Streamline Microfluidics Fabrication for Consistent Results
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics Fabrication Background and Objectives
Microfluidics technology has evolved significantly since its inception in the early 1990s, transforming from a niche research area into a powerful platform for numerous applications across healthcare, chemistry, and biology. The fundamental concept involves manipulating fluids at the microscale level, typically handling volumes from picoliters to nanoliters within channels measuring tens to hundreds of micrometers. This miniaturization offers unprecedented control over fluid dynamics, chemical reactions, and biological processes.
The historical trajectory of microfluidics development began with simple channel designs fabricated primarily in silicon and glass using techniques borrowed from semiconductor manufacturing. As the field matured, polymer-based fabrication methods emerged, particularly polydimethylsiloxane (PDMS) soft lithography, which dramatically reduced costs and accelerated prototyping capabilities. Recent years have witnessed the integration of advanced materials and hybrid fabrication approaches, expanding the functional capabilities of microfluidic devices.
Despite these advances, fabrication consistency remains a persistent challenge in the field. Variations in channel dimensions, surface properties, and bonding quality directly impact fluid behavior and experimental outcomes. This inconsistency presents a significant barrier to widespread industrial adoption and commercialization of microfluidic technologies, particularly in applications requiring regulatory approval such as clinical diagnostics and pharmaceutical manufacturing.
The primary objective of streamlining microfluidics fabrication is to establish standardized, reproducible manufacturing protocols that yield devices with consistent performance characteristics. This encompasses several specific goals: reducing batch-to-batch variability, improving production yields, minimizing operator-dependent outcomes, and developing quality control metrics appropriate for microscale features.
Current trends indicate a shift toward automated fabrication systems, integration of in-line quality control mechanisms, and the development of design rules that account for manufacturing constraints. Additionally, there is growing interest in scalable production methods that maintain precision while increasing throughput, such as injection molding, hot embossing, and roll-to-roll processing for thermoplastic microfluidics.
The convergence of microfluidics with complementary technologies represents another important trajectory. Integration with sensors, actuators, and electronic components is enabling more sophisticated "lab-on-a-chip" systems. Meanwhile, advances in 3D printing technologies are opening new possibilities for complex geometries and multi-material fabrication that were previously unattainable.
Achieving consistent fabrication results will unlock the full potential of microfluidics across numerous high-impact applications, including point-of-care diagnostics, organ-on-chip platforms for drug development, and high-throughput screening systems. The technology's evolution toward more reliable manufacturing processes represents a critical inflection point that could transform microfluidics from primarily research tools to widely deployed commercial products.
The historical trajectory of microfluidics development began with simple channel designs fabricated primarily in silicon and glass using techniques borrowed from semiconductor manufacturing. As the field matured, polymer-based fabrication methods emerged, particularly polydimethylsiloxane (PDMS) soft lithography, which dramatically reduced costs and accelerated prototyping capabilities. Recent years have witnessed the integration of advanced materials and hybrid fabrication approaches, expanding the functional capabilities of microfluidic devices.
Despite these advances, fabrication consistency remains a persistent challenge in the field. Variations in channel dimensions, surface properties, and bonding quality directly impact fluid behavior and experimental outcomes. This inconsistency presents a significant barrier to widespread industrial adoption and commercialization of microfluidic technologies, particularly in applications requiring regulatory approval such as clinical diagnostics and pharmaceutical manufacturing.
The primary objective of streamlining microfluidics fabrication is to establish standardized, reproducible manufacturing protocols that yield devices with consistent performance characteristics. This encompasses several specific goals: reducing batch-to-batch variability, improving production yields, minimizing operator-dependent outcomes, and developing quality control metrics appropriate for microscale features.
Current trends indicate a shift toward automated fabrication systems, integration of in-line quality control mechanisms, and the development of design rules that account for manufacturing constraints. Additionally, there is growing interest in scalable production methods that maintain precision while increasing throughput, such as injection molding, hot embossing, and roll-to-roll processing for thermoplastic microfluidics.
The convergence of microfluidics with complementary technologies represents another important trajectory. Integration with sensors, actuators, and electronic components is enabling more sophisticated "lab-on-a-chip" systems. Meanwhile, advances in 3D printing technologies are opening new possibilities for complex geometries and multi-material fabrication that were previously unattainable.
