SERS Substrates Application in Drug Monitoring Technologies
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SERS Substrate Technology Background and Objectives
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique since its discovery in the 1970s. The phenomenon, based on the enhancement of Raman scattering signals when molecules are adsorbed on roughened metal surfaces, has evolved from a scientific curiosity to a valuable tool with diverse applications. The historical development of SERS technology has been marked by significant breakthroughs in understanding the underlying physical mechanisms, particularly the electromagnetic and chemical enhancement effects that contribute to signal amplification by factors of 10^6 to 10^14.
In the context of drug monitoring technologies, SERS substrates represent a convergence of nanotechnology, materials science, and analytical chemistry. The evolution of SERS substrates has progressed from simple roughened electrodes to sophisticated engineered nanostructures with precisely controlled geometries and compositions. This technological advancement has been driven by the increasing demand for sensitive, rapid, and non-destructive methods for detecting and monitoring pharmaceutical compounds in various matrices.
The primary objective of SERS substrate technology in drug monitoring is to develop highly sensitive, reproducible, and stable platforms capable of detecting therapeutic drugs at clinically relevant concentrations. This includes monitoring drug levels in biological fluids for personalized medicine applications, detecting illicit drugs for forensic purposes, and quality control in pharmaceutical manufacturing. The technology aims to overcome limitations of traditional drug monitoring methods such as chromatography and mass spectrometry, which often require complex sample preparation, expensive equipment, and specialized operators.
Current technological trends in SERS substrates focus on enhancing sensitivity through optimized nanostructure design, improving reproducibility through standardized fabrication processes, and expanding applicability through surface functionalization strategies. The integration of SERS with microfluidic systems and portable Raman spectrometers represents a significant direction toward point-of-care applications, enabling real-time drug monitoring in clinical settings.
The global research landscape shows accelerating interest in SERS-based drug monitoring, with publications increasing exponentially over the past decade. This growth reflects both the maturation of fundamental SERS technology and the recognition of its potential to address unmet needs in therapeutic drug monitoring, particularly for drugs with narrow therapeutic windows where precise concentration monitoring is critical for patient safety and treatment efficacy.
Looking forward, the technical goals for SERS substrates in drug monitoring include achieving single-molecule detection sensitivity for a broader range of pharmaceutical compounds, developing multiplexed detection capabilities for simultaneous monitoring of multiple drugs, and creating user-friendly integrated systems that can be deployed in diverse healthcare settings, from sophisticated hospital laboratories to resource-limited environments.
In the context of drug monitoring technologies, SERS substrates represent a convergence of nanotechnology, materials science, and analytical chemistry. The evolution of SERS substrates has progressed from simple roughened electrodes to sophisticated engineered nanostructures with precisely controlled geometries and compositions. This technological advancement has been driven by the increasing demand for sensitive, rapid, and non-destructive methods for detecting and monitoring pharmaceutical compounds in various matrices.
The primary objective of SERS substrate technology in drug monitoring is to develop highly sensitive, reproducible, and stable platforms capable of detecting therapeutic drugs at clinically relevant concentrations. This includes monitoring drug levels in biological fluids for personalized medicine applications, detecting illicit drugs for forensic purposes, and quality control in pharmaceutical manufacturing. The technology aims to overcome limitations of traditional drug monitoring methods such as chromatography and mass spectrometry, which often require complex sample preparation, expensive equipment, and specialized operators.
Current technological trends in SERS substrates focus on enhancing sensitivity through optimized nanostructure design, improving reproducibility through standardized fabrication processes, and expanding applicability through surface functionalization strategies. The integration of SERS with microfluidic systems and portable Raman spectrometers represents a significant direction toward point-of-care applications, enabling real-time drug monitoring in clinical settings.
The global research landscape shows accelerating interest in SERS-based drug monitoring, with publications increasing exponentially over the past decade. This growth reflects both the maturation of fundamental SERS technology and the recognition of its potential to address unmet needs in therapeutic drug monitoring, particularly for drugs with narrow therapeutic windows where precise concentration monitoring is critical for patient safety and treatment efficacy.
Looking forward, the technical goals for SERS substrates in drug monitoring include achieving single-molecule detection sensitivity for a broader range of pharmaceutical compounds, developing multiplexed detection capabilities for simultaneous monitoring of multiple drugs, and creating user-friendly integrated systems that can be deployed in diverse healthcare settings, from sophisticated hospital laboratories to resource-limited environments.
