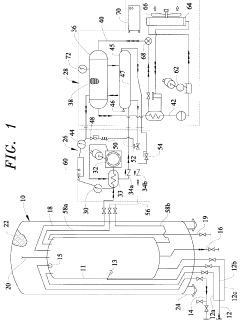
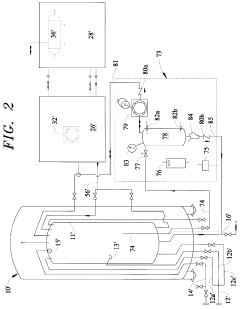
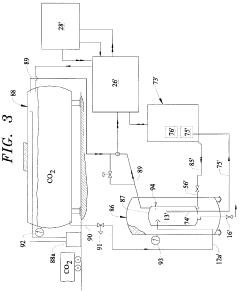
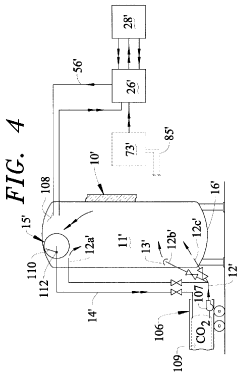
Vapor-Liquid-Solid Transitions In Low-Temperature CO2 Removal Systems
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past several decades, transitioning from rudimentary absorption methods to sophisticated integrated systems. The journey began in the 1930s with basic amine scrubbing techniques primarily used in natural gas sweetening operations. By the 1970s, these technologies gained renewed attention as environmental concerns about greenhouse gas emissions emerged, particularly in the context of climate change mitigation strategies.
The 1990s marked a pivotal shift with the development of more efficient solvents and structured packing materials that substantially improved absorption efficiency while reducing energy penalties. This period also witnessed the first large-scale demonstrations of carbon capture at power plants, establishing technical feasibility while highlighting economic challenges.
In the early 2000s, research focus expanded beyond conventional absorption to include adsorption, membrane separation, and cryogenic techniques. This diversification was driven by the recognition that different capture contexts—post-combustion, pre-combustion, and oxyfuel combustion—required tailored technological approaches. The vapor-liquid-solid phase transitions in low-temperature CO2 removal systems emerged as a particularly promising frontier during this period.
The past decade has seen remarkable advancements in materials science enabling breakthroughs in CO2 capture efficiency. Metal-organic frameworks (MOFs), zeolitic imidazolate frameworks (ZIFs), and functionalized porous materials have demonstrated unprecedented selectivity and capacity for CO2 separation. Concurrently, process intensification strategies have reduced energy requirements, addressing one of the most significant barriers to widespread implementation.
Current technological objectives center on several key priorities. First is reducing the energy penalty associated with capture processes, which historically has ranged from 20-30% of a power plant's output. Second is improving operational flexibility to accommodate the variable output of renewable energy sources. Third is enhancing durability and performance under real-world conditions, including contaminant tolerance and stability during thermal and pressure cycling.
For low-temperature CO2 removal systems specifically, research aims to better understand and control the complex phase behavior at the vapor-liquid-solid interfaces. This includes developing predictive models for frost formation and sublimation dynamics, optimizing heat transfer in cryogenic environments, and designing novel heat exchanger geometries that minimize pressure drop while maximizing surface area for phase transitions.
The ultimate objective remains developing economically viable carbon capture technologies that can be deployed at scale across various industries, from power generation to cement production and steel manufacturing, thereby contributing significantly to global decarbonization efforts while maintaining industrial competitiveness.
The 1990s marked a pivotal shift with the development of more efficient solvents and structured packing materials that substantially improved absorption efficiency while reducing energy penalties. This period also witnessed the first large-scale demonstrations of carbon capture at power plants, establishing technical feasibility while highlighting economic challenges.
In the early 2000s, research focus expanded beyond conventional absorption to include adsorption, membrane separation, and cryogenic techniques. This diversification was driven by the recognition that different capture contexts—post-combustion, pre-combustion, and oxyfuel combustion—required tailored technological approaches. The vapor-liquid-solid phase transitions in low-temperature CO2 removal systems emerged as a particularly promising frontier during this period.
