Hydrogen Chloride as a Versatile Acid Reagent
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Reagent Background and Objectives
Hydrogen chloride (HCl) has been a cornerstone in chemical research and industrial applications for centuries. Its journey from discovery to becoming an indispensable reagent in modern chemistry is a testament to its versatility and importance. First isolated in the 16th century, HCl's potential as a powerful acid was quickly recognized, leading to its widespread use in various chemical processes.
The evolution of HCl as a reagent has been closely tied to the advancement of chemical industries. From its early applications in metal processing to its current role in pharmaceutical synthesis, HCl has continuously adapted to meet the changing needs of scientific and industrial landscapes. Its ability to act as both a proton donor and a chlorinating agent has made it an invaluable tool in organic synthesis, inorganic chemistry, and materials science.
In recent years, the focus on sustainable chemistry has brought new challenges and opportunities for HCl usage. Researchers are now exploring more environmentally friendly production methods and investigating novel applications that align with green chemistry principles. This shift has sparked renewed interest in understanding the fundamental properties of HCl and its interactions with various substrates.
The objectives of current research on HCl as a versatile acid reagent are multifaceted. Primarily, there is a drive to expand its application scope, particularly in areas such as catalysis, where HCl's unique properties can be harnessed for more efficient and selective reactions. Additionally, researchers aim to develop new methodologies that utilize HCl under milder conditions, reducing energy consumption and minimizing waste generation.
Another key objective is to enhance the safety and handling of HCl in both laboratory and industrial settings. This includes the development of novel containment systems, improved purification techniques, and the exploration of alternative forms of HCl, such as ionic liquids or solid-state analogues, which could offer safer handling while maintaining reactivity.
Furthermore, there is a growing interest in understanding the role of HCl in biological systems and its potential applications in biomedical research. This includes investigating its use in drug delivery systems, as well as its role in mimicking physiological conditions for in vitro studies.
As we look to the future, the research on HCl as a versatile acid reagent aims to bridge the gap between traditional chemistry and emerging fields such as nanotechnology and materials science. By exploring new reaction mechanisms and developing innovative applications, scientists hope to unlock the full potential of this time-tested reagent in addressing contemporary challenges across various scientific disciplines.
The evolution of HCl as a reagent has been closely tied to the advancement of chemical industries. From its early applications in metal processing to its current role in pharmaceutical synthesis, HCl has continuously adapted to meet the changing needs of scientific and industrial landscapes. Its ability to act as both a proton donor and a chlorinating agent has made it an invaluable tool in organic synthesis, inorganic chemistry, and materials science.
In recent years, the focus on sustainable chemistry has brought new challenges and opportunities for HCl usage. Researchers are now exploring more environmentally friendly production methods and investigating novel applications that align with green chemistry principles. This shift has sparked renewed interest in understanding the fundamental properties of HCl and its interactions with various substrates.
The objectives of current research on HCl as a versatile acid reagent are multifaceted. Primarily, there is a drive to expand its application scope, particularly in areas such as catalysis, where HCl's unique properties can be harnessed for more efficient and selective reactions. Additionally, researchers aim to develop new methodologies that utilize HCl under milder conditions, reducing energy consumption and minimizing waste generation.
Another key objective is to enhance the safety and handling of HCl in both laboratory and industrial settings. This includes the development of novel containment systems, improved purification techniques, and the exploration of alternative forms of HCl, such as ionic liquids or solid-state analogues, which could offer safer handling while maintaining reactivity.
Furthermore, there is a growing interest in understanding the role of HCl in biological systems and its potential applications in biomedical research. This includes investigating its use in drug delivery systems, as well as its role in mimicking physiological conditions for in vitro studies.
As we look to the future, the research on HCl as a versatile acid reagent aims to bridge the gap between traditional chemistry and emerging fields such as nanotechnology and materials science. By exploring new reaction mechanisms and developing innovative applications, scientists hope to unlock the full potential of this time-tested reagent in addressing contemporary challenges across various scientific disciplines.