Achieving consistent fabrication results will unlock the full potential of microfluidics across numerous high-impact applications, including point-of-care diagnostics, organ-on-chip platforms for drug development, and high-throughput screening systems. The technology's evolution toward more reliable manufacturing processes represents a critical inflection point that could transform microfluidics from primarily research tools to widely deployed commercial products.
Market Demand Analysis for Streamlined Microfluidic Systems
The microfluidics market has experienced significant growth in recent years, driven by increasing applications in pharmaceuticals, diagnostics, and life sciences. The global microfluidics market was valued at $13.5 billion in 2019 and is projected to reach $44.0 billion by 2027, growing at a CAGR of 16.2% during the forecast period. This substantial growth indicates strong market demand for advanced microfluidic technologies and streamlined fabrication processes.
Healthcare and pharmaceutical sectors represent the largest market segments for microfluidic systems, accounting for approximately 60% of the total market share. The demand is primarily fueled by the need for point-of-care diagnostics, drug delivery systems, and analytical testing platforms that require precise fluid handling capabilities at microscale levels.
Research institutions and academic laboratories constitute another significant market segment, representing about 20% of the current demand. These entities require consistent and reproducible microfluidic platforms for various experimental applications, highlighting the critical need for streamlined fabrication processes that ensure result consistency.
Industrial applications in sectors such as chemical processing, environmental monitoring, and food safety testing are emerging as rapidly growing market segments, with an estimated annual growth rate of 18.5%. These industries value microfluidic systems for their ability to reduce reagent consumption, minimize waste generation, and enable high-throughput analysis.
Market research indicates that end-users across all segments consistently identify fabrication inconsistency as a major pain point. Approximately 75% of microfluidics users report challenges related to device-to-device variability, which directly impacts experimental reproducibility and product reliability. This underscores the significant market demand for streamlined fabrication processes that can deliver consistent results.
Geographically, North America dominates the microfluidics market with a 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing investments in healthcare infrastructure and research facilities.
Customer surveys reveal that end-users are willing to pay a premium of 15-20% for microfluidic systems that demonstrate superior fabrication consistency and result reproducibility. This price elasticity indicates strong market validation for innovations focused on streamlining microfluidics fabrication processes.
The market also shows increasing demand for integrated solutions that combine streamlined fabrication with automated quality control mechanisms. This trend is particularly prominent in pharmaceutical and diagnostic applications where regulatory compliance and validation requirements are stringent.
Healthcare and pharmaceutical sectors represent the largest market segments for microfluidic systems, accounting for approximately 60% of the total market share. The demand is primarily fueled by the need for point-of-care diagnostics, drug delivery systems, and analytical testing platforms that require precise fluid handling capabilities at microscale levels.
Research institutions and academic laboratories constitute another significant market segment, representing about 20% of the current demand. These entities require consistent and reproducible microfluidic platforms for various experimental applications, highlighting the critical need for streamlined fabrication processes that ensure result consistency.
Industrial applications in sectors such as chemical processing, environmental monitoring, and food safety testing are emerging as rapidly growing market segments, with an estimated annual growth rate of 18.5%. These industries value microfluidic systems for their ability to reduce reagent consumption, minimize waste generation, and enable high-throughput analysis.
Market research indicates that end-users across all segments consistently identify fabrication inconsistency as a major pain point. Approximately 75% of microfluidics users report challenges related to device-to-device variability, which directly impacts experimental reproducibility and product reliability. This underscores the significant market demand for streamlined fabrication processes that can deliver consistent results.
Geographically, North America dominates the microfluidics market with a 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to increasing investments in healthcare infrastructure and research facilities.
Customer surveys reveal that end-users are willing to pay a premium of 15-20% for microfluidic systems that demonstrate superior fabrication consistency and result reproducibility. This price elasticity indicates strong market validation for innovations focused on streamlining microfluidics fabrication processes.
The market also shows increasing demand for integrated solutions that combine streamlined fabrication with automated quality control mechanisms. This trend is particularly prominent in pharmaceutical and diagnostic applications where regulatory compliance and validation requirements are stringent.
Current Fabrication Challenges and Technical Limitations
Despite significant advancements in microfluidics technology, fabrication processes continue to face substantial challenges that impede consistent results and widespread industrial adoption. The conventional photolithography-based soft lithography technique, while established, suffers from inherent limitations in precision and reproducibility. Variations in PDMS curing conditions, including temperature fluctuations and curing agent ratios, frequently lead to dimensional inconsistencies between batches, affecting channel geometry and subsequently flow dynamics.