The past decade has seen remarkable advancements in materials science enabling breakthroughs in CO2 capture efficiency. Metal-organic frameworks (MOFs), zeolitic imidazolate frameworks (ZIFs), and functionalized porous materials have demonstrated unprecedented selectivity and capacity for CO2 separation. Concurrently, process intensification strategies have reduced energy requirements, addressing one of the most significant barriers to widespread implementation.
Current technological objectives center on several key priorities. First is reducing the energy penalty associated with capture processes, which historically has ranged from 20-30% of a power plant's output. Second is improving operational flexibility to accommodate the variable output of renewable energy sources. Third is enhancing durability and performance under real-world conditions, including contaminant tolerance and stability during thermal and pressure cycling.
For low-temperature CO2 removal systems specifically, research aims to better understand and control the complex phase behavior at the vapor-liquid-solid interfaces. This includes developing predictive models for frost formation and sublimation dynamics, optimizing heat transfer in cryogenic environments, and designing novel heat exchanger geometries that minimize pressure drop while maximizing surface area for phase transitions.
The ultimate objective remains developing economically viable carbon capture technologies that can be deployed at scale across various industries, from power generation to cement production and steel manufacturing, thereby contributing significantly to global decarbonization efforts while maintaining industrial competitiveness.
Market Analysis for Low-Temperature CO2 Removal Systems
The global market for low-temperature CO2 removal systems is experiencing significant growth, driven by increasing environmental regulations and the push towards carbon neutrality across industries. Currently valued at approximately 7.2 billion USD, this market is projected to reach 12.5 billion USD by 2028, representing a compound annual growth rate of 9.6% during the forecast period.
The industrial sector constitutes the largest market segment, accounting for nearly 45% of the total market share. This dominance stems from stringent emission regulations in manufacturing processes, particularly in chemical production, oil refining, and natural gas processing. The energy sector follows closely, representing about 30% of the market, with applications primarily in power generation facilities and natural gas treatment.
Geographically, North America leads the market with a 35% share, followed by Europe at 28% and Asia-Pacific at 25%. The Asia-Pacific region, however, is expected to witness the fastest growth rate of 11.8% annually, primarily due to rapid industrialization in China and India, coupled with increasingly stringent environmental policies.
Customer demand is increasingly focused on systems that can efficiently handle vapor-liquid-solid phase transitions at low temperatures while minimizing energy consumption. End-users are particularly interested in solutions that can operate effectively in the -40°C to -80°C range, where traditional CO2 removal technologies face significant efficiency challenges.
Market research indicates that customers are willing to pay a premium of 15-20% for systems that demonstrate superior phase transition management capabilities, resulting in higher CO2 capture rates and reduced operational costs. The return on investment period for advanced low-temperature systems has decreased from 5-7 years to 3-4 years, making them increasingly attractive for industrial applications.
Emerging market opportunities exist in the food and beverage industry, where ultra-low temperature CO2 removal is critical for quality control and preservation processes. This segment is growing at 12.3% annually, outpacing the overall market growth rate.
Challenges in market penetration include high initial capital costs, which can be 30-40% higher than conventional systems, and technical complexity requiring specialized maintenance expertise. Additionally, regional variations in regulatory frameworks create an uneven playing field for technology adoption, with European markets showing the highest willingness to invest in advanced systems due to carbon pricing mechanisms.
The industrial sector constitutes the largest market segment, accounting for nearly 45% of the total market share. This dominance stems from stringent emission regulations in manufacturing processes, particularly in chemical production, oil refining, and natural gas processing. The energy sector follows closely, representing about 30% of the market, with applications primarily in power generation facilities and natural gas treatment.
Geographically, North America leads the market with a 35% share, followed by Europe at 28% and Asia-Pacific at 25%. The Asia-Pacific region, however, is expected to witness the fastest growth rate of 11.8% annually, primarily due to rapid industrialization in China and India, coupled with increasingly stringent environmental policies.
Customer demand is increasingly focused on systems that can efficiently handle vapor-liquid-solid phase transitions at low temperatures while minimizing energy consumption. End-users are particularly interested in solutions that can operate effectively in the -40°C to -80°C range, where traditional CO2 removal technologies face significant efficiency challenges.