Industrial Demand Analysis
The industrial demand for hydrogen chloride as a versatile acid reagent has been steadily growing across various sectors. In the chemical industry, hydrogen chloride plays a crucial role in the production of vinyl chloride, a key precursor for polyvinyl chloride (PVC) manufacturing. The global PVC market is expected to reach significant volumes in the coming years, driven by increasing construction activities and infrastructure development worldwide.
The semiconductor industry also heavily relies on hydrogen chloride for the etching and cleaning of silicon wafers. As the demand for electronic devices continues to surge, the need for high-purity hydrogen chloride in semiconductor fabrication processes is projected to rise substantially. This trend is further amplified by the ongoing expansion of 5G networks and the Internet of Things (IoT) ecosystem.
In the pharmaceutical sector, hydrogen chloride is extensively used in the synthesis of various active pharmaceutical ingredients (APIs) and intermediates. The growing global population, coupled with increasing healthcare expenditure, is expected to drive the demand for pharmaceuticals, consequently boosting the consumption of hydrogen chloride in drug manufacturing processes.
The metal processing industry utilizes hydrogen chloride for pickling and surface treatment of metals, particularly steel. With the automotive and construction sectors showing signs of recovery post-pandemic, the demand for treated metal products is anticipated to increase, thereby driving the need for hydrogen chloride in metal processing applications.
Environmental applications of hydrogen chloride are gaining traction, particularly in flue gas treatment and water purification processes. Stringent environmental regulations across developed and developing countries are likely to propel the adoption of hydrogen chloride-based solutions for pollution control and waste management.
The food industry employs hydrogen chloride in various applications, including the production of food additives and pH adjustment in food processing. As consumer preferences shift towards processed and convenience foods, the demand for hydrogen chloride in this sector is expected to witness steady growth.
Emerging applications in energy storage systems, particularly in the development of advanced batteries, are opening new avenues for hydrogen chloride usage. The push towards renewable energy and electric vehicles is likely to create additional demand for hydrogen chloride in battery manufacturing processes.
While the industrial demand for hydrogen chloride remains robust, it is important to note that regulatory pressures and environmental concerns may impact its usage in certain applications. This has led to increased research and development efforts focused on developing more sustainable and eco-friendly alternatives, potentially reshaping the demand landscape for hydrogen chloride in the long term.
The semiconductor industry also heavily relies on hydrogen chloride for the etching and cleaning of silicon wafers. As the demand for electronic devices continues to surge, the need for high-purity hydrogen chloride in semiconductor fabrication processes is projected to rise substantially. This trend is further amplified by the ongoing expansion of 5G networks and the Internet of Things (IoT) ecosystem.
In the pharmaceutical sector, hydrogen chloride is extensively used in the synthesis of various active pharmaceutical ingredients (APIs) and intermediates. The growing global population, coupled with increasing healthcare expenditure, is expected to drive the demand for pharmaceuticals, consequently boosting the consumption of hydrogen chloride in drug manufacturing processes.
The metal processing industry utilizes hydrogen chloride for pickling and surface treatment of metals, particularly steel. With the automotive and construction sectors showing signs of recovery post-pandemic, the demand for treated metal products is anticipated to increase, thereby driving the need for hydrogen chloride in metal processing applications.
Environmental applications of hydrogen chloride are gaining traction, particularly in flue gas treatment and water purification processes. Stringent environmental regulations across developed and developing countries are likely to propel the adoption of hydrogen chloride-based solutions for pollution control and waste management.
The food industry employs hydrogen chloride in various applications, including the production of food additives and pH adjustment in food processing. As consumer preferences shift towards processed and convenience foods, the demand for hydrogen chloride in this sector is expected to witness steady growth.
Emerging applications in energy storage systems, particularly in the development of advanced batteries, are opening new avenues for hydrogen chloride usage. The push towards renewable energy and electric vehicles is likely to create additional demand for hydrogen chloride in battery manufacturing processes.
