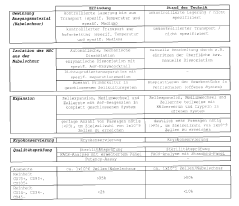
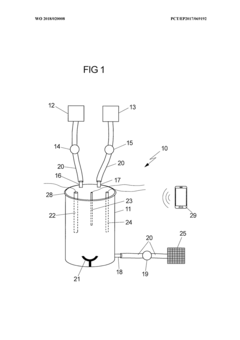
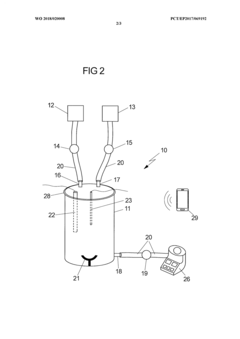
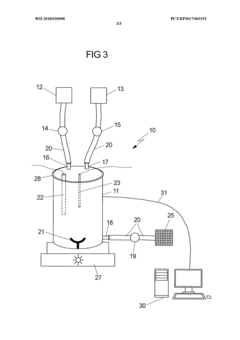
Validation of closed-system cell washing to replace open centrifugation steps in GMP workflows
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell Washing Technology Background and Objectives
Cell washing is a critical process in cell therapy manufacturing that involves the removal of unwanted components from cell suspensions while maintaining cell viability and functionality. Historically, this process has relied heavily on open centrifugation methods, which have been the industry standard for decades due to their effectiveness and relative simplicity. However, as cell therapies have advanced toward commercialization, the limitations of open systems have become increasingly apparent, particularly in Good Manufacturing Practice (GMP) environments where contamination risks must be minimized.
The evolution of cell washing technology has progressed from manual open processes to semi-automated systems, and now toward fully closed automated solutions. This progression reflects the industry's growing recognition of the need for standardization, reproducibility, and contamination control in cell therapy manufacturing. The transition began in earnest during the early 2000s when the first cell therapies were approaching clinical trials, accelerating significantly in the 2010s as regulatory frameworks for cell therapies became more defined.
Current technological objectives in closed-system cell washing focus on achieving comparable or superior cell recovery rates and viability compared to traditional centrifugation while eliminating open processing steps. Additional goals include reducing processing time, minimizing operator variability, enabling integration with other closed manufacturing steps, and providing comprehensive documentation for regulatory compliance. These objectives align with the broader industry trend toward fully automated, closed-system cell therapy manufacturing platforms.
The technical challenges being addressed include maintaining optimal washing efficiency without compromising cell viability, developing systems compatible with diverse cell types (from fragile primary cells to robust cell lines), and ensuring scalability from research quantities to commercial production volumes. Particular attention is being paid to the development of single-use components that eliminate cross-contamination risks while maintaining cost-effectiveness.
Looking forward, the field aims to validate closed-system cell washing technologies that can be seamlessly integrated into end-to-end manufacturing platforms, with real-time monitoring capabilities and process analytical technology (PAT) integration. The ultimate objective is to establish robust, standardized washing protocols that meet stringent regulatory requirements while supporting the commercial viability of advanced cell therapies through improved efficiency, reduced costs, and enhanced product quality.
The evolution of cell washing technology has progressed from manual open processes to semi-automated systems, and now toward fully closed automated solutions. This progression reflects the industry's growing recognition of the need for standardization, reproducibility, and contamination control in cell therapy manufacturing. The transition began in earnest during the early 2000s when the first cell therapies were approaching clinical trials, accelerating significantly in the 2010s as regulatory frameworks for cell therapies became more defined.
Current technological objectives in closed-system cell washing focus on achieving comparable or superior cell recovery rates and viability compared to traditional centrifugation while eliminating open processing steps. Additional goals include reducing processing time, minimizing operator variability, enabling integration with other closed manufacturing steps, and providing comprehensive documentation for regulatory compliance. These objectives align with the broader industry trend toward fully automated, closed-system cell therapy manufacturing platforms.
