to adopt more sustainable practices, the lack of uniform global standards and incentives can hinder the transition to greener production methods. Harmonizing regulations and providing appropriate incentives could accelerate the adoption of eco-friendly ethyl acetate production technologies.
In conclusion, the ethyl acetate industry is at a crossroads, balancing traditional production methods with the need for more sustainable alternatives. While progress has been made in developing eco-friendly solutions, significant challenges remain in terms of economic competitiveness, raw material sourcing, technical scalability, and regulatory support. Addressing these challenges will be crucial for the industry's transition towards more sustainable and environmentally responsible ethyl acetate production.
Existing Green Production Methods for Ethyl Acetate
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of catalysts to improve yield and efficiency. The purification methods aim to obtain high-quality ethyl acetate suitable for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These processes aim to improve yield, efficiency, and purity of the final product.
- Applications of ethyl acetate in industrial processes: Ethyl acetate is utilized in diverse industrial applications, such as solvent extraction, coating formulations, and as a reaction medium. Its properties make it suitable for use in various manufacturing processes and chemical syntheses.
- Ethyl acetate in pharmaceutical and cosmetic formulations: The use of ethyl acetate in pharmaceutical and cosmetic products is explored, including its role as a solvent, excipient, or active ingredient. Its applications range from drug delivery systems to personal care products.
- Environmental and safety considerations for ethyl acetate: Research focuses on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener synthesis methods, reducing emissions, and enhancing handling procedures.
- Novel derivatives and modifications of ethyl acetate: Innovations in creating new derivatives or modified forms of ethyl acetate are presented. These modifications aim to enhance its properties or create new compounds with unique characteristics for specific applications.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes as a solvent, reactant, or intermediate. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. Its use in extraction processes and as a reaction medium is also highlighted.Expand Specific Solutions03 Ethyl acetate in formulations and compositions
Ethyl acetate is incorporated into various formulations and compositions for different purposes. These include adhesives, coatings, inks, and personal care products. Its properties as a solvent and its compatibility with other ingredients make it valuable in these applications.Expand Specific Solutions04 Recovery and recycling of ethyl acetate
Methods for recovering and recycling ethyl acetate from industrial processes are described. These techniques aim to reduce waste, improve process efficiency, and minimize environmental impact. Separation and purification steps are often involved in the recovery process.Expand Specific Solutions05 Ethyl acetate in sustainable and green chemistry
The use of ethyl acetate in sustainable and green chemistry applications is explored. This includes its role as a more environmentally friendly solvent alternative, its production from renewable resources, and its use in processes designed to reduce environmental impact.Expand Specific Solutions
Key Players in Ethyl Acetate Industry
The research on ethyl acetate for eco-friendly industrial solutions is in a growth phase, with increasing market size driven by demand for sustainable alternatives. The global ethyl acetate market is projected to expand significantly due to its versatile applications in various industries. Technologically, the field is advancing, with companies like Celanese International Corp., China Petroleum & Chemical Corp., and Eastman Chemical Co. leading innovation. These firms are developing improved production processes and exploring bio-based feedstocks to enhance the environmental profile of ethyl acetate. Academic institutions such as Nanjing Normal University and Tianjin University are contributing to fundamental research, while specialized chemical companies like Taixing Jinjiang Chemical Industry Co. Ltd. are focusing on application-specific developments, indicating a maturing technological landscape.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for eco-friendly ethyl acetate production using renewable feedstocks. Their approach involves the esterification of ethanol derived from biomass with acetic acid produced through fermentation[1]. This method significantly reduces the carbon footprint compared to traditional petrochemical routes. The company has also implemented advanced catalytic systems that enhance reaction efficiency and selectivity, resulting in higher yields and reduced energy consumption[3]. Additionally, Celanese has integrated membrane separation technology to purify the final product, minimizing waste and improving overall process sustainability[5].
Strengths: Renewable feedstock utilization, reduced carbon emissions, high product purity. Weaknesses: Potentially higher production costs, dependence on biomass availability.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has pioneered a green chemistry approach to ethyl acetate production, focusing on process intensification and waste reduction. Their method employs a novel reactive distillation technology that combines reaction and separation steps, significantly improving energy efficiency[2]. The company has also developed a proprietary catalyst system that enables lower reaction temperatures and pressures, further reducing the environmental impact[4]. Eastman's process incorporates a closed-loop solvent recovery system, minimizing emissions and maximizing resource utilization. Furthermore, they have implemented advanced process control strategies to optimize production parameters in real-time, ensuring consistent product quality while minimizing energy consumption[6].
Strengths: Energy-efficient process, reduced emissions, high product consistency. Weaknesses: Complex technology implementation, potential scalability challenges.