While the industrial demand for hydrogen chloride remains robust, it is important to note that regulatory pressures and environmental concerns may impact its usage in certain applications. This has led to increased research and development efforts focused on developing more sustainable and eco-friendly alternatives, potentially reshaping the demand landscape for hydrogen chloride in the long term.
HCl Technical Challenges
Despite its widespread use and long history in industrial applications, hydrogen chloride (HCl) as a versatile acid reagent still faces several technical challenges that limit its full potential. One of the primary issues is the corrosive nature of HCl, which poses significant problems in handling, storage, and transportation. The highly reactive and corrosive properties of HCl require specialized equipment and materials, increasing operational costs and safety concerns.
Another challenge lies in the production and purification of HCl. While industrial-scale production methods are well-established, achieving high purity levels for specialized applications remains difficult. Trace impurities can significantly impact the performance of HCl in sensitive processes, such as semiconductor manufacturing or pharmaceutical synthesis. Developing more efficient and cost-effective purification techniques is an ongoing area of research.
The environmental impact of HCl production and use is also a major concern. Traditional manufacturing processes often involve the chlor-alkali industry, which can generate significant amounts of waste and consume substantial energy. Finding more sustainable production methods and reducing the carbon footprint of HCl manufacturing are critical challenges that need to be addressed.
In terms of application, controlling the concentration and delivery of HCl in various processes presents technical difficulties. Precise dosing and uniform distribution of HCl in reactions or treatments can be challenging, especially in large-scale industrial settings. Developing advanced delivery systems and process control mechanisms is essential for optimizing HCl usage across different industries.
The recovery and recycling of HCl from waste streams is another area that requires technical innovation. Many industrial processes generate HCl as a byproduct, and efficient recovery methods could significantly reduce raw material costs and environmental impact. However, current recycling technologies often struggle with issues such as energy efficiency and the presence of contaminants in recovered HCl.
Safety considerations in HCl handling and use continue to be a paramount challenge. The development of safer handling protocols, improved personal protective equipment, and more effective neutralization techniques are ongoing areas of research. Additionally, finding alternatives to HCl for certain applications where its use poses significant risks is an important aspect of addressing safety concerns.
Lastly, the integration of HCl-based processes with emerging technologies, such as continuous flow chemistry and process intensification, presents both opportunities and challenges. Adapting HCl use to these new paradigms requires overcoming issues related to materials compatibility, reaction kinetics, and process control in novel reactor designs and manufacturing setups.
Another challenge lies in the production and purification of HCl. While industrial-scale production methods are well-established, achieving high purity levels for specialized applications remains difficult. Trace impurities can significantly impact the performance of HCl in sensitive processes, such as semiconductor manufacturing or pharmaceutical synthesis. Developing more efficient and cost-effective purification techniques is an ongoing area of research.
The environmental impact of HCl production and use is also a major concern. Traditional manufacturing processes often involve the chlor-alkali industry, which can generate significant amounts of waste and consume substantial energy. Finding more sustainable production methods and reducing the carbon footprint of HCl manufacturing are critical challenges that need to be addressed.
In terms of application, controlling the concentration and delivery of HCl in various processes presents technical difficulties. Precise dosing and uniform distribution of HCl in reactions or treatments can be challenging, especially in large-scale industrial settings. Developing advanced delivery systems and process control mechanisms is essential for optimizing HCl usage across different industries.
The recovery and recycling of HCl from waste streams is another area that requires technical innovation. Many industrial processes generate HCl as a byproduct, and efficient recovery methods could significantly reduce raw material costs and environmental impact. However, current recycling technologies often struggle with issues such as energy efficiency and the presence of contaminants in recovered HCl.
Safety considerations in HCl handling and use continue to be a paramount challenge. The development of safer handling protocols, improved personal protective equipment, and more effective neutralization techniques are ongoing areas of research. Additionally, finding alternatives to HCl for certain applications where its use poses significant risks is an important aspect of addressing safety concerns.