The technical challenges being addressed include maintaining optimal washing efficiency without compromising cell viability, developing systems compatible with diverse cell types (from fragile primary cells to robust cell lines), and ensuring scalability from research quantities to commercial production volumes. Particular attention is being paid to the development of single-use components that eliminate cross-contamination risks while maintaining cost-effectiveness.
Looking forward, the field aims to validate closed-system cell washing technologies that can be seamlessly integrated into end-to-end manufacturing platforms, with real-time monitoring capabilities and process analytical technology (PAT) integration. The ultimate objective is to establish robust, standardized washing protocols that meet stringent regulatory requirements while supporting the commercial viability of advanced cell therapies through improved efficiency, reduced costs, and enhanced product quality.
Market Analysis for Closed-System Cell Processing
The global market for closed-system cell processing technologies is experiencing significant growth, driven primarily by the expanding cell and gene therapy sector. Current market valuations place this segment at approximately $1.2 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 18-20% over the next five years. This robust growth trajectory reflects the increasing adoption of advanced cell processing technologies across pharmaceutical and biotechnology industries.
Demand for closed-system cell washing solutions is particularly strong in regions with established biopharmaceutical manufacturing capabilities, including North America, Western Europe, and parts of Asia-Pacific. North America currently dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%, with the remainder distributed across other regions.
Key market drivers include stringent regulatory requirements for GMP compliance, growing emphasis on contamination control, and the need for process standardization in cell therapy manufacturing. The FDA and EMA have both issued guidance documents emphasizing the importance of closed processing systems to minimize contamination risks, thereby accelerating market adoption. Additionally, the labor-intensive nature of traditional open centrifugation methods has created strong economic incentives for automation and closed systems.
Customer segments in this market include pharmaceutical companies developing cell therapies, contract development and manufacturing organizations (CDMOs), academic research institutions, and hospital-based cell processing facilities. Among these, pharmaceutical companies and CDMOs represent the largest and fastest-growing segments, collectively accounting for over 70% of market demand.
Cost considerations remain significant, with closed-system cell washing technologies typically requiring initial investments of $50,000-$200,000 per unit, depending on throughput capacity and automation features. However, return on investment analyses demonstrate potential cost savings of 30-40% over three years when factoring reduced clean room requirements, decreased contamination rates, and improved process efficiency.
Market challenges include integration with existing workflows, validation requirements, and the need for specialized training. Early adopters report implementation periods of 3-6 months before achieving optimal operational efficiency, highlighting the importance of comprehensive validation protocols and staff training programs.
Future market growth is expected to be fueled by technological innovations addressing current limitations, expansion of cell therapy applications, and increasing adoption in emerging markets. Particular growth opportunities exist in point-of-care settings and decentralized manufacturing models, where compact, user-friendly closed systems could significantly expand the accessibility of advanced cell therapies.
Demand for closed-system cell washing solutions is particularly strong in regions with established biopharmaceutical manufacturing capabilities, including North America, Western Europe, and parts of Asia-Pacific. North America currently dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%, with the remainder distributed across other regions.
Key market drivers include stringent regulatory requirements for GMP compliance, growing emphasis on contamination control, and the need for process standardization in cell therapy manufacturing. The FDA and EMA have both issued guidance documents emphasizing the importance of closed processing systems to minimize contamination risks, thereby accelerating market adoption. Additionally, the labor-intensive nature of traditional open centrifugation methods has created strong economic incentives for automation and closed systems.
Customer segments in this market include pharmaceutical companies developing cell therapies, contract development and manufacturing organizations (CDMOs), academic research institutions, and hospital-based cell processing facilities. Among these, pharmaceutical companies and CDMOs represent the largest and fastest-growing segments, collectively accounting for over 70% of market demand.
Cost considerations remain significant, with closed-system cell washing technologies typically requiring initial investments of $50,000-$200,000 per unit, depending on throughput capacity and automation features. However, return on investment analyses demonstrate potential cost savings of 30-40% over three years when factoring reduced clean room requirements, decreased contamination rates, and improved process efficiency.
Market challenges include integration with existing workflows, validation requirements, and the need for specialized training. Early adopters report implementation periods of 3-6 months before achieving optimal operational efficiency, highlighting the importance of comprehensive validation protocols and staff training programs.