Innovative Approaches in Ethyl Acetate Synthesis
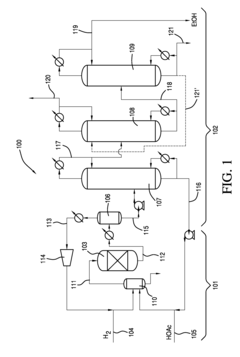
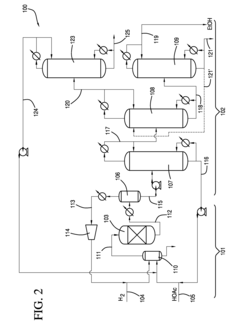
Process for producing an ethyl acetate solvent and co-production of ethanol
PatentInactiveUS20110190531A1
Innovation
- A process involving the hydrogenation of acetic acid in the presence of a catalyst, followed by a series of distillation columns to separate and recover ethanol and ethyl acetate solvent, with specific catalyst compositions and conditions to optimize ethanol and ethyl acetate production, including the use of platinum-based catalysts and modified silica supports.
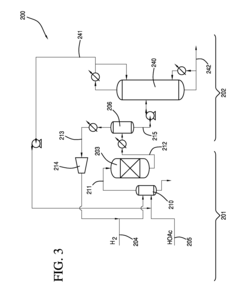
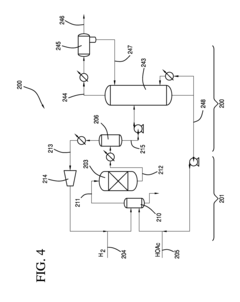
Processes for making ethyl acetate from acetic acid
PatentInactiveEP2493607A1
Innovation
- A process involving hydrogenation of acetic acid using catalysts composed of metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which achieve high selectivity to ethyl acetate while minimizing by-product formation.
Environmental Impact Assessment
The environmental impact assessment of ethyl acetate in eco-friendly industrial solutions reveals both positive and negative aspects. On the positive side, ethyl acetate is considered a more environmentally friendly solvent compared to many traditional alternatives. It has lower toxicity and is biodegradable, which reduces its long-term environmental persistence. The production of ethyl acetate through sustainable methods, such as using bio-based feedstocks, can significantly reduce its carbon footprint.
However, the environmental impact of ethyl acetate is not negligible. Its production still involves chemical processes that consume energy and resources. The manufacturing process may generate waste and emissions, although these are generally less harmful than those associated with more toxic solvents. Ethyl acetate is also volatile, contributing to atmospheric VOC (Volatile Organic Compound) levels, which can participate in the formation of ground-level ozone and smog.
In terms of water pollution, ethyl acetate has a relatively low impact due to its high biodegradability. It breaks down quickly in aquatic environments, reducing the risk of long-term contamination. However, large spills or improper disposal can still cause short-term damage to aquatic ecosystems.
The use of ethyl acetate in industrial applications can lead to improved environmental outcomes when replacing more harmful solvents. Its lower toxicity reduces the risk to workers and surrounding communities. Additionally, its high recovery and recycling potential in many applications contribute to a more circular economy, reducing overall waste and resource consumption.
Life cycle assessments of ethyl acetate-based solutions generally show favorable results compared to traditional alternatives. The reduced environmental impact during production, use, and disposal phases often outweighs the initial resource investment. This is particularly true when ethyl acetate is produced from renewable sources or industrial by-products.
Despite its eco-friendly profile, the large-scale use of ethyl acetate still requires careful management. Proper handling, storage, and disposal practices are essential to minimize environmental risks. Industrial users must implement effective recovery and recycling systems to maximize the environmental benefits of using ethyl acetate.
In conclusion, while ethyl acetate offers significant environmental advantages over many traditional solvents, its use still requires responsible management and continuous improvement in production and application technologies to fully realize its potential as an eco-friendly industrial solution.
However, the environmental impact of ethyl acetate is not negligible. Its production still involves chemical processes that consume energy and resources. The manufacturing process may generate waste and emissions, although these are generally less harmful than those associated with more toxic solvents. Ethyl acetate is also volatile, contributing to atmospheric VOC (Volatile Organic Compound) levels, which can participate in the formation of ground-level ozone and smog.
In terms of water pollution, ethyl acetate has a relatively low impact due to its high biodegradability. It breaks down quickly in aquatic environments, reducing the risk of long-term contamination. However, large spills or improper disposal can still cause short-term damage to aquatic ecosystems.
The use of ethyl acetate in industrial applications can lead to improved environmental outcomes when replacing more harmful solvents. Its lower toxicity reduces the risk to workers and surrounding communities. Additionally, its high recovery and recycling potential in many applications contribute to a more circular economy, reducing overall waste and resource consumption.
