Vacuum Pump Optimization for High-Purity Pharmaceutical Synthesis
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vacuum Pump Evolution
The evolution of vacuum pumps in the context of high-purity pharmaceutical synthesis has been marked by significant technological advancements and a continuous drive for improved performance. The journey began with simple mechanical pumps in the early 20th century, which provided basic vacuum capabilities but were limited in their ability to achieve high levels of purity.
As the pharmaceutical industry's demands for higher purity and more precise control over synthesis environments grew, so did the sophistication of vacuum pump technology. The mid-20th century saw the introduction of oil-sealed rotary vane pumps, which offered improved vacuum levels and reliability. However, these pumps faced challenges with oil contamination, a critical issue in pharmaceutical applications where even trace impurities could compromise product quality.
The late 20th century brought about a paradigm shift with the development of oil-free technologies. Dry scroll pumps and diaphragm pumps emerged as game-changers, eliminating the risk of oil contamination and providing cleaner vacuum environments. These innovations were particularly crucial for high-purity pharmaceutical synthesis, where maintaining a contamination-free environment is paramount.
The turn of the millennium saw further refinements in vacuum pump technology. Magnetically levitated turbomolecular pumps were introduced, offering ultra-high vacuum capabilities with minimal vibration and wear. This technology proved invaluable for sensitive pharmaceutical processes requiring extreme precision and purity.
In recent years, the focus has shifted towards energy efficiency and smart control systems. Variable speed drives have been integrated into vacuum pumps, allowing for dynamic adjustment of pump performance based on process requirements. This not only optimizes energy consumption but also enhances process control, a critical factor in pharmaceutical synthesis.
The latest frontier in vacuum pump evolution for pharmaceutical applications is the integration of Industry 4.0 principles. Smart vacuum pumps equipped with sensors and connectivity features now offer real-time monitoring, predictive maintenance, and data analytics capabilities. These advancements enable pharmaceutical manufacturers to optimize their processes, ensure consistent quality, and comply with stringent regulatory requirements.
Looking ahead, the evolution of vacuum pumps for high-purity pharmaceutical synthesis is likely to continue along the paths of increased efficiency, enhanced purity, and greater integration with digital systems. Emerging technologies such as advanced materials for pump components and AI-driven control algorithms are poised to further revolutionize the field, promising even higher levels of performance and reliability in the pursuit of pharmaceutical excellence.
As the pharmaceutical industry's demands for higher purity and more precise control over synthesis environments grew, so did the sophistication of vacuum pump technology. The mid-20th century saw the introduction of oil-sealed rotary vane pumps, which offered improved vacuum levels and reliability. However, these pumps faced challenges with oil contamination, a critical issue in pharmaceutical applications where even trace impurities could compromise product quality.
The late 20th century brought about a paradigm shift with the development of oil-free technologies. Dry scroll pumps and diaphragm pumps emerged as game-changers, eliminating the risk of oil contamination and providing cleaner vacuum environments. These innovations were particularly crucial for high-purity pharmaceutical synthesis, where maintaining a contamination-free environment is paramount.
The turn of the millennium saw further refinements in vacuum pump technology. Magnetically levitated turbomolecular pumps were introduced, offering ultra-high vacuum capabilities with minimal vibration and wear. This technology proved invaluable for sensitive pharmaceutical processes requiring extreme precision and purity.
In recent years, the focus has shifted towards energy efficiency and smart control systems. Variable speed drives have been integrated into vacuum pumps, allowing for dynamic adjustment of pump performance based on process requirements. This not only optimizes energy consumption but also enhances process control, a critical factor in pharmaceutical synthesis.
The latest frontier in vacuum pump evolution for pharmaceutical applications is the integration of Industry 4.0 principles. Smart vacuum pumps equipped with sensors and connectivity features now offer real-time monitoring, predictive maintenance, and data analytics capabilities. These advancements enable pharmaceutical manufacturers to optimize their processes, ensure consistent quality, and comply with stringent regulatory requirements.
Looking ahead, the evolution of vacuum pumps for high-purity pharmaceutical synthesis is likely to continue along the paths of increased efficiency, enhanced purity, and greater integration with digital systems. Emerging technologies such as advanced materials for pump components and AI-driven control algorithms are poised to further revolutionize the field, promising even higher levels of performance and reliability in the pursuit of pharmaceutical excellence.
