UHMWPE's Contributions to High-Performance Biometric Devices
AUG 6, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
UHMWPE in Biometrics: Background and Objectives
Ultra-high-molecular-weight polyethylene (UHMWPE) has emerged as a revolutionary material in the field of biometric devices, offering unique properties that significantly enhance performance and reliability. The evolution of UHMWPE in biometrics can be traced back to its initial applications in medical implants, where its exceptional wear resistance and biocompatibility were first recognized.
As biometric technology advanced, the need for materials that could withstand repeated use while maintaining accuracy became paramount. UHMWPE's high strength-to-weight ratio, coupled with its resistance to abrasion and chemicals, made it an ideal candidate for integration into various biometric devices. This marked the beginning of UHMWPE's journey in the biometrics sector, transitioning from medical applications to security and identification systems.
The primary objective of incorporating UHMWPE into biometric devices is to improve durability and precision. Biometric sensors, such as fingerprint scanners and iris recognition systems, require materials that can maintain their structural integrity and surface properties over extended periods of use. UHMWPE's ability to resist deformation and maintain its shape under stress contributes significantly to the longevity and consistent performance of these devices.
Another crucial goal is to enhance the user experience in biometric authentication processes. UHMWPE's smooth surface and low friction coefficient allow for more comfortable and efficient interactions between users and biometric devices. This is particularly important in high-traffic areas where rapid and reliable identification is essential, such as airports, secure facilities, and smart devices.
The development of UHMWPE in biometrics also aims to address environmental concerns. Its resistance to moisture, chemicals, and UV radiation makes it suitable for outdoor and harsh environment applications, expanding the potential deployment scenarios for biometric systems. This aligns with the growing trend of integrating biometric authentication into various aspects of daily life, from mobile devices to smart home systems.
As research continues, the focus is shifting towards exploring new composites and manufacturing techniques that can further enhance UHMWPE's properties for biometric applications. This includes improving its optical clarity for use in iris scanners, increasing its electrical conductivity for touch-sensitive applications, and developing nano-engineered surfaces for more accurate fingerprint recognition.
The ongoing technological evolution in this field is driven by the need for more secure, efficient, and user-friendly biometric systems. UHMWPE's contributions to high-performance biometric devices represent a significant step forward in achieving these objectives, paving the way for more advanced and reliable identification technologies in the future.
As biometric technology advanced, the need for materials that could withstand repeated use while maintaining accuracy became paramount. UHMWPE's high strength-to-weight ratio, coupled with its resistance to abrasion and chemicals, made it an ideal candidate for integration into various biometric devices. This marked the beginning of UHMWPE's journey in the biometrics sector, transitioning from medical applications to security and identification systems.
The primary objective of incorporating UHMWPE into biometric devices is to improve durability and precision. Biometric sensors, such as fingerprint scanners and iris recognition systems, require materials that can maintain their structural integrity and surface properties over extended periods of use. UHMWPE's ability to resist deformation and maintain its shape under stress contributes significantly to the longevity and consistent performance of these devices.
Another crucial goal is to enhance the user experience in biometric authentication processes. UHMWPE's smooth surface and low friction coefficient allow for more comfortable and efficient interactions between users and biometric devices. This is particularly important in high-traffic areas where rapid and reliable identification is essential, such as airports, secure facilities, and smart devices.
The development of UHMWPE in biometrics also aims to address environmental concerns. Its resistance to moisture, chemicals, and UV radiation makes it suitable for outdoor and harsh environment applications, expanding the potential deployment scenarios for biometric systems. This aligns with the growing trend of integrating biometric authentication into various aspects of daily life, from mobile devices to smart home systems.
As research continues, the focus is shifting towards exploring new composites and manufacturing techniques that can further enhance UHMWPE's properties for biometric applications. This includes improving its optical clarity for use in iris scanners, increasing its electrical conductivity for touch-sensitive applications, and developing nano-engineered surfaces for more accurate fingerprint recognition.