Market Demand Analysis for SERS-Based Drug Monitoring
The global market for SERS-based drug monitoring technologies is experiencing significant growth, driven by increasing concerns about medication adherence, therapeutic drug monitoring, and drug abuse detection. According to recent market analyses, the therapeutic drug monitoring market is projected to reach $2.7 billion by 2025, with SERS-based technologies capturing an expanding share due to their superior sensitivity and specificity compared to traditional methods.
Healthcare providers worldwide are seeking more efficient point-of-care testing solutions that can deliver rapid results while maintaining high accuracy. This demand is particularly pronounced in hospital settings where therapeutic drug monitoring for antibiotics, immunosuppressants, and antiepileptic drugs requires precise concentration measurements to ensure optimal dosing and minimize adverse effects. The ability of SERS substrates to detect drug molecules at nanomolar or even picomolar concentrations addresses this critical clinical need.
The pharmaceutical industry represents another significant market segment, with growing investment in drug development monitoring tools. SERS technology offers valuable capabilities for tracking drug metabolism, biodistribution, and pharmacokinetics during preclinical and clinical trials. This application alone is estimated to generate over $500 million in market value by 2027, as pharmaceutical companies increasingly adopt advanced analytical techniques to accelerate drug development pipelines.
Law enforcement and forensic agencies constitute a rapidly expanding market for SERS-based drug detection technologies. The global drug testing market, valued at approximately $6.3 billion, is increasingly turning toward more sensitive detection methods capable of identifying novel synthetic drugs and lower concentration samples. SERS substrates offer particular advantages in this field due to their ability to provide chemical fingerprints of substances with minimal sample preparation.
Consumer health monitoring represents an emerging application area with substantial growth potential. The wearable medical device market, currently valued at $21.3 billion, is beginning to incorporate SERS-based sensors for continuous drug monitoring applications. This trend aligns with the broader shift toward personalized medicine and patient-centered healthcare delivery models.
Regional market analysis indicates that North America currently dominates the SERS-based drug monitoring market, accounting for approximately 40% of global revenue. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by increasing healthcare expenditure, expanding research infrastructure, and growing awareness about precision medicine approaches in countries like China, Japan, and South Korea.
Healthcare providers worldwide are seeking more efficient point-of-care testing solutions that can deliver rapid results while maintaining high accuracy. This demand is particularly pronounced in hospital settings where therapeutic drug monitoring for antibiotics, immunosuppressants, and antiepileptic drugs requires precise concentration measurements to ensure optimal dosing and minimize adverse effects. The ability of SERS substrates to detect drug molecules at nanomolar or even picomolar concentrations addresses this critical clinical need.
The pharmaceutical industry represents another significant market segment, with growing investment in drug development monitoring tools. SERS technology offers valuable capabilities for tracking drug metabolism, biodistribution, and pharmacokinetics during preclinical and clinical trials. This application alone is estimated to generate over $500 million in market value by 2027, as pharmaceutical companies increasingly adopt advanced analytical techniques to accelerate drug development pipelines.
Law enforcement and forensic agencies constitute a rapidly expanding market for SERS-based drug detection technologies. The global drug testing market, valued at approximately $6.3 billion, is increasingly turning toward more sensitive detection methods capable of identifying novel synthetic drugs and lower concentration samples. SERS substrates offer particular advantages in this field due to their ability to provide chemical fingerprints of substances with minimal sample preparation.
Consumer health monitoring represents an emerging application area with substantial growth potential. The wearable medical device market, currently valued at $21.3 billion, is beginning to incorporate SERS-based sensors for continuous drug monitoring applications. This trend aligns with the broader shift toward personalized medicine and patient-centered healthcare delivery models.
Regional market analysis indicates that North America currently dominates the SERS-based drug monitoring market, accounting for approximately 40% of global revenue. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by increasing healthcare expenditure, expanding research infrastructure, and growing awareness about precision medicine approaches in countries like China, Japan, and South Korea.
Current SERS Substrate Development Status and Challenges
Surface-Enhanced Raman Spectroscopy (SERS) substrate technology has witnessed significant advancements globally, with research institutions and commercial entities actively pursuing innovations in this field. Currently, the development of SERS substrates for drug monitoring applications faces several critical challenges despite promising progress. The primary technical hurdle remains achieving consistent enhancement factors across substrate surfaces, as variations in nanoscale features can lead to significant performance inconsistencies, compromising reliability in clinical drug monitoring scenarios.
Sensitivity limitations present another substantial challenge, particularly when detecting drugs at physiologically relevant concentrations (often nanomolar or lower). While laboratory demonstrations have shown impressive detection limits, translating these capabilities to real-world biological matrices containing proteins, salts, and other interfering compounds remains problematic. The complex nature of biological samples often leads to non-specific binding and signal interference, reducing the effectiveness of many current SERS substrates.