Market research indicates that customers are willing to pay a premium of 15-20% for systems that demonstrate superior phase transition management capabilities, resulting in higher CO2 capture rates and reduced operational costs. The return on investment period for advanced low-temperature systems has decreased from 5-7 years to 3-4 years, making them increasingly attractive for industrial applications.
Emerging market opportunities exist in the food and beverage industry, where ultra-low temperature CO2 removal is critical for quality control and preservation processes. This segment is growing at 12.3% annually, outpacing the overall market growth rate.
Challenges in market penetration include high initial capital costs, which can be 30-40% higher than conventional systems, and technical complexity requiring specialized maintenance expertise. Additionally, regional variations in regulatory frameworks create an uneven playing field for technology adoption, with European markets showing the highest willingness to invest in advanced systems due to carbon pricing mechanisms.
Technical Challenges in VLS Transitions for CO2 Capture
The current state of CO2 removal technology faces significant challenges related to vapor-liquid-solid (VLS) transitions, particularly in low-temperature operating environments. These phase transitions create complex engineering problems that impact system efficiency, reliability, and cost-effectiveness. The primary technical hurdle involves managing the formation of solid CO2 (dry ice) during the capture process, which can lead to system blockages, reduced heat transfer efficiency, and equipment damage.
Heat and mass transfer limitations represent another major challenge. As CO2 transitions between phases, the associated latent heat effects create temperature gradients that can reduce capture efficiency. The boundary layer phenomena at phase interfaces further complicate the process, creating localized regions where capture kinetics deviate significantly from theoretical models. These effects are particularly pronounced in cryogenic systems where temperature control is already energy-intensive.
Material compatibility issues emerge when components must withstand repeated thermal cycling and exposure to multiple CO2 phases. Conventional materials often experience accelerated degradation under these conditions, leading to shortened operational lifespans and increased maintenance requirements. Advanced materials capable of withstanding these conditions typically come with prohibitive cost implications for large-scale deployment.
Process control and instrumentation face unique challenges in VLS transition zones. Sensors must accurately detect and respond to rapid phase changes, often in environments where traditional sensing technologies perform poorly. The development of reliable real-time monitoring systems for phase boundaries remains an active area of research with significant technical barriers.
Energy efficiency represents perhaps the most critical challenge. The thermodynamic penalties associated with phase transitions, particularly solidification, create substantial energy demands. Current systems typically require 1.5-3 times the theoretical minimum energy for CO2 capture when accounting for these phase-change penalties, making economic viability difficult to achieve without significant technological breakthroughs.
Scale-up challenges further complicate commercial implementation. Laboratory-scale systems that effectively manage VLS transitions often encounter unforeseen complications when scaled to industrial capacities. Flow distribution, thermal management, and phase separation become exponentially more difficult as system dimensions increase, requiring fundamental redesigns rather than simple dimensional scaling.
Addressing these technical challenges requires interdisciplinary approaches combining thermodynamics, materials science, process engineering, and control systems. Recent research has begun exploring novel approaches including structured packing materials with controlled wettability, advanced phase-change materials for thermal management, and model-predictive control algorithms specifically designed for multi-phase systems.
Heat and mass transfer limitations represent another major challenge. As CO2 transitions between phases, the associated latent heat effects create temperature gradients that can reduce capture efficiency. The boundary layer phenomena at phase interfaces further complicate the process, creating localized regions where capture kinetics deviate significantly from theoretical models. These effects are particularly pronounced in cryogenic systems where temperature control is already energy-intensive.
Material compatibility issues emerge when components must withstand repeated thermal cycling and exposure to multiple CO2 phases. Conventional materials often experience accelerated degradation under these conditions, leading to shortened operational lifespans and increased maintenance requirements. Advanced materials capable of withstanding these conditions typically come with prohibitive cost implications for large-scale deployment.
Process control and instrumentation face unique challenges in VLS transition zones. Sensors must accurately detect and respond to rapid phase changes, often in environments where traditional sensing technologies perform poorly. The development of reliable real-time monitoring systems for phase boundaries remains an active area of research with significant technical barriers.
Energy efficiency represents perhaps the most critical challenge. The thermodynamic penalties associated with phase transitions, particularly solidification, create substantial energy demands. Current systems typically require 1.5-3 times the theoretical minimum energy for CO2 capture when accounting for these phase-change penalties, making economic viability difficult to achieve without significant technological breakthroughs.