Surface treatment processes present another critical challenge, as achieving uniform hydrophilicity or hydrophobicity across microchannels remains difficult to standardize. This inconsistency directly impacts fluid behavior, particularly in droplet-based applications where surface properties significantly influence droplet formation and stability. The manual nature of many fabrication steps further compounds these issues, introducing operator-dependent variations that compromise experimental reproducibility.
Integration of functional components such as sensors, electrodes, and valves into microfluidic devices presents complex fabrication hurdles. Current techniques often require multiple processing steps with precise alignment requirements, increasing fabrication complexity and failure rates. The bonding process between PDMS and glass or other substrates frequently results in channel deformation or incomplete sealing, leading to leakage and device failure.
Material limitations constitute another significant barrier. While PDMS remains the dominant material due to its optical transparency and ease of use, it suffers from solvent absorption, protein adsorption, and evaporation issues that can alter experimental conditions. Alternative materials like thermoplastics offer potential solutions but introduce their own fabrication challenges, including more complex molding requirements and less favorable surface modification properties.
Scaling production from laboratory prototypes to commercial manufacturing encounters substantial obstacles. Current fabrication methods are predominantly optimized for small-scale production, with limited throughput and high unit costs. The transition to high-volume manufacturing necessitates significant process modifications that often compromise the precise features achieved in laboratory settings.
Quality control methodologies remain underdeveloped for microfluidic fabrication. Unlike the semiconductor industry, standardized testing protocols and acceptance criteria are not well established, making quality assurance challenging. Non-destructive testing methods capable of detecting internal defects in assembled devices are particularly lacking, resulting in high rejection rates during final testing phases.
Cross-contamination between fabrication steps represents another persistent challenge, especially in multi-layer devices or those incorporating biological materials. Clean room facilities can mitigate this issue but significantly increase production costs and accessibility barriers for many research groups and small enterprises.
Surface treatment processes present another critical challenge, as achieving uniform hydrophilicity or hydrophobicity across microchannels remains difficult to standardize. This inconsistency directly impacts fluid behavior, particularly in droplet-based applications where surface properties significantly influence droplet formation and stability. The manual nature of many fabrication steps further compounds these issues, introducing operator-dependent variations that compromise experimental reproducibility.
Integration of functional components such as sensors, electrodes, and valves into microfluidic devices presents complex fabrication hurdles. Current techniques often require multiple processing steps with precise alignment requirements, increasing fabrication complexity and failure rates. The bonding process between PDMS and glass or other substrates frequently results in channel deformation or incomplete sealing, leading to leakage and device failure.
Material limitations constitute another significant barrier. While PDMS remains the dominant material due to its optical transparency and ease of use, it suffers from solvent absorption, protein adsorption, and evaporation issues that can alter experimental conditions. Alternative materials like thermoplastics offer potential solutions but introduce their own fabrication challenges, including more complex molding requirements and less favorable surface modification properties.
Scaling production from laboratory prototypes to commercial manufacturing encounters substantial obstacles. Current fabrication methods are predominantly optimized for small-scale production, with limited throughput and high unit costs. The transition to high-volume manufacturing necessitates significant process modifications that often compromise the precise features achieved in laboratory settings.
Quality control methodologies remain underdeveloped for microfluidic fabrication. Unlike the semiconductor industry, standardized testing protocols and acceptance criteria are not well established, making quality assurance challenging. Non-destructive testing methods capable of detecting internal defects in assembled devices are particularly lacking, resulting in high rejection rates during final testing phases.
Cross-contamination between fabrication steps represents another persistent challenge, especially in multi-layer devices or those incorporating biological materials. Clean room facilities can mitigate this issue but significantly increase production costs and accessibility barriers for many research groups and small enterprises.
Current Streamlining Solutions for Microfluidic Fabrication
01 Standardized fabrication processes for microfluidic devices
Standardized fabrication processes are essential for ensuring consistency in microfluidic device production. These processes involve precise control of manufacturing parameters, such as temperature, pressure, and material properties. By implementing standardized protocols and quality control measures, manufacturers can achieve reproducible results across multiple production batches. This approach minimizes variations in channel dimensions, surface properties, and overall device performance.- Standardized fabrication processes for microfluidic devices: Implementing standardized fabrication processes is crucial for ensuring consistency in microfluidic device production. These processes include precise photolithography techniques, controlled etching methods, and standardized bonding procedures. By following well-defined protocols and using automated manufacturing systems, variations between batches can be minimized, resulting in more reliable and reproducible microfluidic devices for research and commercial applications.