Stability issues also plague existing SERS substrate technologies. Many high-performance substrates demonstrate rapid degradation under biological conditions or lose enhancement capabilities during storage, limiting their practical application in routine clinical settings. This instability necessitates either immediate use after production or complex preservation methods, both of which complicate implementation in standard healthcare environments.
Manufacturing scalability represents a significant bottleneck in the widespread adoption of SERS for drug monitoring. Many advanced SERS substrates rely on sophisticated nanofabrication techniques such as electron-beam lithography or focused ion beam milling, which are inherently low-throughput and high-cost. The inability to mass-produce consistent, high-quality substrates at reasonable costs has restricted their application primarily to research settings rather than clinical practice.
Geographically, SERS substrate development exhibits distinct regional characteristics. North American and European research institutions lead in fundamental innovation and novel substrate design, while Asian manufacturers, particularly in China and South Korea, have made significant progress in scaling production technologies. This geographical distribution has created an innovation-implementation gap that slows clinical translation.
Regulatory challenges further complicate the landscape, as SERS-based diagnostic technologies must navigate complex approval pathways before clinical implementation. The lack of standardized testing protocols for SERS substrate performance evaluation makes regulatory compliance particularly challenging, creating additional barriers to commercialization.
Despite these challenges, recent breakthroughs in self-assembled nanostructures, flexible substrate materials, and machine learning-assisted design approaches are gradually addressing these limitations, suggesting promising directions for future development in SERS-based drug monitoring technologies.
Sensitivity limitations present another substantial challenge, particularly when detecting drugs at physiologically relevant concentrations (often nanomolar or lower). While laboratory demonstrations have shown impressive detection limits, translating these capabilities to real-world biological matrices containing proteins, salts, and other interfering compounds remains problematic. The complex nature of biological samples often leads to non-specific binding and signal interference, reducing the effectiveness of many current SERS substrates.
Stability issues also plague existing SERS substrate technologies. Many high-performance substrates demonstrate rapid degradation under biological conditions or lose enhancement capabilities during storage, limiting their practical application in routine clinical settings. This instability necessitates either immediate use after production or complex preservation methods, both of which complicate implementation in standard healthcare environments.
Manufacturing scalability represents a significant bottleneck in the widespread adoption of SERS for drug monitoring. Many advanced SERS substrates rely on sophisticated nanofabrication techniques such as electron-beam lithography or focused ion beam milling, which are inherently low-throughput and high-cost. The inability to mass-produce consistent, high-quality substrates at reasonable costs has restricted their application primarily to research settings rather than clinical practice.
Geographically, SERS substrate development exhibits distinct regional characteristics. North American and European research institutions lead in fundamental innovation and novel substrate design, while Asian manufacturers, particularly in China and South Korea, have made significant progress in scaling production technologies. This geographical distribution has created an innovation-implementation gap that slows clinical translation.
Regulatory challenges further complicate the landscape, as SERS-based diagnostic technologies must navigate complex approval pathways before clinical implementation. The lack of standardized testing protocols for SERS substrate performance evaluation makes regulatory compliance particularly challenging, creating additional barriers to commercialization.
Despite these challenges, recent breakthroughs in self-assembled nanostructures, flexible substrate materials, and machine learning-assisted design approaches are gradually addressing these limitations, suggesting promising directions for future development in SERS-based drug monitoring technologies.
Current SERS Substrate Solutions for Drug Monitoring
01 Metal nanostructure-based SERS substrates
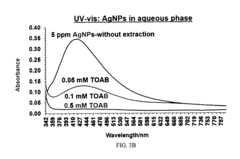
Metal nanostructures, particularly those made of gold, silver, or copper, serve as effective SERS substrates due to their plasmonic properties. These substrates can be fabricated in various forms including nanoparticles, nanorods, and nanopatterned surfaces. The localized surface plasmon resonance generated by these metal nanostructures significantly enhances the Raman signal, allowing for highly sensitive molecular detection. The enhancement factor can reach several orders of magnitude, making these substrates suitable for trace analysis applications.- Metallic nanostructures for SERS substrates: Metallic nanostructures are widely used as SERS substrates due to their plasmonic properties that enhance Raman signals. These structures can be fabricated using various methods including lithography, deposition, and chemical synthesis. The size, shape, and arrangement of these nanostructures can be optimized to achieve maximum enhancement factors. Common materials include gold, silver, and copper nanoparticles or nanopatterned surfaces that provide localized surface plasmon resonance effects.