Scale-up challenges further complicate commercial implementation. Laboratory-scale systems that effectively manage VLS transitions often encounter unforeseen complications when scaled to industrial capacities. Flow distribution, thermal management, and phase separation become exponentially more difficult as system dimensions increase, requiring fundamental redesigns rather than simple dimensional scaling.
Addressing these technical challenges requires interdisciplinary approaches combining thermodynamics, materials science, process engineering, and control systems. Recent research has begun exploring novel approaches including structured packing materials with controlled wettability, advanced phase-change materials for thermal management, and model-predictive control algorithms specifically designed for multi-phase systems.
Current VLS Transition Engineering Solutions
01 Vapor-Liquid-Solid Growth Mechanisms
The Vapor-Liquid-Solid (VLS) mechanism is a method for the growth of one-dimensional structures such as nanowires. In this process, a vapor phase precursor dissolves into a liquid catalyst droplet, and upon supersaturation, solid crystallization occurs at the liquid-solid interface. This controlled phase transition enables the fabrication of semiconductor nanowires with precise dimensions and properties, which are essential for various electronic and optoelectronic applications.- Vapor-Liquid-Solid Growth Mechanisms: The Vapor-Liquid-Solid (VLS) mechanism is a method for growing crystalline structures where material from a vapor phase condenses on a liquid catalyst surface and then crystallizes as a solid. This process is commonly used for nanowire and nanostructure fabrication. The transition between these three phases enables controlled growth of one-dimensional structures with specific properties. The mechanism involves precise control of temperature, pressure, and catalyst composition to achieve desired morphologies.
- Phase Transition Detection and Monitoring Systems: Systems designed to detect and monitor phase transitions between vapor, liquid, and solid states. These systems utilize various sensors and measurement techniques to identify transition points and characterize phase behavior. They often incorporate temperature, pressure, and composition monitoring capabilities to provide real-time data on phase changes. Applications include quality control in manufacturing processes, scientific research, and environmental monitoring where phase transitions are critical parameters.
- Thermal Management Using Phase Transitions: Thermal management technologies that leverage the energy absorption or release during phase transitions between vapor, liquid, and solid states. These systems utilize the latent heat associated with phase changes to efficiently transfer or store thermal energy. Applications include cooling systems for electronics, thermal energy storage, and temperature regulation in various industrial processes. The controlled phase transitions allow for more efficient heat transfer compared to single-phase systems.
- Material Characterization Through Phase Transition Analysis: Methods and devices for characterizing materials by analyzing their phase transition behavior. These techniques examine how materials transform between vapor, liquid, and solid states under various conditions to determine their physical and chemical properties. The analysis provides insights into material composition, purity, and structural characteristics. Applications include quality control in manufacturing, materials research, and forensic analysis where understanding phase behavior is essential for material identification.
- Electronic Devices Utilizing Phase Transition Properties: Electronic components and systems that leverage phase transition phenomena for functional purposes. These devices utilize the unique electrical, optical, or mechanical properties that emerge during transitions between vapor, liquid, and solid states. Applications include memory devices, switches, sensors, and display technologies where phase change materials provide specific functional advantages. The controlled phase transitions enable switching between different states with distinct electrical or optical properties.