Pharma Market Demand
The pharmaceutical industry's demand for high-purity synthesis processes has been steadily increasing, driven by the need for more complex and potent drug molecules. This trend has created a significant market for advanced vacuum pump technologies that can support the stringent requirements of pharmaceutical manufacturing. The global pharmaceutical market is projected to reach $1.5 trillion by 2023, with a compound annual growth rate (CAGR) of 3-6%. Within this market, the demand for high-purity active pharmaceutical ingredients (APIs) is growing at an even faster rate, estimated at 7-9% CAGR.
Vacuum pumps play a crucial role in various pharmaceutical processes, including distillation, crystallization, drying, and filtration. The optimization of these pumps for high-purity synthesis is becoming increasingly important as pharmaceutical companies strive to improve product quality, reduce contamination risks, and enhance overall process efficiency. The market for pharmaceutical-grade vacuum pumps is expected to grow at a CAGR of 6-8% over the next five years, reaching a value of approximately $1.2 billion by 2025.
Key factors driving the demand for optimized vacuum pumps in pharmaceutical synthesis include:
1. Stringent regulatory requirements: Regulatory bodies such as the FDA and EMA are imposing stricter guidelines on pharmaceutical manufacturing processes, necessitating the use of more advanced and reliable vacuum pump technologies.
2. Increasing focus on product quality: Pharmaceutical companies are investing heavily in technologies that can ensure consistent high-purity synthesis, reducing the risk of product recalls and improving patient safety.
3. Cost reduction initiatives: Optimized vacuum pumps can lead to significant energy savings and reduced maintenance costs, aligning with the industry's efforts to improve operational efficiency.
4. Growth in biopharmaceuticals: The rapidly expanding biopharmaceutical sector requires specialized vacuum pump solutions capable of handling sensitive biological materials and maintaining sterile conditions.
5. Adoption of continuous manufacturing: As the industry shifts towards continuous manufacturing processes, there is a growing need for vacuum pumps that can operate reliably for extended periods without compromising performance.
The market demand for vacuum pump optimization in high-purity pharmaceutical synthesis is not uniform across all regions. North America and Europe currently dominate the market, accounting for approximately 60% of the global demand. However, the Asia-Pacific region, particularly China and India, is expected to show the highest growth rate in the coming years, driven by the expansion of their domestic pharmaceutical industries and increasing investments in advanced manufacturing technologies.
In conclusion, the pharmaceutical market's demand for optimized vacuum pumps in high-purity synthesis is robust and growing. This trend presents significant opportunities for vacuum pump manufacturers and technology providers to develop innovative solutions that address the specific needs of the pharmaceutical industry, particularly in areas such as contamination prevention, energy efficiency, and process integration.
Vacuum pumps play a crucial role in various pharmaceutical processes, including distillation, crystallization, drying, and filtration. The optimization of these pumps for high-purity synthesis is becoming increasingly important as pharmaceutical companies strive to improve product quality, reduce contamination risks, and enhance overall process efficiency. The market for pharmaceutical-grade vacuum pumps is expected to grow at a CAGR of 6-8% over the next five years, reaching a value of approximately $1.2 billion by 2025.
Key factors driving the demand for optimized vacuum pumps in pharmaceutical synthesis include:
1. Stringent regulatory requirements: Regulatory bodies such as the FDA and EMA are imposing stricter guidelines on pharmaceutical manufacturing processes, necessitating the use of more advanced and reliable vacuum pump technologies.
2. Increasing focus on product quality: Pharmaceutical companies are investing heavily in technologies that can ensure consistent high-purity synthesis, reducing the risk of product recalls and improving patient safety.
3. Cost reduction initiatives: Optimized vacuum pumps can lead to significant energy savings and reduced maintenance costs, aligning with the industry's efforts to improve operational efficiency.
4. Growth in biopharmaceuticals: The rapidly expanding biopharmaceutical sector requires specialized vacuum pump solutions capable of handling sensitive biological materials and maintaining sterile conditions.
5. Adoption of continuous manufacturing: As the industry shifts towards continuous manufacturing processes, there is a growing need for vacuum pumps that can operate reliably for extended periods without compromising performance.
