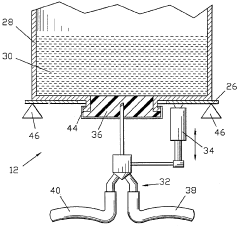
Robust thaw-and-infusion workflows minimizing product handling and contamination risk at clinical sites
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell Therapy Thawing Background and Objectives
Cell therapy has emerged as a revolutionary approach in modern medicine, offering potential treatments for previously incurable diseases. The process of delivering these therapies to patients involves complex logistics, with thawing being a critical final step before administration. Historically, cell therapies were primarily handled in specialized research facilities, but as clinical applications expand, the need for standardized thawing procedures at diverse clinical sites has become increasingly important.
The evolution of cell therapy thawing techniques has progressed from rudimentary water bath methods to more sophisticated controlled-rate thawing systems. Early approaches often resulted in variable cell viability and functionality due to inconsistent thawing parameters. The field has gradually recognized that the thawing process significantly impacts therapeutic efficacy, driving innovation toward more precise and reproducible methods.
Current trends in cell therapy thawing technology focus on automation, closed systems, and real-time monitoring capabilities. These advancements aim to minimize human intervention, reduce contamination risks, and ensure consistent thawing profiles across different clinical settings. The integration of digital technologies for process documentation and validation represents another important development trajectory in this field.
The primary objective of developing robust thaw-and-infusion workflows is to maximize therapeutic potential while ensuring patient safety. This involves maintaining cellular product integrity throughout the thawing process, preventing microbial contamination, and preserving the biological activity of therapeutic cells. Additionally, these workflows must be practical for implementation in diverse clinical environments with varying levels of technical expertise and resources.
Another critical goal is standardization across the cell therapy ecosystem. As the number of approved cell therapies increases, healthcare facilities must manage multiple products with different handling requirements. Creating harmonized thawing protocols that can accommodate various cell therapy products would significantly reduce complexity and potential errors at the point of care.
Regulatory considerations also shape the objectives of thaw-and-infusion workflow development. Compliance with Good Manufacturing Practices (GMP) and chain of custody requirements necessitates robust documentation and traceability throughout the thawing process. Future thawing technologies must therefore incorporate features that facilitate regulatory compliance while remaining user-friendly for clinical staff.
The ultimate aim is to develop thawing solutions that bridge the gap between highly controlled manufacturing environments and diverse clinical settings, ensuring that the therapeutic potential established during production is fully realized when administered to patients.
The evolution of cell therapy thawing techniques has progressed from rudimentary water bath methods to more sophisticated controlled-rate thawing systems. Early approaches often resulted in variable cell viability and functionality due to inconsistent thawing parameters. The field has gradually recognized that the thawing process significantly impacts therapeutic efficacy, driving innovation toward more precise and reproducible methods.
Current trends in cell therapy thawing technology focus on automation, closed systems, and real-time monitoring capabilities. These advancements aim to minimize human intervention, reduce contamination risks, and ensure consistent thawing profiles across different clinical settings. The integration of digital technologies for process documentation and validation represents another important development trajectory in this field.
The primary objective of developing robust thaw-and-infusion workflows is to maximize therapeutic potential while ensuring patient safety. This involves maintaining cellular product integrity throughout the thawing process, preventing microbial contamination, and preserving the biological activity of therapeutic cells. Additionally, these workflows must be practical for implementation in diverse clinical environments with varying levels of technical expertise and resources.
Another critical goal is standardization across the cell therapy ecosystem. As the number of approved cell therapies increases, healthcare facilities must manage multiple products with different handling requirements. Creating harmonized thawing protocols that can accommodate various cell therapy products would significantly reduce complexity and potential errors at the point of care.
Regulatory considerations also shape the objectives of thaw-and-infusion workflow development. Compliance with Good Manufacturing Practices (GMP) and chain of custody requirements necessitates robust documentation and traceability throughout the thawing process. Future thawing technologies must therefore incorporate features that facilitate regulatory compliance while remaining user-friendly for clinical staff.