The ongoing technological evolution in this field is driven by the need for more secure, efficient, and user-friendly biometric systems. UHMWPE's contributions to high-performance biometric devices represent a significant step forward in achieving these objectives, paving the way for more advanced and reliable identification technologies in the future.
Market Analysis for UHMWPE-Enhanced Biometric Devices
The market for UHMWPE-enhanced biometric devices is experiencing significant growth, driven by increasing demand for high-performance security solutions across various sectors. The global biometrics market, which includes UHMWPE-enhanced devices, is projected to expand at a compound annual growth rate (CAGR) of over 13% from 2021 to 2026. This growth is primarily fueled by the rising need for advanced security measures in government, healthcare, banking, and consumer electronics industries.
UHMWPE's unique properties, such as high strength-to-weight ratio, excellent abrasion resistance, and chemical inertness, make it an ideal material for enhancing the durability and performance of biometric devices. The integration of UHMWPE in fingerprint scanners, facial recognition systems, and iris scanners has led to improved device longevity and reliability, particularly in harsh environments.
The healthcare sector represents a significant market opportunity for UHMWPE-enhanced biometric devices. With the increasing adoption of electronic health records and the need for secure patient identification, hospitals and clinics are investing in robust biometric solutions. The market for healthcare biometrics is expected to grow substantially, with UHMWPE-enhanced devices playing a crucial role in this expansion.
In the banking and financial services industry, the demand for UHMWPE-enhanced biometric devices is driven by the need for secure authentication in ATMs, mobile banking applications, and point-of-sale terminals. The integration of UHMWPE in these devices ensures better resistance to wear and tear, reducing maintenance costs and improving overall system reliability.
The consumer electronics market is another key driver for UHMWPE-enhanced biometric devices. With the proliferation of smartphones and tablets incorporating biometric authentication features, manufacturers are seeking materials that can withstand daily use while maintaining high performance. UHMWPE's durability and scratch resistance make it an attractive option for protecting sensitive biometric sensors in these devices.
Geographically, North America and Europe currently dominate the market for UHMWPE-enhanced biometric devices, owing to early adoption of advanced security technologies and stringent regulations. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in smart city projects, digital identity programs, and the rapid expansion of the consumer electronics industry.
Despite the positive market outlook, challenges such as high initial costs and the need for specialized manufacturing processes may hinder the widespread adoption of UHMWPE-enhanced biometric devices. However, ongoing research and development efforts are focused on optimizing production techniques and reducing costs, which is expected to address these challenges in the near future.
UHMWPE's unique properties, such as high strength-to-weight ratio, excellent abrasion resistance, and chemical inertness, make it an ideal material for enhancing the durability and performance of biometric devices. The integration of UHMWPE in fingerprint scanners, facial recognition systems, and iris scanners has led to improved device longevity and reliability, particularly in harsh environments.
The healthcare sector represents a significant market opportunity for UHMWPE-enhanced biometric devices. With the increasing adoption of electronic health records and the need for secure patient identification, hospitals and clinics are investing in robust biometric solutions. The market for healthcare biometrics is expected to grow substantially, with UHMWPE-enhanced devices playing a crucial role in this expansion.
In the banking and financial services industry, the demand for UHMWPE-enhanced biometric devices is driven by the need for secure authentication in ATMs, mobile banking applications, and point-of-sale terminals. The integration of UHMWPE in these devices ensures better resistance to wear and tear, reducing maintenance costs and improving overall system reliability.
The consumer electronics market is another key driver for UHMWPE-enhanced biometric devices. With the proliferation of smartphones and tablets incorporating biometric authentication features, manufacturers are seeking materials that can withstand daily use while maintaining high performance. UHMWPE's durability and scratch resistance make it an attractive option for protecting sensitive biometric sensors in these devices.