Future market growth is expected to be fueled by technological innovations addressing current limitations, expansion of cell therapy applications, and increasing adoption in emerging markets. Particular growth opportunities exist in point-of-care settings and decentralized manufacturing models, where compact, user-friendly closed systems could significantly expand the accessibility of advanced cell therapies.
Current Challenges in GMP Cell Washing Methods
Cell washing is a critical step in Good Manufacturing Practice (GMP) workflows for cell and gene therapies, traditionally performed using open centrifugation methods. These conventional approaches present significant challenges that impact product quality, process efficiency, and regulatory compliance in biopharmaceutical manufacturing environments.
The primary challenge with open centrifugation methods is contamination risk. Open systems expose cell products to environmental contaminants, increasing the likelihood of microbial contamination and cross-contamination between batches. This necessitates stringent clean room facilities with extensive environmental monitoring programs, substantially increasing manufacturing costs and complexity.
Process variability represents another major hurdle. Manual centrifugation techniques are highly operator-dependent, leading to inconsistent cell recovery rates, variable washing efficiency, and unpredictable cell viability outcomes. This variability compromises batch-to-batch consistency, a critical requirement for GMP manufacturing and regulatory approval.
Scalability limitations further constrain traditional centrifugation approaches. As production volumes increase to meet commercial demands, open centrifugation becomes increasingly impractical. The process is labor-intensive, time-consuming, and difficult to scale proportionally, creating bottlenecks in manufacturing workflows and limiting production capacity.
Cell product quality is also adversely affected by conventional centrifugation. The mechanical stress from repeated centrifugation cycles can damage fragile cell membranes, reduce viability, and alter cellular function. These quality impacts are particularly problematic for sensitive cell types used in advanced therapy medicinal products (ATMPs).
From a regulatory perspective, open processing steps face increasing scrutiny from agencies like the FDA and EMA. Regulatory guidelines increasingly favor closed systems that minimize contamination risks and process variability. Manufacturers using open centrifugation face greater documentation burdens and validation challenges to demonstrate process control and product consistency.
Resource utilization presents additional challenges. Open centrifugation requires dedicated equipment, specialized facilities, and highly trained personnel. The process consumes significant clean room space and requires extensive gowning procedures, environmental monitoring, and cleaning validation protocols, all contributing to higher production costs.
Documentation and traceability requirements are more complex with open systems. Each manual intervention point requires detailed standard operating procedures, operator training records, and in-process controls. This documentation burden increases quality assurance costs and regulatory compliance complexity.
The primary challenge with open centrifugation methods is contamination risk. Open systems expose cell products to environmental contaminants, increasing the likelihood of microbial contamination and cross-contamination between batches. This necessitates stringent clean room facilities with extensive environmental monitoring programs, substantially increasing manufacturing costs and complexity.
Process variability represents another major hurdle. Manual centrifugation techniques are highly operator-dependent, leading to inconsistent cell recovery rates, variable washing efficiency, and unpredictable cell viability outcomes. This variability compromises batch-to-batch consistency, a critical requirement for GMP manufacturing and regulatory approval.
Scalability limitations further constrain traditional centrifugation approaches. As production volumes increase to meet commercial demands, open centrifugation becomes increasingly impractical. The process is labor-intensive, time-consuming, and difficult to scale proportionally, creating bottlenecks in manufacturing workflows and limiting production capacity.
Cell product quality is also adversely affected by conventional centrifugation. The mechanical stress from repeated centrifugation cycles can damage fragile cell membranes, reduce viability, and alter cellular function. These quality impacts are particularly problematic for sensitive cell types used in advanced therapy medicinal products (ATMPs).
From a regulatory perspective, open processing steps face increasing scrutiny from agencies like the FDA and EMA. Regulatory guidelines increasingly favor closed systems that minimize contamination risks and process variability. Manufacturers using open centrifugation face greater documentation burdens and validation challenges to demonstrate process control and product consistency.