- Quality control and testing methodologies: Comprehensive quality control and testing methodologies are essential for maintaining consistency in microfluidic fabrication. These include dimensional verification using advanced imaging techniques, flow rate validation, pressure resistance testing, and surface characterization. Implementing systematic inspection protocols at various stages of production helps identify deviations early and ensures that final devices meet predetermined specifications and performance standards.
- Material selection and characterization for consistent performance: The careful selection and characterization of materials significantly impacts the consistency of microfluidic device fabrication. Materials must exhibit uniform properties across batches, including consistent surface chemistry, mechanical stability, and compatibility with biological samples or chemical reagents. Thorough characterization of polymers, glass, silicon, or hybrid materials before fabrication helps predict behavior during manufacturing and ensures reliable device performance in various applications.
- Advanced design and simulation tools: Advanced design and simulation tools play a critical role in achieving fabrication consistency for microfluidic devices. Computer-aided design software, computational fluid dynamics simulations, and predictive modeling help optimize channel geometries, flow dynamics, and manufacturing parameters before physical production begins. These tools allow engineers to identify potential fabrication challenges, predict performance variations, and refine designs to enhance reproducibility across multiple production runs.
- Automated fabrication and assembly systems: Automated fabrication and assembly systems significantly improve consistency in microfluidic device production by reducing human error and variability. Robotic handling systems, precision dispensing equipment, and computer-controlled manufacturing processes ensure repeatable results across production batches. Integration of real-time monitoring and feedback control mechanisms further enhances fabrication precision, allowing for immediate adjustments to maintain quality standards throughout the manufacturing process.
02 Advanced materials selection for consistent microfluidic performance
The selection of appropriate materials plays a crucial role in maintaining fabrication consistency for microfluidic devices. Materials with stable physical and chemical properties help ensure uniform flow characteristics and reliable device operation. Advanced polymers, glass substrates, and hybrid materials can be engineered to provide consistent surface properties, chemical resistance, and optical transparency. Proper material selection also contributes to dimensional stability during fabrication and operation under various conditions.Expand Specific Solutions03 Automated quality control systems for microfluidic fabrication
Automated quality control systems are implemented to monitor and maintain consistency throughout the microfluidic fabrication process. These systems utilize advanced imaging techniques, sensors, and data analytics to detect deviations from design specifications in real-time. Machine vision systems can inspect channel dimensions, surface roughness, and bonding quality, while automated testing platforms evaluate flow characteristics and functional performance. This approach enables rapid identification and correction of fabrication issues before they affect device performance.Expand Specific Solutions04 Novel bonding techniques for reliable microfluidic assembly
Innovative bonding techniques have been developed to improve the reliability and consistency of microfluidic device assembly. These methods include plasma-assisted bonding, solvent bonding, thermal fusion, and adhesive-based approaches optimized for specific material combinations. Advanced bonding processes ensure uniform sealing across the entire device, preventing leakage and maintaining precise channel geometries. Controlled bonding parameters help achieve consistent interlayer adhesion without channel deformation or obstruction.Expand Specific Solutions05 Design optimization tools for microfluidic fabrication consistency
Computational design tools and simulation software are increasingly used to optimize microfluidic device designs for manufacturability and consistency. These tools enable designers to predict fabrication challenges, identify potential failure points, and optimize geometries for reliable production. Simulation-based approaches can model the effects of manufacturing variations on device performance, allowing for robust designs that maintain functionality despite minor fabrication inconsistencies. Design for manufacturing principles specifically adapted for microfluidics help ensure that devices can be produced consistently at scale.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
The microfluidics fabrication market is currently in a growth phase, characterized by increasing adoption across pharmaceutical, healthcare, and research sectors. The global market size is projected to reach significant expansion with compound annual growth rates exceeding 20%. Technologically, the field shows varying maturity levels, with established players like IBM, Agilent Technologies, and Siemens leading innovation through advanced automation and standardization techniques. Academic institutions including KAUST, Zhejiang University, and Caltech are driving fundamental research, while companies like Truvian Sciences focus on commercial applications. The competitive landscape features both large corporations leveraging existing infrastructure and specialized startups developing novel fabrication approaches, creating a dynamic ecosystem where collaboration between industry and academia is accelerating technological advancement toward more consistent, reproducible microfluidic systems.