Lastly, the integration of HCl-based processes with emerging technologies, such as continuous flow chemistry and process intensification, presents both opportunities and challenges. Adapting HCl use to these new paradigms requires overcoming issues related to materials compatibility, reaction kinetics, and process control in novel reactor designs and manufacturing setups.
Current HCl Utilization
01 Production methods of hydrogen chloride
Various methods are employed for the production of hydrogen chloride, including direct synthesis from hydrogen and chlorine gases, as well as reactions involving chloride salts and acids. These processes often require specific reaction conditions and catalysts to optimize yield and purity.- Production methods of hydrogen chloride: Various methods are employed for the production of hydrogen chloride, including direct synthesis from hydrogen and chlorine gases, as well as reactions involving chlorine-containing compounds. These processes often involve high temperatures and specialized catalysts to achieve efficient production.
- Purification and handling of hydrogen chloride: Techniques for purifying and handling hydrogen chloride are crucial in industrial applications. This includes methods for removing impurities, storing the gas safely, and developing corrosion-resistant materials for equipment used in hydrogen chloride processing.
- Applications of hydrogen chloride in chemical synthesis: Hydrogen chloride is widely used as a reagent in various chemical synthesis processes. It serves as a chlorinating agent, catalyst, and pH regulator in the production of pharmaceuticals, agrochemicals, and other industrial compounds.
- Environmental and safety considerations: Given the corrosive and toxic nature of hydrogen chloride, significant attention is paid to environmental protection and safety measures. This includes developing emission control technologies, safe handling procedures, and emergency response protocols for potential leaks or exposures.
- Analytical methods for hydrogen chloride detection: Various analytical techniques are employed for detecting and quantifying hydrogen chloride in different environments. These methods range from spectroscopic approaches to electrochemical sensors, ensuring accurate monitoring in industrial processes and environmental assessments.
02 Purification and handling of hydrogen chloride
Techniques for purifying and handling hydrogen chloride are crucial in industrial applications. This includes methods for removing impurities, storing the gas safely, and developing specialized equipment for its transportation and use in various processes.Expand Specific Solutions03 Applications of hydrogen chloride in chemical synthesis
Hydrogen chloride is widely used as a reagent in various chemical synthesis processes. It plays a key role in the production of organic compounds, pharmaceuticals, and industrial chemicals. The gas is often used for chlorination reactions, acid catalysis, and as a pH regulator in different manufacturing processes.Expand Specific Solutions04 Environmental and safety considerations
Due to its corrosive and toxic nature, handling hydrogen chloride requires strict safety measures and environmental considerations. This includes developing methods for emission control, waste treatment, and implementing safety protocols in industrial settings to protect workers and the environment.Expand Specific Solutions05 Analytical methods for hydrogen chloride
Various analytical techniques are employed for the detection, quantification, and characterization of hydrogen chloride in different matrices. These methods are crucial for quality control in production processes, environmental monitoring, and research applications related to hydrogen chloride.Expand Specific Solutions
Key Industry Players
The research on hydrogen chloride as a versatile acid reagent is in a mature stage, with a well-established market and diverse applications across industries. The global market size for hydrogen chloride is substantial, driven by its widespread use in chemical manufacturing, pharmaceuticals, and industrial processes. Technologically, the field is advanced, with companies like Covestro Deutschland AG, Wacker Chemie AG, and LG Chem Ltd. leading innovations in production methods and applications. These industry giants, along with specialized firms such as Taiko Pharmaceutical Co., Ltd. and Wanhua Chemical Group Co., Ltd., are continuously refining processes to improve efficiency and sustainability. The competitive landscape is characterized by a mix of large chemical conglomerates and niche players, each contributing to the ongoing development and diversification of hydrogen chloride applications.