The market demand for vacuum pump optimization in high-purity pharmaceutical synthesis is not uniform across all regions. North America and Europe currently dominate the market, accounting for approximately 60% of the global demand. However, the Asia-Pacific region, particularly China and India, is expected to show the highest growth rate in the coming years, driven by the expansion of their domestic pharmaceutical industries and increasing investments in advanced manufacturing technologies.
In conclusion, the pharmaceutical market's demand for optimized vacuum pumps in high-purity synthesis is robust and growing. This trend presents significant opportunities for vacuum pump manufacturers and technology providers to develop innovative solutions that address the specific needs of the pharmaceutical industry, particularly in areas such as contamination prevention, energy efficiency, and process integration.
Current Challenges
The optimization of vacuum pumps for high-purity pharmaceutical synthesis faces several significant challenges in the current technological landscape. One of the primary issues is achieving and maintaining ultra-high vacuum levels required for sensitive pharmaceutical processes. Traditional vacuum pump technologies often struggle to reach the extreme low pressures needed without introducing contaminants or causing unwanted chemical reactions.
Another major challenge lies in the prevention of cross-contamination between different batches or products. Pharmaceutical synthesis demands stringent cleanliness standards, and even minute traces of previous materials can compromise product purity. Existing pump designs may have areas where residues can accumulate, making thorough cleaning between processes difficult and time-consuming.
Energy efficiency remains a persistent concern in vacuum pump optimization. High-performance pumps capable of achieving the necessary vacuum levels often consume substantial amounts of energy, leading to increased operational costs and environmental impact. Balancing the need for powerful suction with energy conservation poses a significant engineering challenge.
The issue of heat generation during operation also presents difficulties. Excessive heat can affect the stability of sensitive pharmaceutical compounds and potentially lead to unwanted side reactions. Cooling systems integrated into vacuum pumps must be carefully designed to maintain optimal temperature conditions without introducing additional contamination risks.
Noise and vibration reduction represent another area of concern, particularly in laboratory environments where precision and stability are crucial. The mechanical nature of many vacuum pump designs can produce disruptive noise and vibrations, potentially affecting other sensitive equipment or processes in the vicinity.
Scalability and flexibility pose challenges when optimizing vacuum pumps for pharmaceutical applications. The ability to adjust vacuum levels precisely for different synthesis processes while maintaining consistent performance across various scales of production is essential but technically demanding.
Lastly, the integration of advanced monitoring and control systems into vacuum pump technology presents both opportunities and challenges. Real-time monitoring of vacuum levels, contamination detection, and predictive maintenance capabilities are highly desirable but require sophisticated sensor technologies and data processing systems that can operate reliably in harsh vacuum environments.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, fluid dynamics, control systems, and clean manufacturing techniques. The development of next-generation vacuum pump technologies for high-purity pharmaceutical synthesis must balance these competing demands to deliver solutions that meet the exacting standards of the pharmaceutical industry while improving efficiency and reliability.
Another major challenge lies in the prevention of cross-contamination between different batches or products. Pharmaceutical synthesis demands stringent cleanliness standards, and even minute traces of previous materials can compromise product purity. Existing pump designs may have areas where residues can accumulate, making thorough cleaning between processes difficult and time-consuming.
Energy efficiency remains a persistent concern in vacuum pump optimization. High-performance pumps capable of achieving the necessary vacuum levels often consume substantial amounts of energy, leading to increased operational costs and environmental impact. Balancing the need for powerful suction with energy conservation poses a significant engineering challenge.
The issue of heat generation during operation also presents difficulties. Excessive heat can affect the stability of sensitive pharmaceutical compounds and potentially lead to unwanted side reactions. Cooling systems integrated into vacuum pumps must be carefully designed to maintain optimal temperature conditions without introducing additional contamination risks.
Noise and vibration reduction represent another area of concern, particularly in laboratory environments where precision and stability are crucial. The mechanical nature of many vacuum pump designs can produce disruptive noise and vibrations, potentially affecting other sensitive equipment or processes in the vicinity.
Scalability and flexibility pose challenges when optimizing vacuum pumps for pharmaceutical applications. The ability to adjust vacuum levels precisely for different synthesis processes while maintaining consistent performance across various scales of production is essential but technically demanding.