- Flexible and portable SERS substrates: Flexible SERS substrates enable analysis on non-flat surfaces and can be integrated into portable detection systems. These substrates are typically fabricated on polymer or paper-based materials with deposited metallic nanostructures. The flexibility allows for conformal contact with irregular sample surfaces while maintaining enhancement capabilities. These substrates are particularly useful for field applications, point-of-care diagnostics, and environmental monitoring where traditional rigid substrates may be impractical.
- Semiconductor-based SERS substrates: Semiconductor materials can be used as alternative SERS substrates or in combination with metals to create hybrid structures. These substrates utilize charge-transfer mechanisms in addition to electromagnetic enhancement to amplify Raman signals. Materials such as silicon, titanium dioxide, and zinc oxide can be structured into nanopillars, nanowires, or porous architectures to create effective SERS platforms. The semiconductor-based substrates often offer improved stability and reproducibility compared to pure metallic substrates.
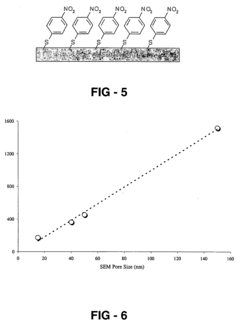
- Fabrication methods for high-sensitivity SERS substrates: Advanced fabrication techniques are employed to create SERS substrates with high sensitivity and reproducibility. These methods include nanolithography, self-assembly, template-assisted growth, and laser ablation. The fabrication processes focus on creating hotspots with intense electromagnetic fields where Raman enhancement is maximized. Control over nanogap dimensions, surface roughness, and structural periodicity is crucial for achieving consistent enhancement factors across the substrate surface.
- Functionalized SERS substrates for specific detection applications: SERS substrates can be functionalized with specific recognition elements to enable selective detection of target analytes. Surface modification with antibodies, aptamers, or molecular receptors allows for highly specific molecular recognition. These functionalized substrates combine the sensitivity of SERS with the selectivity of molecular recognition, making them powerful tools for biosensing, environmental monitoring, and medical diagnostics. The functionalization strategies are designed to maintain the enhancement properties while adding molecular specificity.
02 Fabrication methods for SERS substrates
Various fabrication techniques are employed to create SERS substrates with optimal enhancement properties. These include lithographic methods, chemical synthesis, physical vapor deposition, and template-assisted approaches. Advanced nanofabrication techniques allow for precise control over the size, shape, and spacing of nanostructures, which are critical parameters affecting SERS enhancement. Novel manufacturing processes aim to create reproducible substrates with uniform enhancement factors across the entire surface area.Expand Specific Solutions03 Flexible and portable SERS substrates
Flexible SERS substrates are designed for applications requiring conformable sensing surfaces or portable detection systems. These substrates typically incorporate plasmonic nanostructures on flexible polymer films or paper-based materials. The flexibility allows the substrate to maintain SERS activity while conforming to irregular surfaces, enabling in-situ analysis in various environments. Portable SERS substrates are particularly valuable for field testing, point-of-care diagnostics, and environmental monitoring applications.Expand Specific Solutions04 SERS substrate enhancement strategies
Various strategies are employed to enhance the performance of SERS substrates, including the creation of hot spots, core-shell structures, and hierarchical architectures. Hot spots, regions of extremely high electromagnetic field enhancement, can be engineered by controlling the gap between adjacent nanostructures. Additionally, combining SERS substrates with other materials such as graphene or quantum dots can provide synergistic enhancement effects. These enhancement strategies aim to improve sensitivity, selectivity, and reproducibility of SERS measurements.Expand Specific Solutions05 Application-specific SERS substrates
SERS substrates are increasingly being tailored for specific applications such as biomedical diagnostics, food safety testing, environmental monitoring, and security screening. These specialized substrates may incorporate molecular recognition elements, such as antibodies or aptamers, to enhance selectivity for target analytes. Some designs focus on multiplexed detection capabilities, allowing simultaneous analysis of multiple analytes. Application-specific SERS substrates often balance enhancement factors with other properties such as stability, biocompatibility, and ease of use.Expand Specific Solutions
Key Industry Players in SERS Substrate Manufacturing
Surface-Enhanced Raman Spectroscopy (SERS) substrates for drug monitoring technologies are emerging as a critical analytical tool in the early growth phase of precision medicine. The market is expanding rapidly, projected to reach significant scale as healthcare systems increasingly adopt real-time therapeutic drug monitoring. Technologically, the field shows varying maturity levels across applications, with companies like Hamamatsu Photonics and Corning leading commercial development of advanced substrates, while academic institutions such as Jilin University and Northwestern University drive fundamental research innovations. Research organizations including Naval Research Laboratory and Korea Research Institute of Chemical Technology are bridging the gap between theoretical advancements and practical applications. The competitive landscape features established photonics companies alongside specialized startups, with pharmaceutical entities like Baxter International beginning to integrate these technologies into clinical workflows.