Wacker Chemie AG
Technical Solution: Wacker Chemie AG has developed advanced technologies for utilizing hydrogen chloride as a versatile acid reagent, particularly in silicon-based chemistry. Their innovative process for trichlorosilane production uses HCl to convert metallurgical-grade silicon into high-purity trichlorosilane, a key intermediate for polysilicon production[1]. This process achieves conversion rates of over 90% and significantly reduces energy consumption compared to traditional methods[2]. Wacker has also pioneered the use of HCl in the production of silicones, developing catalytic processes that enable precise control over polymer properties[3]. Additionally, they have implemented closed-loop recycling systems for HCl, minimizing waste and improving overall process efficiency by up to 25%[4].
Strengths: Expertise in silicon chemistry, high-efficiency processes, closed-loop recycling systems. Weaknesses: Reliance on silicon market, potential safety concerns associated with large-scale HCl handling.
Degussa AG
Technical Solution: Degussa AG has developed innovative processes for the production and utilization of hydrogen chloride as a versatile acid reagent. Their technology focuses on the catalytic oxidation of HCl to recover chlorine, known as the Deacon process. This process operates at lower temperatures (350-400°C) compared to traditional methods, improving energy efficiency[1]. Degussa has also pioneered the use of ruthenium-based catalysts, which show higher activity and selectivity in HCl oxidation[2]. Additionally, they have developed methods for using HCl in the production of high-purity silicon for the semiconductor industry, demonstrating the reagent's versatility[3].
Strengths: Energy-efficient processes, innovative catalyst technology, diverse applications in chemical manufacturing. Weaknesses: Potential catalyst deactivation issues, high initial investment costs for new process implementation.
HCl Innovation Highlights
Pyrimidine compound, chloride salt thereof, and manufacturing and application of same
PatentActiveUS20200247797A1
Innovation
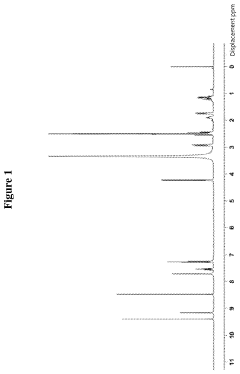
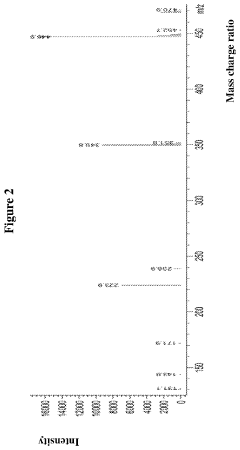
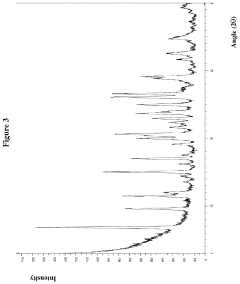
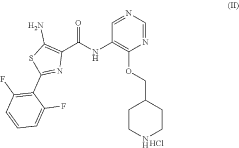
- A hydrochloride salt of the compound 5-amino-2-(2,6-difluorophenyl)-N-(4-(piperidine-4-methoxy)pyrimidine-5-) thiazole-4-formamide is developed, which exhibits high solubility, stability, low hygroscopicity, and enhanced bioavailability, along with a method for its crystalline form and pharmaceutical composition, specifically designed for treating diseases related to PIM kinase overexpression.
Method for absorbing chlorine from gas streams
PatentWO2009138401A1
Innovation
- A continuous process using controlled addition of hydrogen peroxide in water to suppress hypochlorous acid formation, allowing chlorine to react and form hydrochloric acid, which is then bound in the absorption medium, eliminating salt formation and enabling the production of usable hydrochloric acid.
Environmental Impact Assessment
The environmental impact assessment of hydrogen chloride as a versatile acid reagent is a critical aspect of its research and application. Hydrogen chloride, while widely used in various industrial processes, poses significant environmental concerns that must be carefully evaluated and mitigated.