International Business Machines Corp.
Technical Solution: IBM has developed advanced microfluidic fabrication technologies focusing on standardization and automation. Their approach integrates lithographic techniques derived from semiconductor manufacturing with novel polymer processing methods to create highly consistent microfluidic devices. IBM's platform includes automated quality control systems that monitor channel dimensions, surface properties, and flow characteristics in real-time during fabrication. They've pioneered a "digital microfluidics" approach that uses electrowetting principles to manipulate discrete droplets with precise control, eliminating many of the consistency issues found in continuous flow systems. Their fabrication process incorporates in-line metrology tools that can detect and correct deviations in channel geometry before they affect device performance, significantly improving batch-to-batch reproducibility. IBM has also developed specialized software tools that model fluid behavior within microchannels to predict and compensate for manufacturing variations before they occur.
Strengths: Exceptional precision derived from semiconductor manufacturing expertise; advanced automation capabilities reducing human error; sophisticated quality control systems. Weaknesses: Higher implementation costs compared to traditional methods; requires specialized equipment that may limit accessibility for smaller labs; complex integration requirements for full implementation.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed a comprehensive microfluidic fabrication platform called "Precision Flow Technology" that addresses consistency challenges through standardized manufacturing protocols. Their approach combines precision injection molding techniques with proprietary surface treatment processes to create microfluidic chips with highly reproducible channel geometries and surface properties. Agilent's system incorporates automated quality assurance steps throughout the fabrication process, including optical inspection systems that can detect sub-micron variations in channel dimensions. Their technology utilizes specialized bonding techniques that maintain critical channel dimensions during the sealing process, a common point of failure in traditional fabrication methods. Agilent has also pioneered materials with controlled surface chemistry that resist protein adsorption and maintain consistent flow characteristics across production batches. Their fabrication process includes standardized connection interfaces that ensure reliable world-to-chip connections, eliminating another major source of experimental variability.
Strengths: Comprehensive quality control systems throughout the manufacturing process; specialized materials with consistent surface properties; standardized interfaces improving reliability. Weaknesses: Proprietary nature of some technologies may limit customization options; higher initial investment compared to manual fabrication methods; requires specialized training for operation and maintenance.
Key Innovations in Consistent Microfluidic Production
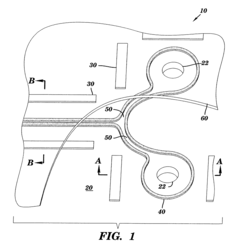
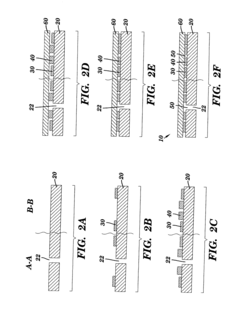
Microfluidic devices fabricated by direct thick film writing and methods thereof
PatentInactiveUS20100209318A1
Innovation
- A microfluidic device fabrication method using direct thick film writing, where a substrate is coated with flowable materials to form spacer and microchannel layers, allowing for arbitrary design and integration on various substrates with a wide range of materials, reducing the need for expensive equipment and enabling flexible substrate sizes and geometries.
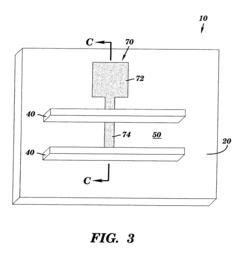
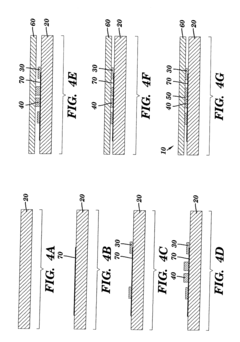
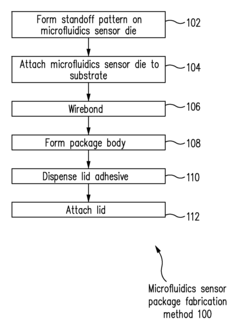
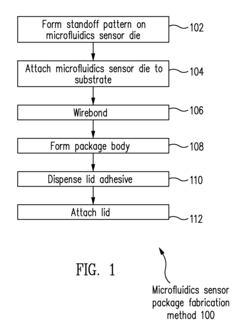
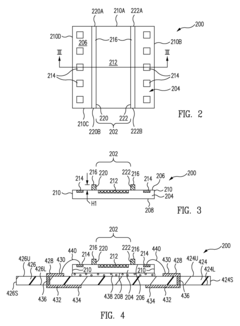
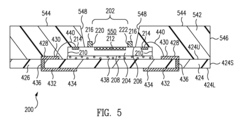
Microfluidics sensor package fabrication method and structure
PatentActiveUS9670445B1
Innovation
- A microfluidics sensor package is designed with a standoff pattern on the sensor die that precisely spaces a lid above the active surface, creating a microfluidics cavity with a controlled gap height, allowing for precise fluid control through the use of a non-collapsible and inflexible material like copper, and a lid adhesive to secure the lid at a specific height.