Geographically, North America and Europe currently dominate the market for UHMWPE-enhanced biometric devices, owing to early adoption of advanced security technologies and stringent regulations. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in smart city projects, digital identity programs, and the rapid expansion of the consumer electronics industry.
Despite the positive market outlook, challenges such as high initial costs and the need for specialized manufacturing processes may hinder the widespread adoption of UHMWPE-enhanced biometric devices. However, ongoing research and development efforts are focused on optimizing production techniques and reducing costs, which is expected to address these challenges in the near future.
Current UHMWPE Applications and Challenges in Biometrics
Ultra-high-molecular-weight polyethylene (UHMWPE) has emerged as a crucial material in the development of high-performance biometric devices. Its unique properties, including exceptional strength, durability, and biocompatibility, have led to its widespread adoption in various biometric applications. Currently, UHMWPE is extensively used in the manufacturing of fingerprint sensors, providing a robust and wear-resistant surface for repeated scans.
In the field of iris recognition, UHMWPE-based coatings are being applied to optical components to enhance their longevity and resistance to environmental factors. These coatings help maintain the clarity and precision of iris imaging systems over extended periods, ensuring consistent performance in diverse operating conditions.
UHMWPE is also finding applications in wearable biometric devices, such as fitness trackers and smartwatches. Its low friction coefficient and high abrasion resistance make it an ideal material for device casings and straps, offering comfort to users while withstanding daily wear and tear.
Despite its numerous advantages, the integration of UHMWPE in biometric devices faces several challenges. One significant hurdle is the material's inherent difficulty in bonding with other substances, which can complicate the manufacturing process and limit design flexibility. Researchers are actively exploring surface modification techniques to improve UHMWPE's adhesion properties without compromising its beneficial characteristics.
Another challenge lies in maintaining the material's performance under extreme conditions. While UHMWPE exhibits excellent stability in most environments, prolonged exposure to high temperatures or certain chemicals can lead to degradation. This necessitates the development of specialized formulations and protective measures to ensure the longevity of UHMWPE-based biometric components in diverse operational settings.
The integration of UHMWPE with electronic components presents another area of ongoing research. As biometric devices become increasingly sophisticated, there is a growing need to seamlessly incorporate UHMWPE into complex electronic systems without compromising signal integrity or device functionality.
Furthermore, the high cost of UHMWPE compared to conventional plastics poses a challenge for widespread adoption in consumer-grade biometric devices. Manufacturers are exploring cost-effective production methods and optimizing material usage to make UHMWPE-enhanced devices more accessible to a broader market.
As the biometrics industry continues to evolve, addressing these challenges will be crucial for fully leveraging UHMWPE's potential in next-generation devices. Ongoing research and development efforts are focused on overcoming these limitations, paving the way for more robust, reliable, and advanced biometric solutions.
In the field of iris recognition, UHMWPE-based coatings are being applied to optical components to enhance their longevity and resistance to environmental factors. These coatings help maintain the clarity and precision of iris imaging systems over extended periods, ensuring consistent performance in diverse operating conditions.
UHMWPE is also finding applications in wearable biometric devices, such as fitness trackers and smartwatches. Its low friction coefficient and high abrasion resistance make it an ideal material for device casings and straps, offering comfort to users while withstanding daily wear and tear.
Despite its numerous advantages, the integration of UHMWPE in biometric devices faces several challenges. One significant hurdle is the material's inherent difficulty in bonding with other substances, which can complicate the manufacturing process and limit design flexibility. Researchers are actively exploring surface modification techniques to improve UHMWPE's adhesion properties without compromising its beneficial characteristics.
Another challenge lies in maintaining the material's performance under extreme conditions. While UHMWPE exhibits excellent stability in most environments, prolonged exposure to high temperatures or certain chemicals can lead to degradation. This necessitates the development of specialized formulations and protective measures to ensure the longevity of UHMWPE-based biometric components in diverse operational settings.