Resource utilization presents additional challenges. Open centrifugation requires dedicated equipment, specialized facilities, and highly trained personnel. The process consumes significant clean room space and requires extensive gowning procedures, environmental monitoring, and cleaning validation protocols, all contributing to higher production costs.
Documentation and traceability requirements are more complex with open systems. Each manual intervention point requires detailed standard operating procedures, operator training records, and in-process controls. This documentation burden increases quality assurance costs and regulatory compliance complexity.
Current Closed-System Cell Washing Solutions
01 Validation protocols for closed-system cell washing
Validation protocols are essential for ensuring the effectiveness and reliability of closed-system cell washing processes. These protocols typically include verification of system integrity, performance qualification, and process validation steps. The validation process ensures that the cell washing system consistently meets predetermined specifications and quality standards, which is critical for applications in cell therapy and transfusion medicine.- Validation protocols for closed-system cell washing: Validation protocols are essential for ensuring the effectiveness and reliability of closed-system cell washing processes. These protocols typically include verification of system integrity, performance qualification, and documentation of process parameters. Validation ensures that the cell washing system consistently removes unwanted substances while maintaining cell viability and functionality. The protocols may include testing for sterility, removal efficiency, and reproducibility of results.
- Automated monitoring and quality control systems: Automated monitoring and quality control systems are implemented in closed-system cell washing to ensure process consistency and compliance with regulatory requirements. These systems continuously track critical parameters such as temperature, centrifugation speed, washing solution volumes, and cycle times. Real-time monitoring allows for immediate detection of deviations and automated documentation of the entire washing process, which is crucial for validation purposes and maintaining Good Manufacturing Practices (GMP) standards.
- Data management and documentation for validation: Comprehensive data management and documentation are critical components of closed-system cell washing validation. This includes electronic record-keeping of all process parameters, equipment calibration records, operator training documentation, and deviation reports. Proper documentation ensures traceability and provides evidence that the cell washing process consistently meets predetermined specifications. Advanced data management systems may incorporate electronic signatures and audit trails to comply with regulatory requirements for cell therapy products.
- Risk assessment and failure mode analysis: Risk assessment and failure mode analysis are essential for identifying potential points of failure in closed-system cell washing processes. This involves systematic evaluation of process steps, equipment components, and operating procedures to identify critical control points. By anticipating possible failure modes and their consequences, appropriate preventive measures can be implemented. This approach helps establish robust validation protocols that address all potential risks to product quality and patient safety.
- Validation of cleaning and sterilization procedures: Validation of cleaning and sterilization procedures is crucial for maintaining the integrity of closed-system cell washing processes. This includes verification that all components contacting the cell product are properly cleaned and sterilized between uses or are single-use disposables. Validation protocols typically include testing for residual cleaning agents, endotoxins, and microbial contamination. These procedures ensure that the cell washing system does not introduce contaminants that could affect cell viability or patient safety.
02 Automated monitoring and quality control systems
Automated monitoring and quality control systems are implemented in closed-system cell washing to ensure process consistency and product quality. These systems include sensors for real-time monitoring of critical parameters, automated data collection, and analysis tools for process verification. The integration of automation in quality control reduces human error and provides comprehensive documentation for regulatory compliance.Expand Specific Solutions03 Contamination prevention and sterility assurance
Closed-system cell washing validation includes measures to prevent contamination and ensure sterility throughout the process. This involves validation of the system's ability to maintain a sterile environment, testing for microbial contamination, and verification of the integrity of the closed system. Sterility assurance is critical for cell-based products intended for therapeutic use to prevent adverse patient outcomes.Expand Specific Solutions04 Process parameter optimization and standardization
Optimization and standardization of process parameters are key components of closed-system cell washing validation. This includes establishing acceptable ranges for critical parameters such as washing time, centrifugation speed, buffer composition, and temperature. Standardized procedures ensure consistency across different batches and operators, which is essential for reproducible results in clinical applications.Expand Specific Solutions05 Regulatory compliance and documentation requirements
Validation of closed-system cell washing processes must meet regulatory compliance standards and documentation requirements. This includes maintaining comprehensive records of validation activities, equipment qualification, operator training, and process verification. Proper documentation is essential for regulatory submissions and inspections, ensuring that the cell washing process meets good manufacturing practice (GMP) standards for therapeutic applications.Expand Specific Solutions
Key Industry Players in Cell Processing Equipment
The closed-system cell washing technology market is currently in a growth phase, with increasing adoption in GMP workflows as organizations seek to replace traditional open centrifugation steps. The market is expanding rapidly due to stringent regulatory requirements for cell therapy manufacturing, with an estimated value exceeding $500 million annually. Leading players in this competitive landscape include Cytiva (Global Life Sciences Solutions USA LLC), which offers advanced automated closed washing systems, alongside Lonza Walkersville and Cellares Corp., who are developing integrated cell processing platforms. Japanese corporations like Kaneka Corp. and Olympus Corp. are contributing specialized equipment, while emerging players such as Redbud Labs and Hrain Biotechnology are introducing innovative microfluidic solutions. Technical maturity varies significantly, with established companies offering validated systems while newer entrants focus on novel approaches to improve efficiency and reduce contamination risks.