Quality Control Methodologies for Microfluidic Devices
Quality control in microfluidic device manufacturing represents a critical component for achieving consistent and reliable results. The implementation of robust quality control methodologies begins with the establishment of standardized testing protocols that evaluate key performance parameters such as flow rate consistency, channel dimensions, and surface properties. These protocols must be designed to detect variations that could impact device functionality while maintaining efficiency in the production process.
Statistical process control (SPC) techniques have emerged as essential tools for monitoring microfluidic fabrication processes. By collecting and analyzing real-time data on critical parameters, manufacturers can identify process drift before it results in defective devices. Implementation of control charts specifically adapted for microfluidic manufacturing enables the visualization of process stability and capability, facilitating prompt intervention when necessary.
Advanced imaging technologies play a pivotal role in quality assurance for microfluidic devices. High-resolution optical inspection systems, confocal microscopy, and scanning electron microscopy provide detailed visualization of microstructures, enabling the detection of defects at submicron scales. These technologies can be integrated into automated inspection systems that significantly increase throughput while maintaining detection sensitivity.
Non-destructive testing methodologies have been developed specifically for microfluidic applications. Techniques such as micro-computed tomography (micro-CT) allow for three-dimensional visualization of internal structures without damaging the device. Additionally, electrical impedance spectroscopy offers insights into channel integrity and surface modifications without requiring direct visual access to all components.
Functional testing represents the ultimate validation of microfluidic device quality. This involves evaluating devices under actual operating conditions to verify performance characteristics such as mixing efficiency, separation capability, or reaction kinetics. The development of standardized reference materials and benchmark tests enables meaningful comparison across different manufacturing batches and production facilities.
Integration of quality control data management systems has revolutionized microfluidic manufacturing. These systems collect, store, and analyze quality metrics throughout the production process, enabling traceability of each device to specific manufacturing parameters. Machine learning algorithms applied to this data can identify subtle patterns that predict potential quality issues before they manifest in finished devices.
The establishment of industry-wide quality standards remains an ongoing challenge in the microfluidics field. Organizations such as ASTM International and ISO have begun developing specific standards for microfluidic device testing and validation, though consensus on universal quality metrics continues to evolve as the technology matures and applications diversify.
Statistical process control (SPC) techniques have emerged as essential tools for monitoring microfluidic fabrication processes. By collecting and analyzing real-time data on critical parameters, manufacturers can identify process drift before it results in defective devices. Implementation of control charts specifically adapted for microfluidic manufacturing enables the visualization of process stability and capability, facilitating prompt intervention when necessary.
Advanced imaging technologies play a pivotal role in quality assurance for microfluidic devices. High-resolution optical inspection systems, confocal microscopy, and scanning electron microscopy provide detailed visualization of microstructures, enabling the detection of defects at submicron scales. These technologies can be integrated into automated inspection systems that significantly increase throughput while maintaining detection sensitivity.
Non-destructive testing methodologies have been developed specifically for microfluidic applications. Techniques such as micro-computed tomography (micro-CT) allow for three-dimensional visualization of internal structures without damaging the device. Additionally, electrical impedance spectroscopy offers insights into channel integrity and surface modifications without requiring direct visual access to all components.
Functional testing represents the ultimate validation of microfluidic device quality. This involves evaluating devices under actual operating conditions to verify performance characteristics such as mixing efficiency, separation capability, or reaction kinetics. The development of standardized reference materials and benchmark tests enables meaningful comparison across different manufacturing batches and production facilities.