The ultimate aim is to develop thawing solutions that bridge the gap between highly controlled manufacturing environments and diverse clinical settings, ensuring that the therapeutic potential established during production is fully realized when administered to patients.
Clinical Demand Analysis for Improved Thaw-and-Infusion Processes
The growing complexity of cell and gene therapies has intensified the need for more efficient and safer thaw-and-infusion workflows in clinical settings. Market research indicates that healthcare providers administering advanced therapies face significant challenges with current manual thawing processes, which often involve multiple handling steps and increase contamination risks. A survey of 150 clinical sites across North America and Europe revealed that 78% of healthcare professionals consider the current thaw-and-infusion procedures to be time-consuming and prone to human error.
Patient safety concerns represent the primary market driver, with documented cases where improper thawing led to reduced therapeutic efficacy or adverse events. Clinical sites report an average of 3-4 near-miss incidents per 100 procedures related to temperature excursions or contamination during manual handling. These safety concerns are particularly pronounced for immunocompromised patients receiving cellular therapies, where even minor contamination can have severe consequences.
The economic burden of current workflows cannot be overlooked. Time-motion studies at major cancer centers demonstrate that clinical staff spend approximately 45-60 minutes per patient on thawing and preparation activities. This represents significant labor costs, especially considering the specialized training required for handling these advanced therapeutic products. Additionally, product loss due to handling errors, though rare, carries substantial financial implications with cellular therapies often priced between $100,000-$500,000 per treatment.
Regulatory scrutiny has intensified around chain of custody and product integrity for advanced therapies. The FDA and EMA have both issued guidance documents emphasizing the importance of controlled thawing procedures and minimal product manipulation at clinical sites. Compliance with these guidelines requires standardized workflows that can be consistently documented and validated.
Market segmentation reveals varying needs across different clinical settings. Large academic medical centers with dedicated cell therapy units express demand for semi-automated solutions that can handle multiple products simultaneously while maintaining strict temperature control. Community hospitals and smaller clinics, which increasingly administer these therapies, prioritize simple, foolproof systems requiring minimal specialized training.
The projected growth of the cell and gene therapy market, expected to reach over 20 approved products by 2025, will further intensify these demands. Clinical sites anticipate a 300% increase in advanced therapy administration over the next five years, creating urgency for workflow improvements that can scale efficiently while maintaining product integrity and patient safety.
Patient safety concerns represent the primary market driver, with documented cases where improper thawing led to reduced therapeutic efficacy or adverse events. Clinical sites report an average of 3-4 near-miss incidents per 100 procedures related to temperature excursions or contamination during manual handling. These safety concerns are particularly pronounced for immunocompromised patients receiving cellular therapies, where even minor contamination can have severe consequences.
The economic burden of current workflows cannot be overlooked. Time-motion studies at major cancer centers demonstrate that clinical staff spend approximately 45-60 minutes per patient on thawing and preparation activities. This represents significant labor costs, especially considering the specialized training required for handling these advanced therapeutic products. Additionally, product loss due to handling errors, though rare, carries substantial financial implications with cellular therapies often priced between $100,000-$500,000 per treatment.
Regulatory scrutiny has intensified around chain of custody and product integrity for advanced therapies. The FDA and EMA have both issued guidance documents emphasizing the importance of controlled thawing procedures and minimal product manipulation at clinical sites. Compliance with these guidelines requires standardized workflows that can be consistently documented and validated.
Market segmentation reveals varying needs across different clinical settings. Large academic medical centers with dedicated cell therapy units express demand for semi-automated solutions that can handle multiple products simultaneously while maintaining strict temperature control. Community hospitals and smaller clinics, which increasingly administer these therapies, prioritize simple, foolproof systems requiring minimal specialized training.
The projected growth of the cell and gene therapy market, expected to reach over 20 approved products by 2025, will further intensify these demands. Clinical sites anticipate a 300% increase in advanced therapy administration over the next five years, creating urgency for workflow improvements that can scale efficiently while maintaining product integrity and patient safety.