Fenwal, Inc.
Technical Solution: Fenwal (now part of Fresenius Kabi) has developed the AMICUS cell separation and washing system, a semi-automated closed platform designed to replace open centrifugation steps in GMP cell processing workflows. Their technology utilizes continuous-flow centrifugation with specialized separation chambers that maintain cellular integrity while efficiently removing unwanted components. The system employs single-use disposable kits that create a sterile closed pathway for all fluid transfers, eliminating exposure to the environment. Fenwal's platform incorporates optical detection technology to precisely control the interface between cell fractions and washing solutions, optimizing both purity and recovery. The AMICUS system has been extensively validated for various cellular applications including stem cell processing, with documented cell recoveries exceeding 85% while achieving >99% removal of unwanted solutes. The technology supports protocol standardization with programmable procedures that ensure consistent processing across multiple operators and manufacturing sites.
Strengths: Established technology with extensive clinical validation; high cell recovery rates; user-friendly interface requiring minimal specialized training; compatible with existing cell processing workflows; relatively lower cost compared to fully automated systems. Weaknesses: Semi-automated approach still requires some operator intervention; limited throughput for high-volume applications; less flexibility for novel cell types compared to newer platforms; requires dedicated floor space.
Global Life Sciences Solutions USA LLC
Technical Solution: Global Life Sciences Solutions (formerly part of GE Healthcare Life Sciences) has pioneered the Sepax C-Pro system, a closed-system cell processing platform specifically designed for GMP-compliant cell therapy manufacturing. Their technology employs a unique combination of continuous counterflow centrifugation and automated fluid management to perform cell washing operations in a completely closed environment. The system utilizes single-use kits with integrated tubing sets that maintain sterility throughout the process. Their proprietary algorithm optimizes washing parameters based on cell type and volume, achieving >90% cell recovery while removing >99% of unwanted solutes. The platform includes comprehensive electronic records for each process step, supporting GMP compliance and process validation. Recent advancements include integration with their Xuri cell expansion systems to create end-to-end closed manufacturing workflows for cell therapies.
Strengths: Extensive validation data supporting GMP compliance; high cell recovery rates; flexible platform adaptable to various cell types; comprehensive electronic documentation system; established global support network. Weaknesses: Requires dedicated equipment and consumables from the same vendor; limited throughput for very high-volume applications; higher operating costs compared to traditional open centrifugation methods.
Technical Analysis of Validation Methodologies
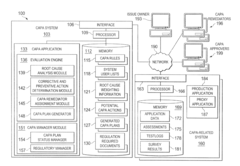
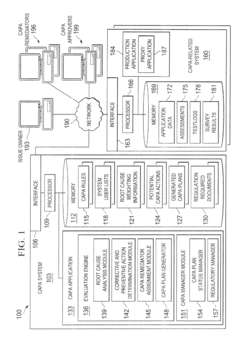
System and Method for CAPA Process Automation
PatentInactiveUS20120136687A1
Innovation
- An automated system that identifies issues, determines root causes, and generates CAPA plans by analyzing data from manufacturing processes, assigning corrective or preventive actions, and monitoring their completion, using a centralized platform with evaluation engines and CAPA management modules.