Jilin University
Technical Solution: Jilin University has developed advanced SERS substrates using noble metal nanostructures for drug monitoring applications. Their technology employs precisely controlled silver and gold nanoparticles arranged in optimized patterns to create "hot spots" that significantly enhance Raman signals. The university's research team has pioneered a novel approach combining electrochemical deposition and nanolithography to create reproducible SERS substrates with enhancement factors exceeding 10^8[1]. Their substrates feature specialized surface modifications that improve selectivity for specific drug molecules, particularly antibiotics and chemotherapy agents in biological fluids. Recent innovations include a microfluidic-integrated SERS platform that enables real-time therapeutic drug monitoring with detection limits in the nanomolar range[2], allowing for precise dosage adjustments in clinical settings. The university has also developed portable SERS devices paired with machine learning algorithms for rapid drug concentration analysis at point-of-care.
Strengths: Exceptional sensitivity with detection limits reaching nanomolar concentrations; highly reproducible manufacturing process ensuring consistent results; specialized surface chemistry for improved drug molecule selectivity. Weaknesses: Higher production costs compared to conventional methods; limited shelf-life of some substrate types; requires specialized equipment for optimal performance in clinical settings.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory has developed sophisticated SERS substrates specifically optimized for drug monitoring in challenging environments. Their proprietary technology utilizes gold-coated silicon nanopillars with precisely controlled dimensions and spacing to create consistent "hot spots" for Raman signal enhancement. These substrates incorporate specialized surface chemistry that resists biofouling in complex biological matrices like blood and urine, making them ideal for therapeutic drug monitoring applications[3]. The laboratory has pioneered a unique fabrication approach combining electron beam lithography and reactive ion etching to achieve enhancement factors exceeding 10^7 consistently across the substrate surface. Their recent innovations include field-deployable SERS platforms integrated with microfluidic sample preparation systems that can detect multiple drug compounds simultaneously at sub-nanogram levels[4]. The technology has been successfully tested for monitoring antimalarial and antibiotic drug levels in military personnel under field conditions, providing rapid results within minutes rather than hours or days required by conventional methods.
Strengths: Exceptional durability in harsh environments; consistent performance across different batches; minimal sample preparation requirements; rapid analysis capabilities suitable for field deployment. Weaknesses: Higher production costs compared to commercial alternatives; requires specialized training for optimal use; limited commercial availability outside military applications.
Critical SERS Enhancement Mechanisms and Patents
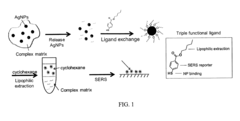
Systems and methods for extraction and surface-enhanced raman spectroscopy detection of metal nanoparticles
PatentInactiveUS20170052123A1
Innovation
- The use of surface-enhanced Raman spectroscopy (SERS) combined with organic substances like ligands and flavonoids to extract and detect MNPs, facilitating their separation and providing a distinct Raman signal for imaging, allowing for nondestructive and sensitive analysis.