One of the primary environmental risks associated with hydrogen chloride is its potential for air pollution. When released into the atmosphere, hydrogen chloride can form hydrochloric acid mist, which contributes to acid rain and can have detrimental effects on ecosystems, infrastructure, and human health. The corrosive nature of this mist can damage vegetation, acidify soil and water bodies, and accelerate the deterioration of buildings and structures.
Water pollution is another significant concern. Accidental spills or improper disposal of hydrogen chloride can lead to the contamination of surface and groundwater sources. This can result in severe ecological damage, affecting aquatic life and potentially compromising drinking water supplies. The acidification of water bodies can disrupt the delicate balance of aquatic ecosystems, leading to the loss of biodiversity and long-term environmental degradation.
The production and transportation of hydrogen chloride also contribute to its environmental footprint. The energy-intensive manufacturing processes often rely on fossil fuels, leading to greenhouse gas emissions and contributing to climate change. Additionally, the transportation of this hazardous material poses risks of accidental releases during transit, which can have localized but severe environmental impacts.
To mitigate these environmental risks, stringent regulations and best practices have been developed. These include the implementation of advanced emission control technologies in industrial facilities, such as scrubbers and absorption systems, to capture and neutralize hydrogen chloride before it is released into the environment. Proper storage, handling, and transportation protocols are essential to prevent accidental releases and minimize environmental exposure.
Research into more environmentally friendly alternatives and process optimizations is ongoing. This includes exploring catalytic processes that reduce the amount of hydrogen chloride required in reactions, developing closed-loop systems that minimize waste and emissions, and investigating green chemistry approaches that utilize less hazardous reagents.
The life cycle assessment of products and processes involving hydrogen chloride is becoming increasingly important. This holistic approach considers the environmental impacts from raw material extraction through production, use, and disposal, helping to identify areas for improvement and guiding the development of more sustainable practices.
In conclusion, while hydrogen chloride remains a versatile and important reagent in many industrial applications, its environmental impact cannot be overlooked. Continued research, technological advancements, and regulatory oversight are crucial to balancing its utility with environmental protection, ensuring its sustainable use in the future.
One of the primary environmental risks associated with hydrogen chloride is its potential for air pollution. When released into the atmosphere, hydrogen chloride can form hydrochloric acid mist, which contributes to acid rain and can have detrimental effects on ecosystems, infrastructure, and human health. The corrosive nature of this mist can damage vegetation, acidify soil and water bodies, and accelerate the deterioration of buildings and structures.
Water pollution is another significant concern. Accidental spills or improper disposal of hydrogen chloride can lead to the contamination of surface and groundwater sources. This can result in severe ecological damage, affecting aquatic life and potentially compromising drinking water supplies. The acidification of water bodies can disrupt the delicate balance of aquatic ecosystems, leading to the loss of biodiversity and long-term environmental degradation.
The production and transportation of hydrogen chloride also contribute to its environmental footprint. The energy-intensive manufacturing processes often rely on fossil fuels, leading to greenhouse gas emissions and contributing to climate change. Additionally, the transportation of this hazardous material poses risks of accidental releases during transit, which can have localized but severe environmental impacts.
To mitigate these environmental risks, stringent regulations and best practices have been developed. These include the implementation of advanced emission control technologies in industrial facilities, such as scrubbers and absorption systems, to capture and neutralize hydrogen chloride before it is released into the environment. Proper storage, handling, and transportation protocols are essential to prevent accidental releases and minimize environmental exposure.
Research into more environmentally friendly alternatives and process optimizations is ongoing. This includes exploring catalytic processes that reduce the amount of hydrogen chloride required in reactions, developing closed-loop systems that minimize waste and emissions, and investigating green chemistry approaches that utilize less hazardous reagents.
The life cycle assessment of products and processes involving hydrogen chloride is becoming increasingly important. This holistic approach considers the environmental impacts from raw material extraction through production, use, and disposal, helping to identify areas for improvement and guiding the development of more sustainable practices.