Device for isolating stem cells from fetal tissues
PatentWO2018020008A1
Innovation
- A device comprising an incubation chamber, pumps, tissue disruption and rinsing solution reservoirs, optional control unit, and means for impurity separation and cell expansion, utilizing mechanical dissociation, enzymatic digestion, and density gradient centrifugation to isolate MSCs in a closed system that enhances yield and quality, ensuring GMP compliance.
Regulatory Compliance and Quality Considerations
Regulatory compliance represents a critical dimension in the validation of closed-system cell washing technologies for GMP workflows. The transition from open centrifugation to closed systems must adhere to stringent regulatory frameworks established by agencies such as the FDA, EMA, and ICH. These frameworks mandate comprehensive validation protocols that demonstrate the closed system's ability to maintain product quality, safety, and efficacy throughout the manufacturing process.
Quality risk management principles, as outlined in ICH Q9, must be systematically applied when implementing closed-system cell washing technologies. This involves identifying potential failure modes, assessing their impact on product quality, and establishing appropriate control strategies. The validation approach should incorporate a thorough risk assessment that evaluates process parameters, equipment design, and operational procedures to ensure consistent performance and compliance with predefined quality attributes.
Documentation requirements for closed-system validation are extensive and must align with GMP guidelines. This includes the development of validation master plans, protocols, and reports that clearly define acceptance criteria, testing methodologies, and data analysis procedures. The documentation should establish traceability between validation activities and regulatory requirements, demonstrating a comprehensive understanding of how the closed system maintains product quality throughout the manufacturing process.
Process analytical technology (PAT) integration represents an emerging consideration in closed-system validation. Real-time monitoring capabilities can enhance quality control by providing continuous verification of critical process parameters. Regulatory agencies increasingly encourage the implementation of PAT as part of a quality-by-design approach, which can streamline validation activities and support continuous process verification.
Personnel training and qualification programs constitute another essential component of regulatory compliance. Operators must demonstrate proficiency in handling closed-system equipment, understanding critical process parameters, and responding to deviations. Training programs should be documented and periodically assessed to ensure ongoing compliance with GMP requirements.
Aseptic processing validation deserves particular attention when implementing closed-system cell washing technologies. Media fill studies, environmental monitoring, and container closure integrity testing must be adapted to the specific characteristics of closed systems. The validation approach should demonstrate that the closed system effectively minimizes contamination risks compared to traditional open centrifugation methods, thereby enhancing product sterility assurance.
Change management protocols must be established to address modifications to closed-system equipment, processes, or components. These protocols should define the level of revalidation required based on the nature and impact of the change, ensuring that regulatory compliance is maintained throughout the product lifecycle.
Quality risk management principles, as outlined in ICH Q9, must be systematically applied when implementing closed-system cell washing technologies. This involves identifying potential failure modes, assessing their impact on product quality, and establishing appropriate control strategies. The validation approach should incorporate a thorough risk assessment that evaluates process parameters, equipment design, and operational procedures to ensure consistent performance and compliance with predefined quality attributes.
Documentation requirements for closed-system validation are extensive and must align with GMP guidelines. This includes the development of validation master plans, protocols, and reports that clearly define acceptance criteria, testing methodologies, and data analysis procedures. The documentation should establish traceability between validation activities and regulatory requirements, demonstrating a comprehensive understanding of how the closed system maintains product quality throughout the manufacturing process.
Process analytical technology (PAT) integration represents an emerging consideration in closed-system validation. Real-time monitoring capabilities can enhance quality control by providing continuous verification of critical process parameters. Regulatory agencies increasingly encourage the implementation of PAT as part of a quality-by-design approach, which can streamline validation activities and support continuous process verification.
Personnel training and qualification programs constitute another essential component of regulatory compliance. Operators must demonstrate proficiency in handling closed-system equipment, understanding critical process parameters, and responding to deviations. Training programs should be documented and periodically assessed to ensure ongoing compliance with GMP requirements.