The integration of UHMWPE with electronic components presents another area of ongoing research. As biometric devices become increasingly sophisticated, there is a growing need to seamlessly incorporate UHMWPE into complex electronic systems without compromising signal integrity or device functionality.
Furthermore, the high cost of UHMWPE compared to conventional plastics poses a challenge for widespread adoption in consumer-grade biometric devices. Manufacturers are exploring cost-effective production methods and optimizing material usage to make UHMWPE-enhanced devices more accessible to a broader market.
As the biometrics industry continues to evolve, addressing these challenges will be crucial for fully leveraging UHMWPE's potential in next-generation devices. Ongoing research and development efforts are focused on overcoming these limitations, paving the way for more robust, reliable, and advanced biometric solutions.
Existing UHMWPE Integration Solutions for Biometrics
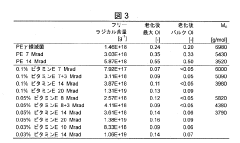
01 Composition and properties of UHMWPE
Ultra-High Molecular Weight Polyethylene (UHMWPE) is a type of polyethylene with extremely long chains, resulting in a very high molecular weight. This unique structure gives UHMWPE exceptional properties, including high impact strength, low friction coefficient, and excellent wear resistance. These characteristics make it suitable for various applications in industries such as medical, aerospace, and manufacturing.- Synthesis and processing of UHMWPE: Various methods for synthesizing and processing Ultra-High Molecular Weight Polyethylene (UHMWPE) are explored. These include polymerization techniques, extrusion processes, and molding methods to produce UHMWPE with desired properties. The focus is on improving the molecular weight, crystallinity, and overall performance of the material.
- UHMWPE composites and blends: Development of UHMWPE-based composites and blends to enhance specific properties. This includes incorporating additives, reinforcing materials, or blending with other polymers to improve mechanical strength, wear resistance, or other functional characteristics. The aim is to create tailored materials for specific applications.
- Surface modification of UHMWPE: Techniques for modifying the surface properties of UHMWPE are investigated. This includes chemical treatments, plasma processing, or grafting methods to alter surface characteristics such as adhesion, wettability, or biocompatibility. The goal is to expand the range of applications for UHMWPE in various industries.
- UHMWPE in medical applications: Utilization of UHMWPE in medical and biomedical applications is explored. This includes the development of UHMWPE-based implants, prosthetics, and medical devices. Research focuses on improving wear resistance, reducing oxidation, and enhancing biocompatibility for long-term use in the human body.
- UHMWPE fibers and films: Production and application of UHMWPE in fiber and film forms are investigated. This includes developing high-strength fibers for use in protective gear, ropes, and textiles, as well as thin films for packaging and membrane applications. The focus is on optimizing drawing and orientation processes to achieve superior mechanical properties.
02 Processing methods for UHMWPE
Various processing methods are used to manufacture UHMWPE products, including compression molding, ram extrusion, and gel spinning. These techniques are designed to overcome the challenges associated with processing high molecular weight polymers, such as high melt viscosity and limited flowability. Advanced processing methods aim to improve the material's properties and expand its range of applications.Expand Specific Solutions03 UHMWPE fiber production and applications
UHMWPE fibers are produced through specialized processes, such as gel spinning, resulting in high-strength, lightweight fibers. These fibers find applications in ballistic protection, cut-resistant gloves, and high-performance ropes and cables. The production methods focus on achieving optimal molecular orientation and crystallinity to maximize the fiber's mechanical properties.Expand Specific Solutions04 Modifications and composites of UHMWPE
Research in UHMWPE focuses on modifying the material or creating composites to enhance its properties further. This includes crosslinking for improved wear resistance, incorporation of nanoparticles for enhanced mechanical properties, and blending with other polymers to create hybrid materials with tailored characteristics. These modifications expand the potential applications of UHMWPE in various fields.Expand Specific Solutions05 Medical applications of UHMWPE
UHMWPE is widely used in medical applications, particularly in orthopedic implants such as hip and knee replacements. Its biocompatibility, low friction, and wear resistance make it an ideal material for articulating surfaces in joint prostheses. Ongoing research focuses on improving the longevity and performance of UHMWPE in medical devices through advanced processing techniques and surface modifications.Expand Specific Solutions
Key Players in UHMWPE and Biometric Device Industry
The market for UHMWPE in high-performance biometric devices is in a growth phase, driven by increasing demand for advanced medical implants and wearable technology. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, UHMWPE applications are maturing, with companies like Smith & Nephew, DePuy Synthes, and Zimmer leading innovation. These firms, along with research institutions such as MIT and USC, are advancing UHMWPE's properties for enhanced biocompatibility and durability. Emerging players like Biomet Manufacturing and Tecres are also contributing to the competitive landscape, focusing on specialized applications and novel manufacturing techniques.