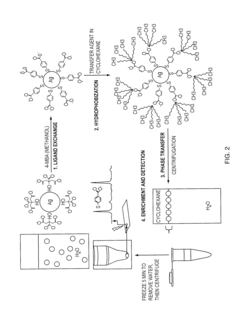
Surface enhanced raman spectroscopy (SERS) substrates exhibiting uniform high enhancement and stability
PatentInactiveUS20060061762A1
Innovation
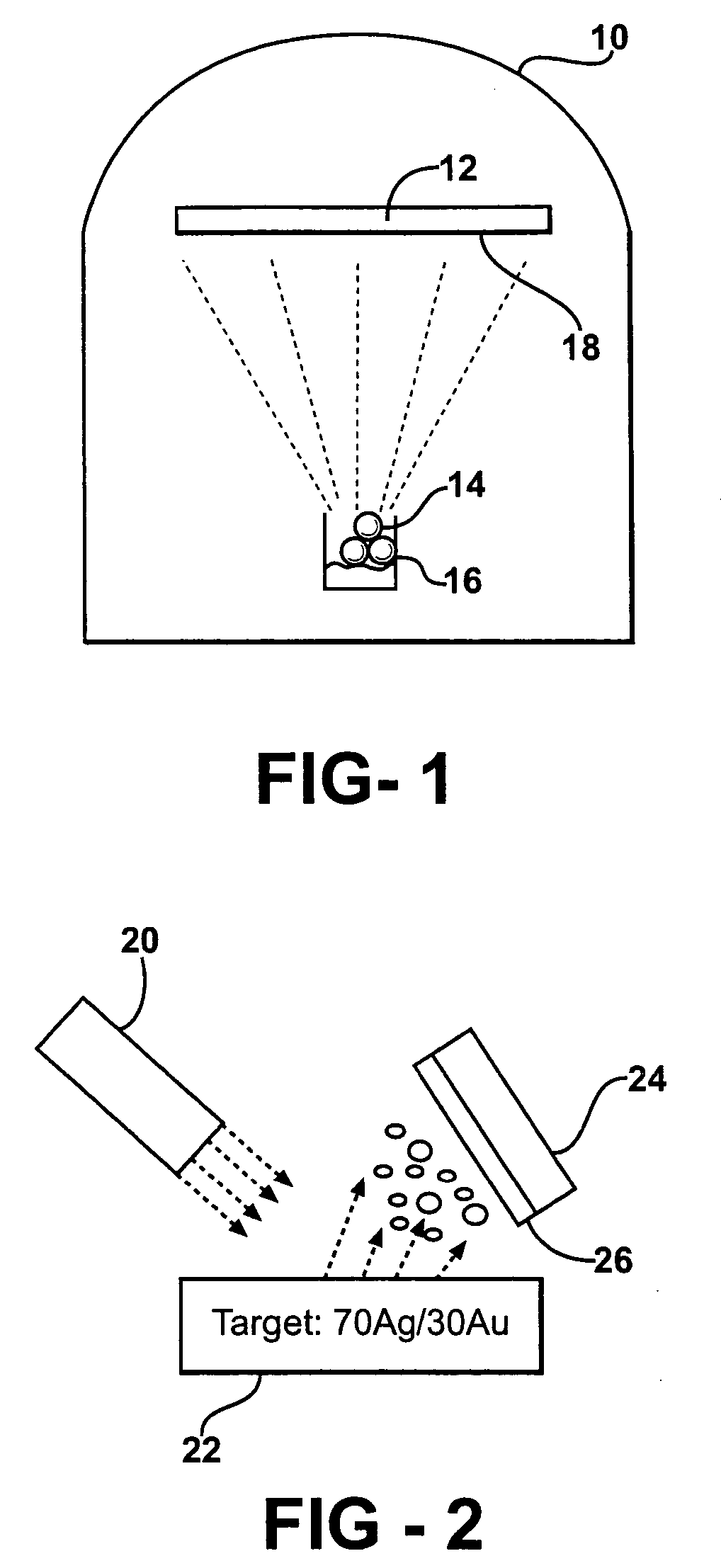
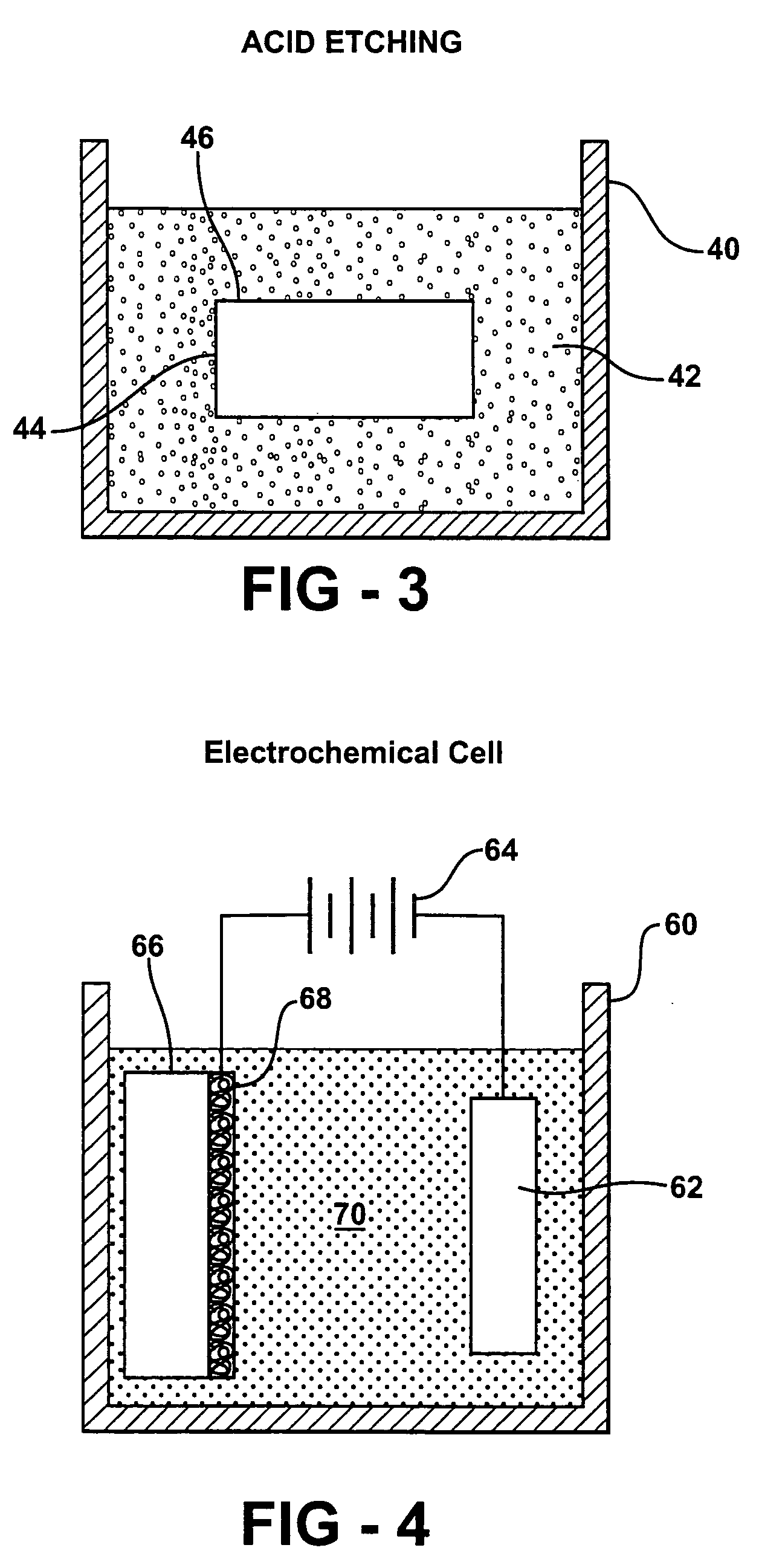
- The development of porous metal substrates, specifically gold substrates created by depositing a gold-silver alloy film and removing the silver through acid etching or electrochemical roughening, resulting in a textured surface that enhances SERS signals uniformly and stably.
Regulatory Framework for Clinical SERS Applications
The regulatory landscape for SERS-based drug monitoring technologies represents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA has established specific pathways for approval of SERS-based diagnostic devices through the Medical Device Regulatory pathway, with most SERS applications currently classified as Class II devices requiring 510(k) clearance. The regulatory requirements focus heavily on analytical validation, clinical validation, and demonstration of clinical utility.
The European Union, under the In Vitro Diagnostic Regulation (IVDR 2017/746), has implemented more stringent requirements for clinical evidence and post-market surveillance of diagnostic technologies including SERS-based systems. This regulation, fully implemented in 2022, places SERS drug monitoring technologies primarily in Class C, requiring conformity assessment by notified bodies and comprehensive technical documentation.
Clinical validation standards for SERS technologies present unique challenges due to the high sensitivity of these methods. Regulatory bodies typically require demonstration of analytical performance metrics including limit of detection, specificity, reproducibility, and robustness across different sample matrices. For drug monitoring applications, comparative studies against gold standard methods such as HPLC-MS are generally mandatory.
Quality control and standardization protocols represent significant regulatory hurdles for SERS technologies. The development of certified reference materials and standardized substrate production methods remains an ongoing challenge that regulatory frameworks are still adapting to address. The International Organization for Standardization (ISO) has begun developing specific standards for nanomaterial-based sensing technologies, which will impact SERS substrate regulation.
Data management and privacy considerations have become increasingly important in the regulatory framework, particularly for technologies that may generate patient-specific drug monitoring profiles. HIPAA compliance in the US and GDPR in Europe impose strict requirements on data handling practices for clinical SERS applications.
Regulatory harmonization efforts are underway through the International Medical Device Regulators Forum (IMDRF), which aims to streamline approval processes across different regions. However, significant disparities remain in how different jurisdictions approach novel analytical technologies like SERS for clinical applications, creating challenges for global commercialization strategies.
The European Union, under the In Vitro Diagnostic Regulation (IVDR 2017/746), has implemented more stringent requirements for clinical evidence and post-market surveillance of diagnostic technologies including SERS-based systems. This regulation, fully implemented in 2022, places SERS drug monitoring technologies primarily in Class C, requiring conformity assessment by notified bodies and comprehensive technical documentation.