Aseptic processing validation deserves particular attention when implementing closed-system cell washing technologies. Media fill studies, environmental monitoring, and container closure integrity testing must be adapted to the specific characteristics of closed systems. The validation approach should demonstrate that the closed system effectively minimizes contamination risks compared to traditional open centrifugation methods, thereby enhancing product sterility assurance.
Change management protocols must be established to address modifications to closed-system equipment, processes, or components. These protocols should define the level of revalidation required based on the nature and impact of the change, ensuring that regulatory compliance is maintained throughout the product lifecycle.
Cost-Benefit Analysis of Technology Implementation
Implementing closed-system cell washing technology in GMP workflows requires substantial initial investment but offers significant long-term financial benefits. The capital expenditure includes purchasing specialized equipment such as automated cell washing systems, which typically range from $50,000 to $250,000 depending on throughput capacity and automation level. Additional costs include facility modifications to accommodate new equipment, validation protocols, staff training, and potential production downtime during implementation.
Despite these upfront costs, the return on investment typically materializes within 12-24 months through multiple efficiency gains. Labor costs decrease significantly as automated closed systems reduce hands-on processing time by 40-60% compared to manual centrifugation methods. A single technician can simultaneously operate multiple closed washing systems, whereas centrifugation requires constant operator attention and intervention.
Consumable expenses show mixed results - while specialized kits for closed systems may cost more per unit than centrifugation tubes, the reduction in contamination events and batch failures generates substantial savings. Industry data indicates contamination rates decrease from approximately 5-8% with open centrifugation to less than 1% with closed systems, potentially saving hundreds of thousands in lost product value annually.
Quality-related economic benefits are particularly compelling. Closed systems demonstrate superior cell recovery rates (typically 85-95% versus 70-85% with centrifugation), improving yield consistency and reducing the need for larger starting materials. The enhanced sterility assurance reduces costly quality investigations, deviations, and batch rejections, with some facilities reporting 30-50% fewer quality events after implementation.
Regulatory compliance benefits translate to financial advantages through streamlined approval processes. Closed systems align with regulatory expectations for contamination control, potentially accelerating facility and process approvals. Several manufacturers report reducing regulatory queries by up to 40% following implementation, accelerating time-to-market for cell therapy products.
Scalability considerations further enhance the economic case. While centrifugation processes face significant challenges when scaling to commercial production volumes, closed washing systems demonstrate more linear scalability with predictable cost increases. This enables more accurate financial forecasting and reduces scale-up risks that could otherwise result in costly process redesigns or validation failures.
Despite these upfront costs, the return on investment typically materializes within 12-24 months through multiple efficiency gains. Labor costs decrease significantly as automated closed systems reduce hands-on processing time by 40-60% compared to manual centrifugation methods. A single technician can simultaneously operate multiple closed washing systems, whereas centrifugation requires constant operator attention and intervention.
Consumable expenses show mixed results - while specialized kits for closed systems may cost more per unit than centrifugation tubes, the reduction in contamination events and batch failures generates substantial savings. Industry data indicates contamination rates decrease from approximately 5-8% with open centrifugation to less than 1% with closed systems, potentially saving hundreds of thousands in lost product value annually.
Quality-related economic benefits are particularly compelling. Closed systems demonstrate superior cell recovery rates (typically 85-95% versus 70-85% with centrifugation), improving yield consistency and reducing the need for larger starting materials. The enhanced sterility assurance reduces costly quality investigations, deviations, and batch rejections, with some facilities reporting 30-50% fewer quality events after implementation.
Regulatory compliance benefits translate to financial advantages through streamlined approval processes. Closed systems align with regulatory expectations for contamination control, potentially accelerating facility and process approvals. Several manufacturers report reducing regulatory queries by up to 40% following implementation, accelerating time-to-market for cell therapy products.
Scalability considerations further enhance the economic case. While centrifugation processes face significant challenges when scaling to commercial production volumes, closed washing systems demonstrate more linear scalability with predictable cost increases. This enables more accurate financial forecasting and reduces scale-up risks that could otherwise result in costly process redesigns or validation failures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!