DePuy Synthes Products, Inc.
Technical Solution: DePuy Synthes has focused on enhancing UHMWPE for use in orthopedic implants and biometric devices. Their MARATHON™ UHMWPE material undergoes a unique irradiation and annealing process, resulting in improved wear resistance without compromising mechanical properties[4]. For biometric applications, they have developed TRUMATCH® Personalized Solutions, which combines 3D printing technology with UHMWPE to create patient-specific implants that can potentially incorporate sensors for biometric data collection[5]. DePuy Synthes has also explored the use of vitamin E-stabilized UHMWPE (AOX™ Antioxidant UHMWPE) to enhance long-term stability and reduce oxidation in implants[6].
Strengths: Customization capabilities, improved wear resistance, and potential for biometric integration. Weaknesses: Higher production costs, need for specialized manufacturing processes.
Biomet Manufacturing LLC
Technical Solution: Biomet has made significant strides in UHMWPE technology for orthopedic applications with potential crossover to biometric devices. Their E1® Antioxidant Infused Technology incorporates vitamin E into UHMWPE, providing oxidative stability and wear resistance[7]. For biometric applications, Biomet has explored the integration of UHMWPE with their Signature™ Personalized Patient Care system, which could potentially allow for the incorporation of sensors within implants for real-time biometric data collection[8]. Additionally, Biomet's ArcomXL® Polyethylene, a highly crosslinked UHMWPE, has shown excellent wear resistance in hip and knee replacements, which could be adapted for use in high-performance biometric devices[9].
Strengths: Enhanced oxidative stability, potential for personalized biometric solutions. Weaknesses: Limited data on long-term performance in biometric applications, potential regulatory hurdles for novel device integration.
Core UHMWPE Innovations for Biometric Performance
High Performance Multimodal Ultra High Molecular Weight Polyethylene
PatentPendingUS20250019532A1
Innovation
- A multimodal polyethylene composition comprising specific weight percentages of low molecular weight, first high molecular weight, and second high molecular weight polyethylene homopolymers or copolymers, produced in a multistage process using a Ziegler-Natta catalyst, which improves mechanical properties and processability by creating a broad molecular weight distribution curve, enhancing abrasion resistance and impact strength.
Ultra-high molecular weight polyethylene for joint surface
PatentActiveJP2017201037A
Innovation
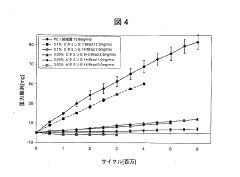
- A method involving the addition of trace amounts (0.02 to 0.12 wt%) of vitamin E to UHMWPE powder before molding, followed by gamma irradiation at doses between 5 and 20 Mrad, without subsequent heat treatment, to create a composition with improved wear resistance, oxidative stability, and mechanical properties.