Lastly, the integration of advanced monitoring and control systems into vacuum pump technology presents both opportunities and challenges. Real-time monitoring of vacuum levels, contamination detection, and predictive maintenance capabilities are highly desirable but require sophisticated sensor technologies and data processing systems that can operate reliably in harsh vacuum environments.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, fluid dynamics, control systems, and clean manufacturing techniques. The development of next-generation vacuum pump technologies for high-purity pharmaceutical synthesis must balance these competing demands to deliver solutions that meet the exacting standards of the pharmaceutical industry while improving efficiency and reliability.
Existing Solutions
01 Improved pump design and configuration
Optimization of vacuum pump design involves enhancing the overall configuration and structure of the pump. This includes modifications to the rotor, stator, and other internal components to improve efficiency and performance. Advanced materials and manufacturing techniques may be employed to reduce friction, increase durability, and optimize fluid flow within the pump.- Improved pump design and configuration: Optimization of vacuum pump design involves enhancing the overall configuration and structure of the pump. This includes modifications to the rotor, stator, and other internal components to improve efficiency and performance. Advanced materials and manufacturing techniques may be employed to reduce friction, increase durability, and optimize fluid flow within the pump.
- Enhanced control systems and monitoring: Implementation of sophisticated control systems and monitoring technologies can significantly optimize vacuum pump operation. This includes the use of sensors, data analytics, and intelligent algorithms to adjust pump parameters in real-time, ensuring optimal performance under varying conditions. Advanced monitoring systems can also predict maintenance needs and prevent unexpected failures.
- Energy efficiency improvements: Focusing on energy efficiency is crucial for vacuum pump optimization. This involves developing pumps with reduced power consumption, improved heat management, and better overall energy utilization. Techniques such as variable speed drives, energy recovery systems, and optimized motor designs can contribute to significant energy savings and improved sustainability.
- Specialized pump designs for specific applications: Tailoring vacuum pump designs to specific industrial applications can lead to significant performance improvements. This includes developing pumps optimized for particular pressure ranges, gas compositions, or operating environments. Specialized designs can enhance efficiency, reduce maintenance requirements, and improve overall process outcomes in targeted applications.
- Integration of smart technologies and IoT: Incorporating smart technologies and Internet of Things (IoT) capabilities into vacuum pump systems can greatly enhance their optimization. This includes remote monitoring and control, predictive maintenance algorithms, and integration with broader industrial automation systems. Such technologies enable real-time performance optimization, improved reliability, and enhanced decision-making capabilities for pump operators.
02 Enhanced sealing and lubrication systems
Vacuum pump optimization can be achieved through improved sealing mechanisms and lubrication systems. This involves developing advanced sealing materials and designs to minimize leakage and maintain vacuum integrity. Optimized lubrication systems ensure proper distribution of lubricants, reducing wear and extending pump life while maintaining efficient operation.Expand Specific Solutions03 Integration of smart control systems
Incorporating smart control systems and sensors into vacuum pumps can significantly enhance their performance and efficiency. These systems allow for real-time monitoring of pump parameters, automatic adjustments to operating conditions, and predictive maintenance. Advanced algorithms and machine learning techniques can be employed to optimize pump operation based on specific application requirements.Expand Specific Solutions04 Energy efficiency and heat management
Optimizing vacuum pumps for energy efficiency involves improving power consumption and heat management. This can include the development of energy-efficient motors, advanced cooling systems, and heat recovery mechanisms. Implementing variable speed drives and optimizing pump sizing for specific applications can also contribute to overall energy savings and improved performance.Expand Specific Solutions05 Application-specific pump customization
Tailoring vacuum pump designs for specific applications can lead to significant performance improvements. This involves optimizing pump characteristics such as flow rate, ultimate pressure, and pumping speed based on the requirements of particular industries or processes. Customization may include modifying pump geometry, materials, or operating parameters to achieve optimal performance in specific operating environments.Expand Specific Solutions
Industry Leaders
The vacuum pump optimization for high-purity pharmaceutical synthesis market is in a growth phase, driven by increasing demand for advanced pharmaceutical manufacturing processes. The global market size is estimated to be in the billions, with steady growth projected. Technologically, the field is moderately mature but continues to evolve, with companies like Edwards Ltd., Pfeiffer Vacuum GmbH, and Air Liquide SA leading innovation. These firms are developing more efficient, precise, and contamination-free vacuum systems tailored for pharmaceutical applications. Emerging players such as BioNTech SE and Genentech, Inc. are also contributing to advancements, particularly in integrating vacuum technology with biotechnology processes.