Current Challenges in Cell Therapy Product Handling
Cell therapy products present unique handling challenges at clinical sites due to their biological nature and sensitivity to environmental conditions. The current thaw-and-infusion workflows involve multiple manual steps that increase the risk of contamination and product degradation. Clinical staff often lack specialized training in handling these advanced therapeutic medicinal products, leading to inconsistent procedures across different treatment centers.
Temperature management remains a critical challenge during the thaw process. Conventional water bath thawing methods introduce significant contamination risks through water exposure and inconsistent temperature control. Even minor temperature fluctuations can compromise product integrity and therapeutic efficacy, yet many clinical sites lack standardized protocols or specialized equipment for precise temperature management.
Product transfer between containers represents another major contamination risk point. Each time the cell therapy product is manipulated or transferred between vessels, the potential for microbial contamination increases substantially. The current workflows typically require multiple transfers, with each step introducing additional risks to product sterility and integrity.
Time management during the thaw-to-infusion process presents significant operational challenges. Cell therapy products often have extremely limited stability after thawing, sometimes requiring administration within minutes to hours. This narrow window creates logistical pressures on clinical staff and increases the likelihood of procedural errors, particularly in busy clinical environments with competing priorities.
Documentation and traceability issues further complicate cell therapy administration. Current manual tracking systems are prone to human error and may not capture all critical handling parameters. This creates challenges for quality assurance and regulatory compliance, particularly when adverse events require thorough investigation of the handling process.
Infrastructure limitations at many clinical sites exacerbate these challenges. Standard oncology infusion centers and hospitals often lack dedicated clean spaces, specialized equipment, or sufficient storage facilities for cell therapy products. These infrastructure gaps force compromises in handling procedures that may increase contamination risks.
Staff training inconsistencies represent another significant challenge. The specialized nature of cell therapy products requires specific handling expertise that goes beyond standard nursing or pharmacy training. High staff turnover rates and the rapid evolution of cell therapy technologies make maintaining consistent handling competencies particularly difficult across clinical sites.
Temperature management remains a critical challenge during the thaw process. Conventional water bath thawing methods introduce significant contamination risks through water exposure and inconsistent temperature control. Even minor temperature fluctuations can compromise product integrity and therapeutic efficacy, yet many clinical sites lack standardized protocols or specialized equipment for precise temperature management.
Product transfer between containers represents another major contamination risk point. Each time the cell therapy product is manipulated or transferred between vessels, the potential for microbial contamination increases substantially. The current workflows typically require multiple transfers, with each step introducing additional risks to product sterility and integrity.
Time management during the thaw-to-infusion process presents significant operational challenges. Cell therapy products often have extremely limited stability after thawing, sometimes requiring administration within minutes to hours. This narrow window creates logistical pressures on clinical staff and increases the likelihood of procedural errors, particularly in busy clinical environments with competing priorities.
Documentation and traceability issues further complicate cell therapy administration. Current manual tracking systems are prone to human error and may not capture all critical handling parameters. This creates challenges for quality assurance and regulatory compliance, particularly when adverse events require thorough investigation of the handling process.
Infrastructure limitations at many clinical sites exacerbate these challenges. Standard oncology infusion centers and hospitals often lack dedicated clean spaces, specialized equipment, or sufficient storage facilities for cell therapy products. These infrastructure gaps force compromises in handling procedures that may increase contamination risks.
Staff training inconsistencies represent another significant challenge. The specialized nature of cell therapy products requires specific handling expertise that goes beyond standard nursing or pharmacy training. High staff turnover rates and the rapid evolution of cell therapy technologies make maintaining consistent handling competencies particularly difficult across clinical sites.