Regulatory Framework for UHMWPE in Biometric Applications
The regulatory framework for UHMWPE in biometric applications is a complex and evolving landscape that reflects the increasing use of this advanced material in high-performance biometric devices. As UHMWPE gains prominence in this field, regulatory bodies worldwide are adapting their guidelines to ensure the safety, efficacy, and reliability of biometric devices incorporating this material.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating biometric devices that utilize UHMWPE. These devices typically fall under Class II medical devices, requiring a 510(k) premarket notification. The FDA's guidance documents for biometric devices emphasize the importance of material biocompatibility, durability, and performance characteristics, all areas where UHMWPE excels.
The European Union's regulatory approach is governed by the Medical Device Regulation (MDR), which came into full effect in May 2021. Under the MDR, biometric devices incorporating UHMWPE must undergo a conformity assessment process, including a thorough evaluation of the material's properties and its interaction with biological systems. The regulation places a strong emphasis on post-market surveillance and the ongoing assessment of device performance and safety.
In Asia, countries like Japan and China have their own regulatory frameworks for biometric devices. The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established guidelines that address the use of advanced materials like UHMWPE in biometric applications. These guidelines often focus on material stability, long-term performance, and potential biological interactions.
International standards organizations, such as the International Organization for Standardization (ISO), have developed specific standards relevant to UHMWPE in biometric devices. ISO 10993, which addresses the biological evaluation of medical devices, is particularly relevant for assessing the biocompatibility of UHMWPE in biometric applications.
As the field of biometrics continues to advance, regulatory bodies are increasingly focusing on the cybersecurity aspects of biometric devices. This includes ensuring the integrity and security of biometric data collected and processed by devices incorporating UHMWPE components. Regulations are evolving to address potential vulnerabilities and to establish robust data protection measures.
The regulatory landscape also considers the environmental impact of UHMWPE in biometric devices. With growing emphasis on sustainability, regulations are beginning to address the lifecycle management of these devices, including considerations for recycling or safe disposal of UHMWPE components at the end of their useful life.
As UHMWPE's role in biometric devices expands, regulatory frameworks are likely to continue evolving. Future regulations may focus on standardizing testing methodologies specific to UHMWPE in biometric applications, establishing clearer guidelines for long-term performance assessment, and addressing emerging concerns related to novel biometric technologies that leverage this advanced material.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating biometric devices that utilize UHMWPE. These devices typically fall under Class II medical devices, requiring a 510(k) premarket notification. The FDA's guidance documents for biometric devices emphasize the importance of material biocompatibility, durability, and performance characteristics, all areas where UHMWPE excels.
The European Union's regulatory approach is governed by the Medical Device Regulation (MDR), which came into full effect in May 2021. Under the MDR, biometric devices incorporating UHMWPE must undergo a conformity assessment process, including a thorough evaluation of the material's properties and its interaction with biological systems. The regulation places a strong emphasis on post-market surveillance and the ongoing assessment of device performance and safety.
In Asia, countries like Japan and China have their own regulatory frameworks for biometric devices. The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established guidelines that address the use of advanced materials like UHMWPE in biometric applications. These guidelines often focus on material stability, long-term performance, and potential biological interactions.
International standards organizations, such as the International Organization for Standardization (ISO), have developed specific standards relevant to UHMWPE in biometric devices. ISO 10993, which addresses the biological evaluation of medical devices, is particularly relevant for assessing the biocompatibility of UHMWPE in biometric applications.
As the field of biometrics continues to advance, regulatory bodies are increasingly focusing on the cybersecurity aspects of biometric devices. This includes ensuring the integrity and security of biometric data collected and processed by devices incorporating UHMWPE components. Regulations are evolving to address potential vulnerabilities and to establish robust data protection measures.
The regulatory landscape also considers the environmental impact of UHMWPE in biometric devices. With growing emphasis on sustainability, regulations are beginning to address the lifecycle management of these devices, including considerations for recycling or safe disposal of UHMWPE components at the end of their useful life.
As UHMWPE's role in biometric devices expands, regulatory frameworks are likely to continue evolving. Future regulations may focus on standardizing testing methodologies specific to UHMWPE in biometric applications, establishing clearer guidelines for long-term performance assessment, and addressing emerging concerns related to novel biometric technologies that leverage this advanced material.