Edwards Ltd.
Technical Solution: Edwards Ltd. has developed advanced vacuum pump technologies specifically tailored for high-purity pharmaceutical synthesis. Their GXS dry screw vacuum pump series incorporates innovative features such as temperature management systems and optimized screw profiles to maintain consistent performance in demanding pharmaceutical applications[1]. The company has also introduced the nXDS scroll pump, which utilizes a hermetically sealed, oil-free design to prevent contamination risks in sensitive processes[2]. Edwards' pumps are equipped with intelligent control systems that allow for real-time monitoring and adjustment of pump parameters, ensuring optimal vacuum conditions throughout the synthesis process[3]. Additionally, their vacuum systems often integrate multiple pump technologies in series or parallel configurations to achieve the ultra-high vacuum levels required for certain pharmaceutical reactions while maintaining energy efficiency[4].
Strengths: Specialized designs for pharmaceutical applications, contamination-free operation, intelligent control systems. Weaknesses: Higher initial cost compared to general-purpose pumps, may require specialized maintenance.
Air Liquide SA
Technical Solution: Air Liquide SA has developed comprehensive vacuum and gas management solutions for high-purity pharmaceutical synthesis. Their ALOHA™ (Advanced Liquid Organic Hydrogen carriers for Applications) technology offers a novel approach to hydrogen storage and delivery, which is crucial in many pharmaceutical synthesis processes[1]. Air Liquide has also introduced the Turbo-V pump series, which combines turbomolecular and drag pumping stages to achieve high vacuum levels while maintaining a compact footprint[2]. The company's expertise in gas purification has led to the development of advanced filtration and purification systems that ensure ultra-high purity gas supply to vacuum chambers, critical for maintaining product quality in pharmaceutical synthesis[3]. Additionally, Air Liquide offers customized vacuum system designs that integrate their pumps with specialized gas delivery systems, optimizing overall process efficiency and product yield[4].
Strengths: Integrated gas and vacuum solutions, advanced hydrogen management technology, customized system designs. Weaknesses: May have a stronger focus on gas management compared to pure vacuum technology, potentially higher costs for fully integrated systems.
Key Innovations
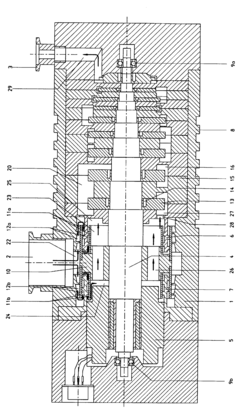
Vacuum pump
PatentInactiveEP1067290A2
Innovation
- A compact, single-unit vacuum pump design featuring a parallel arrangement of gas friction pumps for high-vacuum operation and a side channel pump for atmospheric pressure, with undivided stator discs and clamping rings for optimal axial play, eliminating lubricant use in the high-vacuum side and reducing size and complexity.
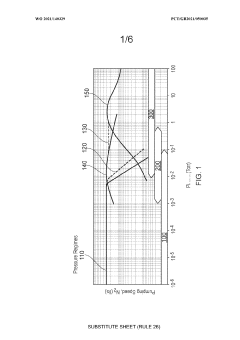
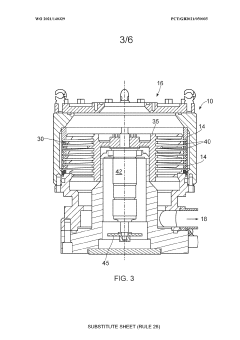
Vacuum pump, vacuum pump set for evacuating a semiconductor processing chamber and method of evacuating a semiconductor processing chamber
PatentWO2021140329A1
Innovation
- A vacuum pump with a rotor and stator design featuring angled blades on a helical path, magnetically levitated bearings, and high transparency perforated elements, allowing effective pumping between 1 mbar and 5 × 10^2 mbar, backed by a roots blower for enhanced performance across the transitional flow regime.