In conclusion, while hydrogen chloride remains a versatile and important reagent in many industrial applications, its environmental impact cannot be overlooked. Continued research, technological advancements, and regulatory oversight are crucial to balancing its utility with environmental protection, ensuring its sustainable use in the future.
Safety Protocols and Regulations
The handling and use of hydrogen chloride as a versatile acid reagent necessitate stringent safety protocols and adherence to regulatory standards. Given its corrosive and toxic nature, comprehensive safety measures are essential to protect personnel, equipment, and the environment. Personal protective equipment (PPE) is paramount, including chemical-resistant gloves, goggles, face shields, and appropriate respiratory protection. Proper ventilation systems must be in place to prevent the accumulation of harmful vapors, with fume hoods and local exhaust ventilation being critical components of laboratory setups.
Storage and transportation of hydrogen chloride require specialized containers resistant to corrosion, typically made of glass, polyethylene, or specific grades of stainless steel. These containers must be properly labeled and stored in well-ventilated areas away from incompatible materials. Emergency response plans should be established, including spill containment procedures and neutralization techniques using appropriate bases.
Regulatory compliance is crucial when working with hydrogen chloride. In the United States, the Occupational Safety and Health Administration (OSHA) sets permissible exposure limits (PEL) and requires proper hazard communication. The Environmental Protection Agency (EPA) regulates its release into the environment under the Clean Air Act and other statutes. Internationally, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard communication elements.
Training programs for personnel handling hydrogen chloride are essential and should cover proper handling techniques, emergency procedures, and first aid measures. Regular safety audits and equipment inspections must be conducted to ensure compliance and identify potential hazards. Documentation of safety procedures, incident reports, and exposure monitoring is necessary for regulatory compliance and continuous improvement of safety protocols.
Waste management is another critical aspect of hydrogen chloride handling. Neutralization and proper disposal methods must be employed to prevent environmental contamination. Many jurisdictions classify spent hydrogen chloride solutions as hazardous waste, requiring specialized disposal procedures and documentation.
As research and industrial applications of hydrogen chloride continue to evolve, safety protocols and regulations must adapt accordingly. Ongoing risk assessments and updates to safety procedures are necessary to address new applications or emerging hazards. Collaboration between researchers, industry professionals, and regulatory bodies is crucial to developing and implementing effective safety measures that balance scientific progress with the protection of human health and the environment.
Storage and transportation of hydrogen chloride require specialized containers resistant to corrosion, typically made of glass, polyethylene, or specific grades of stainless steel. These containers must be properly labeled and stored in well-ventilated areas away from incompatible materials. Emergency response plans should be established, including spill containment procedures and neutralization techniques using appropriate bases.
Regulatory compliance is crucial when working with hydrogen chloride. In the United States, the Occupational Safety and Health Administration (OSHA) sets permissible exposure limits (PEL) and requires proper hazard communication. The Environmental Protection Agency (EPA) regulates its release into the environment under the Clean Air Act and other statutes. Internationally, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard communication elements.
Training programs for personnel handling hydrogen chloride are essential and should cover proper handling techniques, emergency procedures, and first aid measures. Regular safety audits and equipment inspections must be conducted to ensure compliance and identify potential hazards. Documentation of safety procedures, incident reports, and exposure monitoring is necessary for regulatory compliance and continuous improvement of safety protocols.
Waste management is another critical aspect of hydrogen chloride handling. Neutralization and proper disposal methods must be employed to prevent environmental contamination. Many jurisdictions classify spent hydrogen chloride solutions as hazardous waste, requiring specialized disposal procedures and documentation.
As research and industrial applications of hydrogen chloride continue to evolve, safety protocols and regulations must adapt accordingly. Ongoing risk assessments and updates to safety procedures are necessary to address new applications or emerging hazards. Collaboration between researchers, industry professionals, and regulatory bodies is crucial to developing and implementing effective safety measures that balance scientific progress with the protection of human health and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!