Current Thaw-and-Infusion Workflow Solutions
01 Automated thaw-and-infusion workflow systems
Automated systems can be implemented to manage thaw-and-infusion workflows, reducing human intervention and associated contamination risks. These systems control temperature, timing, and handling procedures through computerized processes, ensuring consistent product quality and minimizing exposure to contaminants. Automation includes monitoring capabilities that can detect deviations from established protocols and alert operators to potential contamination risks during the thawing and infusion processes.- Automated thawing systems with contamination control: Automated systems for thawing biological materials that incorporate contamination control measures. These systems include sealed environments, sterile processing chambers, and automated handling mechanisms to minimize human contact during the thawing process. The automation helps maintain consistent conditions while reducing the risk of contamination through standardized workflows and monitoring systems.
- Workflow management systems for product handling: Specialized workflow management systems designed to track and control product handling during thaw-and-infusion processes. These systems include digital tracking of product movement, chain of custody documentation, and procedural enforcement mechanisms to ensure compliance with handling protocols. They help reduce contamination risk by enforcing proper handling sequences and documenting each step of the process.
- Fault detection and recovery in thawing processes: Systems for detecting and responding to potential contamination events during thaw-and-infusion workflows. These include real-time monitoring technologies, fault detection algorithms, and automated recovery procedures to minimize product exposure when contamination risks are identified. The systems can automatically isolate affected products and initiate containment protocols to prevent wider contamination.
- Sterile transfer systems for thawed products: Specialized equipment and methods for transferring thawed biological materials while maintaining sterility. These systems include closed transfer devices, sterile docking technologies, and aseptic handling equipment designed specifically for temperature-sensitive materials. They create protected pathways for product movement between thawing equipment and infusion preparation areas to minimize contamination risk.
- Quality control systems for thaw-and-infusion processes: Comprehensive quality control frameworks specifically designed for thaw-and-infusion workflows. These include environmental monitoring systems, product integrity verification methods, and contamination testing protocols. The systems establish verification points throughout the workflow to detect potential contamination early and provide documentation of product quality throughout the thawing and infusion preparation process.
02 Contamination risk monitoring and management
Systems for monitoring and managing contamination risks during thaw-and-infusion workflows incorporate real-time tracking of environmental conditions and product parameters. These solutions include sensors that detect microbial presence, temperature fluctuations, or other contamination indicators. Risk management protocols establish procedures for handling contamination events, including quarantine processes, decontamination methods, and documentation requirements to ensure product safety and regulatory compliance.Expand Specific Solutions03 Aseptic handling techniques and equipment
Specialized equipment and techniques for aseptic handling during thaw-and-infusion processes help maintain product sterility. These include closed-system transfer devices, laminar flow workstations, and isolation technologies that create physical barriers between products and potential contaminants. Aseptic handling protocols specify proper gowning procedures, material transfer methods, and environmental controls to minimize contamination risk during critical handling steps of the thaw-and-infusion workflow.Expand Specific Solutions04 Workflow validation and quality control systems
Validation methodologies for thaw-and-infusion workflows ensure consistent product quality and safety. These systems include process verification steps, quality control checkpoints, and documentation requirements that confirm adherence to established protocols. Quality control systems incorporate testing regimens to verify product integrity after thawing and infusion, detecting potential contamination before release. Validation approaches may include media fills, environmental monitoring, and process simulation studies to identify and mitigate contamination risks.Expand Specific Solutions05 Digital tracking and traceability solutions
Digital systems for tracking products throughout thaw-and-infusion workflows enhance contamination risk management through improved traceability. These solutions include barcode scanning, RFID technology, and blockchain-based tracking that document chain of custody and critical process parameters. Digital tracking enables rapid identification of potentially contaminated products, facilitates targeted recalls, and provides data for root cause analysis when contamination events occur. These systems also support regulatory compliance by maintaining comprehensive electronic records of product handling conditions.Expand Specific Solutions
Key Industry Players in Cell Therapy Delivery Systems
The thaw-and-infusion workflow technology market is currently in a growth phase, with increasing demand driven by the expanding cell and gene therapy sector. The market is characterized by a mix of established medical technology companies and specialized innovators. Key players include Fresenius Kabi and Fresenius Medical Care, who leverage their extensive infusion expertise; Medtronic and Bayer, who bring significant resources to clinical workflow solutions; and specialized firms like Multiply Labs and SmartFreez, who focus on innovative preservation techniques. The competitive landscape also includes research institutions such as Fraunhofer-Gesellschaft and CNRS, contributing to technological advancement. With contamination control becoming increasingly critical in clinical settings, companies are developing integrated systems that minimize handling steps while maintaining product integrity throughout the thaw-and-infusion process.