Regulatory Compliance
Regulatory compliance is a critical aspect of vacuum pump optimization for high-purity pharmaceutical synthesis. The pharmaceutical industry is subject to stringent regulations to ensure product safety, efficacy, and quality. These regulations extend to the equipment and processes used in drug manufacturing, including vacuum pumps.
In the United States, the Food and Drug Administration (FDA) oversees pharmaceutical production through Good Manufacturing Practices (GMP) regulations. These guidelines require that equipment used in drug manufacturing be designed, constructed, and maintained to prevent contamination and ensure consistent product quality. Vacuum pumps must meet specific cleanliness standards and be constructed of materials that do not interact with or contaminate the pharmaceutical products.
The European Medicines Agency (EMA) enforces similar regulations in the European Union through its GMP guidelines. These regulations emphasize the importance of equipment qualification and validation, which includes vacuum pumps used in pharmaceutical synthesis. Manufacturers must demonstrate that their vacuum pumps can consistently perform within specified parameters and maintain the required level of purity.
International Organization for Standardization (ISO) standards also play a crucial role in regulatory compliance for vacuum pump optimization. ISO 14644, for instance, provides guidelines for cleanroom classification and monitoring, which directly impacts the design and operation of vacuum pumps in pharmaceutical manufacturing environments.
Regulatory bodies also require comprehensive documentation of equipment specifications, maintenance procedures, and performance data. This documentation is essential for demonstrating compliance during regulatory inspections and audits. Vacuum pump manufacturers and pharmaceutical companies must maintain detailed records of pump performance, maintenance activities, and any deviations from standard operating procedures.
Environmental regulations are another important consideration in vacuum pump optimization. Many jurisdictions have strict guidelines on emissions and waste disposal, which can impact the selection and operation of vacuum pumps. For example, the use of oil-sealed pumps may be restricted in certain applications due to potential environmental concerns, leading to a preference for dry pumps in high-purity pharmaceutical synthesis.
Compliance with these regulations often necessitates the implementation of advanced monitoring and control systems for vacuum pumps. Real-time data logging, automated alarm systems, and predictive maintenance protocols are becoming increasingly important to ensure consistent performance and regulatory adherence.
As regulations continue to evolve, vacuum pump manufacturers and pharmaceutical companies must stay informed of changes and adapt their technologies accordingly. This may involve redesigning pump components, implementing new materials, or developing novel control systems to meet increasingly stringent regulatory requirements.
In the United States, the Food and Drug Administration (FDA) oversees pharmaceutical production through Good Manufacturing Practices (GMP) regulations. These guidelines require that equipment used in drug manufacturing be designed, constructed, and maintained to prevent contamination and ensure consistent product quality. Vacuum pumps must meet specific cleanliness standards and be constructed of materials that do not interact with or contaminate the pharmaceutical products.
The European Medicines Agency (EMA) enforces similar regulations in the European Union through its GMP guidelines. These regulations emphasize the importance of equipment qualification and validation, which includes vacuum pumps used in pharmaceutical synthesis. Manufacturers must demonstrate that their vacuum pumps can consistently perform within specified parameters and maintain the required level of purity.
International Organization for Standardization (ISO) standards also play a crucial role in regulatory compliance for vacuum pump optimization. ISO 14644, for instance, provides guidelines for cleanroom classification and monitoring, which directly impacts the design and operation of vacuum pumps in pharmaceutical manufacturing environments.
Regulatory bodies also require comprehensive documentation of equipment specifications, maintenance procedures, and performance data. This documentation is essential for demonstrating compliance during regulatory inspections and audits. Vacuum pump manufacturers and pharmaceutical companies must maintain detailed records of pump performance, maintenance activities, and any deviations from standard operating procedures.
Environmental regulations are another important consideration in vacuum pump optimization. Many jurisdictions have strict guidelines on emissions and waste disposal, which can impact the selection and operation of vacuum pumps. For example, the use of oil-sealed pumps may be restricted in certain applications due to potential environmental concerns, leading to a preference for dry pumps in high-purity pharmaceutical synthesis.
Compliance with these regulations often necessitates the implementation of advanced monitoring and control systems for vacuum pumps. Real-time data logging, automated alarm systems, and predictive maintenance protocols are becoming increasingly important to ensure consistent performance and regulatory adherence.