Clinical validation standards for SERS technologies present unique challenges due to the high sensitivity of these methods. Regulatory bodies typically require demonstration of analytical performance metrics including limit of detection, specificity, reproducibility, and robustness across different sample matrices. For drug monitoring applications, comparative studies against gold standard methods such as HPLC-MS are generally mandatory.
Quality control and standardization protocols represent significant regulatory hurdles for SERS technologies. The development of certified reference materials and standardized substrate production methods remains an ongoing challenge that regulatory frameworks are still adapting to address. The International Organization for Standardization (ISO) has begun developing specific standards for nanomaterial-based sensing technologies, which will impact SERS substrate regulation.
Data management and privacy considerations have become increasingly important in the regulatory framework, particularly for technologies that may generate patient-specific drug monitoring profiles. HIPAA compliance in the US and GDPR in Europe impose strict requirements on data handling practices for clinical SERS applications.
Regulatory harmonization efforts are underway through the International Medical Device Regulators Forum (IMDRF), which aims to streamline approval processes across different regions. However, significant disparities remain in how different jurisdictions approach novel analytical technologies like SERS for clinical applications, creating challenges for global commercialization strategies.
Cost-Benefit Analysis of SERS Implementation
Implementing SERS technology for drug monitoring requires careful evaluation of its economic implications. The initial investment in SERS substrates and associated analytical equipment represents a significant capital expenditure, with high-quality commercial SERS substrates ranging from $50-200 per unit and specialized Raman spectrometers costing between $30,000-100,000. However, these costs must be weighed against the substantial benefits of SERS-based drug monitoring.
The primary economic advantage lies in the reduced sample volume requirements. SERS can detect drug compounds at picogram levels, potentially reducing reagent costs by 60-80% compared to conventional methods like HPLC or mass spectrometry. This translates to approximately $15-25 savings per test in consumable materials alone.
Operational efficiency presents another significant benefit. SERS analysis typically requires 5-10 minutes per sample compared to 30-60 minutes for traditional methods, increasing laboratory throughput by 300-500%. This efficiency gain can generate additional revenue of $50,000-150,000 annually for medium-sized clinical laboratories processing 50-100 samples daily.
From a healthcare system perspective, SERS implementation offers substantial downstream cost savings. Real-time therapeutic drug monitoring enables precise medication dosing, potentially reducing adverse drug reactions by 15-25%. Considering that adverse drug events cost healthcare systems approximately $30 billion annually in the US alone, even a modest reduction represents significant savings.
The technology lifecycle must also factor into cost-benefit calculations. While SERS substrates have limited shelf lives (typically 6-12 months), recent advancements in substrate stability have extended usability periods. Additionally, the reusability of certain substrate types (particularly metal-coated nanostructures) for multiple measurements can further amortize costs.
Return on investment (ROI) analysis indicates that most healthcare facilities can expect to recoup SERS implementation costs within 18-24 months, with smaller clinics potentially requiring 30-36 months. This timeline becomes more favorable when considering the intangible benefits of improved patient outcomes and reduced hospitalization rates due to more precise drug monitoring.
The primary economic advantage lies in the reduced sample volume requirements. SERS can detect drug compounds at picogram levels, potentially reducing reagent costs by 60-80% compared to conventional methods like HPLC or mass spectrometry. This translates to approximately $15-25 savings per test in consumable materials alone.
Operational efficiency presents another significant benefit. SERS analysis typically requires 5-10 minutes per sample compared to 30-60 minutes for traditional methods, increasing laboratory throughput by 300-500%. This efficiency gain can generate additional revenue of $50,000-150,000 annually for medium-sized clinical laboratories processing 50-100 samples daily.
From a healthcare system perspective, SERS implementation offers substantial downstream cost savings. Real-time therapeutic drug monitoring enables precise medication dosing, potentially reducing adverse drug reactions by 15-25%. Considering that adverse drug events cost healthcare systems approximately $30 billion annually in the US alone, even a modest reduction represents significant savings.
The technology lifecycle must also factor into cost-benefit calculations. While SERS substrates have limited shelf lives (typically 6-12 months), recent advancements in substrate stability have extended usability periods. Additionally, the reusability of certain substrate types (particularly metal-coated nanostructures) for multiple measurements can further amortize costs.
Return on investment (ROI) analysis indicates that most healthcare facilities can expect to recoup SERS implementation costs within 18-24 months, with smaller clinics potentially requiring 30-36 months. This timeline becomes more favorable when considering the intangible benefits of improved patient outcomes and reduced hospitalization rates due to more precise drug monitoring.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!