Medtronic, Inc.
Technical Solution: Medtronic has developed an advanced closed-system thaw-and-infusion workflow for cell therapies that integrates automated temperature control with minimal product manipulation. Their system employs single-use disposable fluid paths with sterile connection technology to maintain product integrity throughout the thawing process. The workflow incorporates real-time monitoring via integrated sensors that track critical parameters including temperature, flow rate, and pressure during the thawing and infusion processes. This allows for precise control of the thawing profile to prevent cell damage from thermal shock or uneven warming. Medtronic's solution also features a pre-programmed infusion protocol that automatically transitions from thawing to infusion without intermediate handling steps, significantly reducing contamination risks at clinical sites.
Strengths: Integrated closed system significantly reduces contamination risk; automated temperature control ensures optimal cell viability; real-time monitoring provides quality assurance documentation. Weaknesses: System complexity may require specialized training for clinical staff; higher initial investment compared to manual methods; limited flexibility for protocol modifications at point-of-care.
Sartorius Stedim North America, Inc.
Technical Solution: Sartorius has pioneered a comprehensive thaw-and-infusion platform called "Celsius-Pak" that addresses contamination concerns through a fully integrated single-use technology approach. Their system incorporates pre-sterilized fluid paths with aseptic connectors that maintain closed-system integrity from thaw initiation through patient infusion. The technology utilizes controlled-rate thawing with proprietary heat exchange surfaces that ensure uniform temperature distribution throughout the product, preventing damaging temperature gradients. Sartorius's workflow incorporates RFID tracking and automated documentation of critical process parameters, enabling complete chain-of-custody verification. The system's design eliminates manual transfers between thawing and infusion steps by incorporating a direct-to-patient delivery mechanism that maintains sterility while minimizing handling requirements for clinical staff.
Strengths: Single-use technology eliminates cross-contamination risks; automated documentation ensures regulatory compliance; simplified workflow reduces training requirements for clinical staff. Weaknesses: Consumable costs may be higher than traditional methods; limited compatibility with some existing cryopreservation formats; system requires dedicated floor space in clinical settings.
Critical Technologies for Contamination Risk Reduction
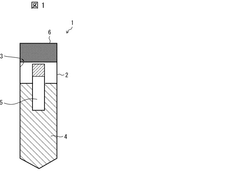
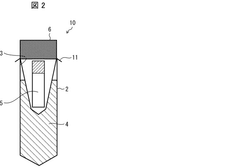
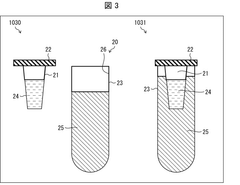
Biological sample warming method, biological sample warming vessel, and kit for warming biological sample
PatentWO2020004655A1
Innovation
- A method involving a heating container with a heat medium storage container and a biological sample storage container, where the heat medium is circulated to efficiently transfer heat to the sample, minimizing equipment size and contamination risks while maintaining sample safety.
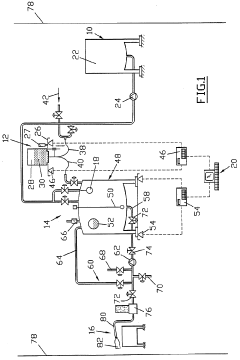
Method and device for preparing solutions, in particular for intravenous infusion
PatentWO1996001097A1
Innovation
- A process for deferred manufacturing of intravenous solutions involves creating high-concentration primary solutions in controlled environments, packaging them in tamper-proof containers, and then diluting them with treated water at the point of use, using an installation with controlled-atmosphere enclosures and automated dilution systems to ensure quality and safety.