As regulations continue to evolve, vacuum pump manufacturers and pharmaceutical companies must stay informed of changes and adapt their technologies accordingly. This may involve redesigning pump components, implementing new materials, or developing novel control systems to meet increasingly stringent regulatory requirements.
Energy Efficiency
Energy efficiency is a critical aspect of vacuum pump optimization in high-purity pharmaceutical synthesis. The pharmaceutical industry's increasing focus on sustainability and cost reduction has driven the need for more energy-efficient vacuum pump solutions. Traditional vacuum pumps often consume significant amounts of energy, contributing to high operational costs and environmental impact.
Recent advancements in vacuum pump technology have led to the development of more energy-efficient models. Variable speed drives (VSDs) have emerged as a key innovation, allowing pumps to adjust their speed and power consumption based on the specific process requirements. This dynamic adjustment significantly reduces energy waste during periods of lower demand, resulting in substantial energy savings over time.
Heat recovery systems have also been integrated into modern vacuum pump designs. These systems capture and repurpose the heat generated during pump operation, which can be utilized for other processes within the pharmaceutical facility. This not only improves overall energy efficiency but also reduces the cooling requirements for the pump itself, further decreasing energy consumption.
The implementation of intelligent control systems has revolutionized vacuum pump energy management. These systems use advanced algorithms and real-time monitoring to optimize pump performance, ensuring that energy consumption is precisely matched to process needs. By continuously analyzing operating conditions and adjusting pump parameters, these control systems can achieve energy savings of up to 50% compared to conventional fixed-speed pumps.
Dry vacuum pump technology has gained prominence in pharmaceutical applications due to its inherent energy efficiency. Unlike oil-sealed pumps, dry pumps eliminate the need for oil changes and disposal, reducing maintenance costs and environmental impact. Moreover, their design allows for better heat management and lower friction, resulting in improved energy efficiency and reduced power consumption.
The adoption of high-efficiency motors, such as IE4 and IE5 rated motors, has further enhanced the energy performance of vacuum pumps. These motors offer superior efficiency levels, minimizing energy losses and heat generation during operation. When combined with optimized pump designs and advanced control systems, they contribute to significant reductions in overall energy consumption.
Manufacturers are also focusing on improving the aerodynamics of pump components to reduce internal friction and enhance energy efficiency. Advanced computational fluid dynamics (CFD) simulations are being employed to optimize impeller designs and flow paths, resulting in pumps that require less power to achieve the same level of vacuum performance.
Recent advancements in vacuum pump technology have led to the development of more energy-efficient models. Variable speed drives (VSDs) have emerged as a key innovation, allowing pumps to adjust their speed and power consumption based on the specific process requirements. This dynamic adjustment significantly reduces energy waste during periods of lower demand, resulting in substantial energy savings over time.
Heat recovery systems have also been integrated into modern vacuum pump designs. These systems capture and repurpose the heat generated during pump operation, which can be utilized for other processes within the pharmaceutical facility. This not only improves overall energy efficiency but also reduces the cooling requirements for the pump itself, further decreasing energy consumption.
The implementation of intelligent control systems has revolutionized vacuum pump energy management. These systems use advanced algorithms and real-time monitoring to optimize pump performance, ensuring that energy consumption is precisely matched to process needs. By continuously analyzing operating conditions and adjusting pump parameters, these control systems can achieve energy savings of up to 50% compared to conventional fixed-speed pumps.
Dry vacuum pump technology has gained prominence in pharmaceutical applications due to its inherent energy efficiency. Unlike oil-sealed pumps, dry pumps eliminate the need for oil changes and disposal, reducing maintenance costs and environmental impact. Moreover, their design allows for better heat management and lower friction, resulting in improved energy efficiency and reduced power consumption.
The adoption of high-efficiency motors, such as IE4 and IE5 rated motors, has further enhanced the energy performance of vacuum pumps. These motors offer superior efficiency levels, minimizing energy losses and heat generation during operation. When combined with optimized pump designs and advanced control systems, they contribute to significant reductions in overall energy consumption.
Manufacturers are also focusing on improving the aerodynamics of pump components to reduce internal friction and enhance energy efficiency. Advanced computational fluid dynamics (CFD) simulations are being employed to optimize impeller designs and flow paths, resulting in pumps that require less power to achieve the same level of vacuum performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!