Environmental Impact of UHMWPE in Biometric Devices
The environmental impact of Ultra-High Molecular Weight Polyethylene (UHMWPE) in biometric devices is a crucial consideration as these technologies become increasingly prevalent. UHMWPE, known for its exceptional strength-to-weight ratio and durability, has found significant applications in high-performance biometric devices. However, its environmental footprint warrants careful examination.
UHMWPE's production process involves energy-intensive polymerization, which contributes to greenhouse gas emissions. The raw materials, primarily derived from fossil fuels, raise concerns about resource depletion and carbon footprint. Despite these initial environmental costs, the longevity of UHMWPE products often offsets the impact over time, as they require less frequent replacement compared to alternative materials.
In biometric devices, UHMWPE's durability translates to extended product lifecycles, potentially reducing electronic waste. This aligns with sustainable design principles and circular economy goals. However, the material's high resistance to degradation poses challenges for end-of-life management. UHMWPE does not biodegrade easily, potentially contributing to long-term plastic pollution if not properly managed.
Recycling UHMWPE from biometric devices presents both opportunities and challenges. While technically recyclable, the complex nature of biometric devices often makes material separation difficult. Advanced recycling technologies, such as chemical recycling, show promise in recovering UHMWPE from mixed waste streams, but these processes are not yet widely available.
The use of UHMWPE in biometric devices may indirectly contribute to reduced environmental impact in certain applications. For instance, in wearable biometric devices, UHMWPE's lightweight properties can lead to improved energy efficiency, potentially extending battery life and reducing the frequency of charging cycles.
Water resistance is another key property of UHMWPE that impacts its environmental profile in biometric applications. This characteristic enhances device longevity in diverse environments, potentially reducing the need for protective casings made from less environmentally friendly materials. However, this same property can make UHMWPE-containing devices more persistent in aquatic environments if improperly disposed of.
As the biometric device industry evolves, there is growing interest in developing bio-based alternatives to UHMWPE that maintain its high-performance characteristics while reducing environmental impact. Research into sustainable polymer production and green chemistry approaches may lead to more environmentally friendly options in the future.
UHMWPE's production process involves energy-intensive polymerization, which contributes to greenhouse gas emissions. The raw materials, primarily derived from fossil fuels, raise concerns about resource depletion and carbon footprint. Despite these initial environmental costs, the longevity of UHMWPE products often offsets the impact over time, as they require less frequent replacement compared to alternative materials.
In biometric devices, UHMWPE's durability translates to extended product lifecycles, potentially reducing electronic waste. This aligns with sustainable design principles and circular economy goals. However, the material's high resistance to degradation poses challenges for end-of-life management. UHMWPE does not biodegrade easily, potentially contributing to long-term plastic pollution if not properly managed.
Recycling UHMWPE from biometric devices presents both opportunities and challenges. While technically recyclable, the complex nature of biometric devices often makes material separation difficult. Advanced recycling technologies, such as chemical recycling, show promise in recovering UHMWPE from mixed waste streams, but these processes are not yet widely available.
The use of UHMWPE in biometric devices may indirectly contribute to reduced environmental impact in certain applications. For instance, in wearable biometric devices, UHMWPE's lightweight properties can lead to improved energy efficiency, potentially extending battery life and reducing the frequency of charging cycles.
Water resistance is another key property of UHMWPE that impacts its environmental profile in biometric applications. This characteristic enhances device longevity in diverse environments, potentially reducing the need for protective casings made from less environmentally friendly materials. However, this same property can make UHMWPE-containing devices more persistent in aquatic environments if improperly disposed of.
As the biometric device industry evolves, there is growing interest in developing bio-based alternatives to UHMWPE that maintain its high-performance characteristics while reducing environmental impact. Research into sustainable polymer production and green chemistry approaches may lead to more environmentally friendly options in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!