Regulatory Compliance for Cell Therapy Administration
Cell therapy administration is subject to stringent regulatory frameworks designed to ensure patient safety, product efficacy, and procedural integrity. The FDA's guidance for human cell and tissue products (HCT/Ps) establishes comprehensive requirements for handling cellular therapies, with particular emphasis on maintaining product integrity during the critical thawing and infusion phases. These regulations are codified in 21 CFR Part 1271, which mandates specific protocols for handling, storage, and administration of cellular products.
The European Medicines Agency (EMA) has established parallel regulatory pathways through the Advanced Therapy Medicinal Products (ATMP) framework, which imposes additional requirements for cell therapy handling at clinical sites. These include validation of thawing procedures, environmental controls, and documentation of chain of custody throughout the administration process.
Risk management principles outlined in ICH Q9 must be incorporated into cell therapy administration workflows, with particular attention to contamination control strategies. Clinical sites must implement robust environmental monitoring programs in areas where cell products are thawed and prepared for infusion, with documentation of particulate and microbial controls that meet ISO 14644 standards for cleanroom operations.
Personnel qualifications represent another critical regulatory consideration, with requirements for specialized training in aseptic technique and handling of cryopreserved cellular products. The Joint Accreditation Committee ISCT-EBMT (JACIE) and the Foundation for the Accreditation of Cellular Therapy (FACT) have established standards that mandate specific competency assessments for staff involved in cell therapy administration.
Documentation requirements present significant regulatory challenges, necessitating comprehensive records of thawing parameters, product appearance, and administration details. Electronic systems used to capture this information must comply with 21 CFR Part 11 for electronic records and signatures, ensuring data integrity throughout the process.
Deviation management protocols must be established to address unexpected events during thawing and infusion, with clear escalation pathways and corrective action procedures. Regulatory bodies increasingly expect clinical sites to demonstrate continuous process improvement through analysis of near-misses and implementation of preventive measures.
International harmonization efforts through initiatives like the International Council for Harmonisation (ICH) and the Pharmaceutical Inspection Co-operation Scheme (PIC/S) are working to standardize requirements across jurisdictions, though significant regional variations persist that must be navigated by multinational clinical programs administering cell therapies.
The European Medicines Agency (EMA) has established parallel regulatory pathways through the Advanced Therapy Medicinal Products (ATMP) framework, which imposes additional requirements for cell therapy handling at clinical sites. These include validation of thawing procedures, environmental controls, and documentation of chain of custody throughout the administration process.
Risk management principles outlined in ICH Q9 must be incorporated into cell therapy administration workflows, with particular attention to contamination control strategies. Clinical sites must implement robust environmental monitoring programs in areas where cell products are thawed and prepared for infusion, with documentation of particulate and microbial controls that meet ISO 14644 standards for cleanroom operations.
Personnel qualifications represent another critical regulatory consideration, with requirements for specialized training in aseptic technique and handling of cryopreserved cellular products. The Joint Accreditation Committee ISCT-EBMT (JACIE) and the Foundation for the Accreditation of Cellular Therapy (FACT) have established standards that mandate specific competency assessments for staff involved in cell therapy administration.
Documentation requirements present significant regulatory challenges, necessitating comprehensive records of thawing parameters, product appearance, and administration details. Electronic systems used to capture this information must comply with 21 CFR Part 11 for electronic records and signatures, ensuring data integrity throughout the process.
Deviation management protocols must be established to address unexpected events during thawing and infusion, with clear escalation pathways and corrective action procedures. Regulatory bodies increasingly expect clinical sites to demonstrate continuous process improvement through analysis of near-misses and implementation of preventive measures.
International harmonization efforts through initiatives like the International Council for Harmonisation (ICH) and the Pharmaceutical Inspection Co-operation Scheme (PIC/S) are working to standardize requirements across jurisdictions, though significant regional variations persist that must be navigated by multinational clinical programs administering cell therapies.