Integration of quality control data management systems has revolutionized microfluidic manufacturing. These systems collect, store, and analyze quality metrics throughout the production process, enabling traceability of each device to specific manufacturing parameters. Machine learning algorithms applied to this data can identify subtle patterns that predict potential quality issues before they manifest in finished devices.
The establishment of industry-wide quality standards remains an ongoing challenge in the microfluidics field. Organizations such as ASTM International and ISO have begun developing specific standards for microfluidic device testing and validation, though consensus on universal quality metrics continues to evolve as the technology matures and applications diversify.
Standardization Efforts in Microfluidics Manufacturing
The microfluidics industry has recognized that inconsistent manufacturing processes represent a significant barrier to widespread adoption and commercialization. In response, several standardization initiatives have emerged across the globe. The International Organization for Standardization (ISO) established the Technical Committee 48 (TC 48) specifically addressing microfluidic devices, which has published guidelines on terminology, materials compatibility, and testing protocols. These standards provide manufacturers with clear benchmarks for quality assurance and performance validation.
Industry consortia have also played a crucial role in standardization efforts. The Microfluidics Consortium, comprising leading academic institutions and industrial partners, has developed open-source design specifications for common microfluidic components such as channels, valves, and pumps. These specifications include recommended dimensions, tolerances, and fabrication parameters that have been empirically validated across multiple production facilities.
Material standardization represents another significant advancement in the field. The development of certified reference materials for microfluidic applications ensures consistency in device performance regardless of manufacturing location. Companies like Dolomite Microfluidics and uFluidix have introduced standardized polymer blends with documented physical properties, chemical resistance profiles, and optical characteristics specifically optimized for microfluidic applications.
Fabrication process standardization has focused on establishing reproducible protocols for common manufacturing techniques. The NIST (National Institute of Standards and Technology) has published detailed procedural guidelines for soft lithography, hot embossing, and injection molding specifically tailored to microfluidic device production. These guidelines include equipment calibration procedures, environmental control parameters, and quality control checkpoints that significantly reduce batch-to-batch variability.
Metrology standards have also been developed to ensure consistent quality assessment across the industry. Advanced characterization techniques including white light interferometry, confocal microscopy, and automated dimensional inspection have been standardized with specific protocols for microfluidic features. These standardized measurement approaches enable meaningful comparison of devices produced by different manufacturers or production runs.
Interconnection standards represent a particularly important development for system integration. The standardization of fluidic ports, connectors, and interfaces allows for modular design approaches and interoperability between components from different suppliers. The Microfluidic Connection Standard (MCS) has gained significant industry adoption, specifying dimensions, thread types, and sealing mechanisms for reliable fluid transfer between microfluidic modules.
Industry consortia have also played a crucial role in standardization efforts. The Microfluidics Consortium, comprising leading academic institutions and industrial partners, has developed open-source design specifications for common microfluidic components such as channels, valves, and pumps. These specifications include recommended dimensions, tolerances, and fabrication parameters that have been empirically validated across multiple production facilities.
Material standardization represents another significant advancement in the field. The development of certified reference materials for microfluidic applications ensures consistency in device performance regardless of manufacturing location. Companies like Dolomite Microfluidics and uFluidix have introduced standardized polymer blends with documented physical properties, chemical resistance profiles, and optical characteristics specifically optimized for microfluidic applications.
Fabrication process standardization has focused on establishing reproducible protocols for common manufacturing techniques. The NIST (National Institute of Standards and Technology) has published detailed procedural guidelines for soft lithography, hot embossing, and injection molding specifically tailored to microfluidic device production. These guidelines include equipment calibration procedures, environmental control parameters, and quality control checkpoints that significantly reduce batch-to-batch variability.
Metrology standards have also been developed to ensure consistent quality assessment across the industry. Advanced characterization techniques including white light interferometry, confocal microscopy, and automated dimensional inspection have been standardized with specific protocols for microfluidic features. These standardized measurement approaches enable meaningful comparison of devices produced by different manufacturers or production runs.
Interconnection standards represent a particularly important development for system integration. The standardization of fluidic ports, connectors, and interfaces allows for modular design approaches and interoperability between components from different suppliers. The Microfluidic Connection Standard (MCS) has gained significant industry adoption, specifying dimensions, thread types, and sealing mechanisms for reliable fluid transfer between microfluidic modules.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!