Life cycle assessments of ethyl acetate-based solutions generally show favorable results compared to traditional alternatives. The reduced environmental impact during production, use, and disposal phases often outweighs the initial resource investment. This is particularly true when ethyl acetate is produced from renewable sources or industrial by-products.
Despite its eco-friendly profile, the large-scale use of ethyl acetate still requires careful management. Proper handling, storage, and disposal practices are essential to minimize environmental risks. Industrial users must implement effective recovery and recycling systems to maximize the environmental benefits of using ethyl acetate.
In conclusion, while ethyl acetate offers significant environmental advantages over many traditional solvents, its use still requires responsible management and continuous improvement in production and application technologies to fully realize its potential as an eco-friendly industrial solution.
Regulatory Framework for Green Chemical Production
The regulatory framework for green chemical production plays a crucial role in promoting the use of eco-friendly industrial solutions, such as ethyl acetate. As governments and international organizations increasingly prioritize environmental protection and sustainable development, the chemical industry faces stricter regulations and guidelines for producing and using chemicals.
In the context of ethyl acetate production, regulatory bodies have implemented various measures to ensure its environmentally responsible manufacture and application. These regulations typically focus on reducing emissions, minimizing waste, and promoting the use of renewable feedstocks. For instance, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation requires manufacturers and importers to assess and manage the risks associated with chemicals, including ethyl acetate.
Many countries have also established specific guidelines for volatile organic compound (VOC) emissions, which directly impact ethyl acetate production and use. These regulations often set limits on VOC content in products and require the implementation of best available techniques (BAT) to minimize emissions during manufacturing processes.
The United States Environmental Protection Agency (EPA) has developed the Green Chemistry Program, which encourages the design of chemical products and processes that reduce or eliminate the generation of hazardous substances. This program provides a framework for developing more sustainable production methods for chemicals like ethyl acetate, emphasizing the use of renewable resources and energy-efficient processes.
In addition to national regulations, international standards such as ISO 14001 for environmental management systems provide guidelines for organizations to improve their environmental performance. These standards encourage companies to adopt lifecycle thinking and consider the environmental impact of their products from raw material extraction to disposal.
The regulatory landscape also includes incentives for green chemical production. Many governments offer tax breaks, grants, or other financial incentives to companies that invest in cleaner technologies or develop more sustainable chemical processes. These incentives aim to accelerate the transition towards greener industrial practices, including the production of eco-friendly solvents like ethyl acetate.
As the demand for sustainable solutions grows, regulatory bodies are increasingly collaborating with industry stakeholders to develop and refine guidelines for green chemical production. This collaborative approach ensures that regulations remain practical and effective while driving innovation in the chemical industry. For ethyl acetate and similar solvents, this may lead to the development of new production methods that further reduce environmental impact and improve overall sustainability.
In the context of ethyl acetate production, regulatory bodies have implemented various measures to ensure its environmentally responsible manufacture and application. These regulations typically focus on reducing emissions, minimizing waste, and promoting the use of renewable feedstocks. For instance, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation requires manufacturers and importers to assess and manage the risks associated with chemicals, including ethyl acetate.
Many countries have also established specific guidelines for volatile organic compound (VOC) emissions, which directly impact ethyl acetate production and use. These regulations often set limits on VOC content in products and require the implementation of best available techniques (BAT) to minimize emissions during manufacturing processes.
The United States Environmental Protection Agency (EPA) has developed the Green Chemistry Program, which encourages the design of chemical products and processes that reduce or eliminate the generation of hazardous substances. This program provides a framework for developing more sustainable production methods for chemicals like ethyl acetate, emphasizing the use of renewable resources and energy-efficient processes.
In addition to national regulations, international standards such as ISO 14001 for environmental management systems provide guidelines for organizations to improve their environmental performance. These standards encourage companies to adopt lifecycle thinking and consider the environmental impact of their products from raw material extraction to disposal.
The regulatory landscape also includes incentives for green chemical production. Many governments offer tax breaks, grants, or other financial incentives to companies that invest in cleaner technologies or develop more sustainable chemical processes. These incentives aim to accelerate the transition towards greener industrial practices, including the production of eco-friendly solvents like ethyl acetate.
As the demand for sustainable solutions grows, regulatory bodies are increasingly collaborating with industry stakeholders to develop and refine guidelines for green chemical production. This collaborative approach ensures that regulations remain practical and effective while driving innovation in the chemical industry. For ethyl acetate and similar solvents, this may lead to the development of new production methods that further reduce environmental impact and improve overall sustainability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!