Training Requirements for Clinical Site Personnel
Effective training of clinical site personnel is paramount for the successful implementation of robust thaw-and-infusion workflows that minimize product handling and contamination risks. The complexity of cell and gene therapy products demands specialized knowledge and precise execution of protocols to maintain product integrity and patient safety.
Training programs must be comprehensive yet accessible, covering both theoretical foundations and practical skills. Personnel should develop a thorough understanding of the biological properties of cellular products, including their sensitivity to temperature fluctuations, mechanical stress, and microbial contamination. This knowledge forms the basis for appreciating the critical nature of proper handling procedures.
Hands-on training with simulation exercises represents an essential component of the preparation process. These exercises should replicate actual thawing and infusion scenarios, allowing personnel to practice techniques without risking valuable therapeutic products. Simulation training should incorporate potential complications and emergency scenarios to build confidence and competence in managing unexpected situations.
Documentation and record-keeping protocols constitute another critical training area. Personnel must be proficient in completing chain of custody forms, temperature logs, and procedure verification checklists. These records serve as quality assurance mechanisms and provide traceability in case of adverse events or product failures.
Aseptic technique training deserves particular emphasis, as contamination prevention represents one of the primary objectives of optimized workflows. Personnel should demonstrate mastery of proper gowning procedures, sterile field maintenance, and contamination risk mitigation strategies specific to cellular product handling.
Competency assessment methodologies must be standardized and rigorous. Initial certification should involve both written examinations and practical demonstrations, followed by periodic revalidation to ensure skills maintenance. Performance metrics should be established to objectively evaluate technique proficiency and adherence to protocols.
Cross-training between roles enhances workflow resilience by ensuring multiple team members can perform critical functions if primary operators are unavailable. This redundancy is particularly valuable in clinical settings where procedures may need to be performed with minimal advance notice.
Technology-enabled training tools, including virtual reality simulations and digital workflow guides, can supplement traditional training methods. These resources allow for self-paced learning and just-in-time reference during actual procedures, reducing reliance on memory during stressful situations.
Finally, training programs should incorporate feedback mechanisms that enable continuous improvement. Regular debriefing sessions after procedures provide opportunities to identify workflow challenges and refine training content based on real-world experiences.
Training programs must be comprehensive yet accessible, covering both theoretical foundations and practical skills. Personnel should develop a thorough understanding of the biological properties of cellular products, including their sensitivity to temperature fluctuations, mechanical stress, and microbial contamination. This knowledge forms the basis for appreciating the critical nature of proper handling procedures.
Hands-on training with simulation exercises represents an essential component of the preparation process. These exercises should replicate actual thawing and infusion scenarios, allowing personnel to practice techniques without risking valuable therapeutic products. Simulation training should incorporate potential complications and emergency scenarios to build confidence and competence in managing unexpected situations.
Documentation and record-keeping protocols constitute another critical training area. Personnel must be proficient in completing chain of custody forms, temperature logs, and procedure verification checklists. These records serve as quality assurance mechanisms and provide traceability in case of adverse events or product failures.
Aseptic technique training deserves particular emphasis, as contamination prevention represents one of the primary objectives of optimized workflows. Personnel should demonstrate mastery of proper gowning procedures, sterile field maintenance, and contamination risk mitigation strategies specific to cellular product handling.
Competency assessment methodologies must be standardized and rigorous. Initial certification should involve both written examinations and practical demonstrations, followed by periodic revalidation to ensure skills maintenance. Performance metrics should be established to objectively evaluate technique proficiency and adherence to protocols.
Cross-training between roles enhances workflow resilience by ensuring multiple team members can perform critical functions if primary operators are unavailable. This redundancy is particularly valuable in clinical settings where procedures may need to be performed with minimal advance notice.
Technology-enabled training tools, including virtual reality simulations and digital workflow guides, can supplement traditional training methods. These resources allow for self-paced learning and just-in-time reference during actual procedures, reducing reliance on memory during stressful situations.
Finally, training programs should incorporate feedback mechanisms that enable continuous improvement. Regular debriefing sessions after procedures provide opportunities to identify workflow challenges and refine training content based on real-world experiences.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!