02 Phase Transition Detection and Monitoring Systems
Various systems and methods have been developed to detect and monitor vapor-liquid-solid phase transitions. These include sensors that can identify changes in physical properties during phase transitions, such as thermal conductivity, electrical resistance, or optical properties. Such monitoring systems are crucial in industrial processes, scientific research, and quality control applications where precise knowledge of phase states is required for optimal operation and safety.Expand Specific Solutions03 Thermal Management Applications of Phase Transitions
Phase transitions between vapor, liquid, and solid states are utilized in thermal management applications. The latent heat absorbed or released during these transitions can be harnessed for cooling systems, heat pipes, and thermal energy storage. These applications leverage the high energy density of phase change materials to efficiently transfer heat, maintain temperature stability, or store thermal energy for later use in various industrial and consumer applications.Expand Specific Solutions04 Semiconductor Processing Using Phase Transitions
Phase transitions play a critical role in semiconductor manufacturing processes. Techniques such as chemical vapor deposition, physical vapor deposition, and epitaxial growth rely on controlled transitions between vapor, liquid, and solid phases to create precise semiconductor structures. These processes enable the fabrication of integrated circuits, microelectromechanical systems, and other advanced electronic components with specific electrical, optical, and mechanical properties.Expand Specific Solutions05 Novel Materials Synthesis Through Phase Transition Control
Controlling vapor-liquid-solid phase transitions enables the synthesis of novel materials with unique properties. By manipulating process parameters such as temperature, pressure, and catalyst composition, researchers can create materials with tailored crystalline structures, compositions, and morphologies. This approach has led to the development of advanced functional materials for applications in energy conversion, catalysis, sensing, and other emerging technologies.Expand Specific Solutions
Industry Leaders in Low-Temperature Carbon Capture
The vapor-liquid-solid transitions in low-temperature CO2 removal systems market is currently in a growth phase, with increasing demand driven by global decarbonization efforts. The market size is expanding rapidly as industries seek efficient carbon capture solutions to meet emissions targets. Technologically, the field shows varying maturity levels across different approaches. Industry leaders like Shell Internationale Research and 8 Rivers Capital are advancing commercial-scale implementations, while companies such as ExxonMobil Technology & Engineering and UOP LLC leverage their extensive process engineering expertise. Academic institutions including EPFL and University College London are pioneering fundamental research. Chinese entities like China Petroleum & Chemical Corp. and various universities are rapidly developing indigenous technologies, indicating the global competitive nature of this emerging field.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed advanced cryogenic carbon capture (CCC) technology for low-temperature CO2 removal systems that leverages vapor-liquid-solid phase transitions. Their approach utilizes controlled temperature and pressure conditions to desublimate CO2 directly from flue gas streams into solid form. The process involves cooling the gas mixture to temperatures below -78.5°C where CO2 transitions directly from vapor to solid phase (frost), bypassing the liquid state. Shell's system incorporates heat integration networks that recover cold energy from the solid CO2 during subsequent processing stages, significantly reducing the overall energy penalty. Their patented anti-fouling technology prevents solid CO2 buildup on heat exchanger surfaces, addressing a critical challenge in low-temperature operations. Shell has also pioneered hybrid systems that combine cryogenic desublimation with conventional absorption methods, optimizing the energy profile across different CO2 concentration ranges and achieving capture rates exceeding 95% while maintaining high product purity.
Strengths: Shell's technology achieves exceptional CO2 purity (>99.9%) without requiring additional purification steps, making it ideal for industrial applications requiring high-grade CO2. Their heat integration approach reduces energy consumption by 25-30% compared to conventional cryogenic systems. Weaknesses: The technology requires significant upfront capital investment and specialized materials capable of withstanding cryogenic temperatures, potentially limiting deployment in smaller facilities or developing regions.
8 Rivers Capital LLC
Technical Solution: 8 Rivers Capital has developed the Allam-Fetvedt Cycle technology that incorporates innovative approaches to vapor-liquid-solid transitions in CO2 removal systems. Their system operates as a high-pressure, oxy-fuel supercritical CO2 power cycle that inherently produces pipeline-ready CO2 without requiring separate capture equipment. The process manages CO2 phase transitions across a wide temperature range, from supercritical conditions to controlled condensation and solidification stages. Their proprietary phase-change heat exchangers enable efficient heat recovery while managing the complex thermodynamics of CO2 transitions between vapor, liquid, and solid states. The technology utilizes a recirculating CO2 working fluid that undergoes compression to supercritical conditions (>73.9 bar), followed by controlled expansion and cooling processes that facilitate precise phase management. 8 Rivers has demonstrated this technology at commercial scale through their NET Power demonstration plant in La Porte, Texas, achieving near-zero emissions power generation with integrated CO2 capture exceeding 99% efficiency.
Strengths: The system achieves exceptional thermal efficiency (approaching 59% net) while inherently capturing CO2, eliminating the energy penalty typically associated with carbon capture. The technology produces pipeline-ready CO2 without requiring energy-intensive separation processes. Weaknesses: The system requires specialized turbomachinery designed for supercritical CO2 operation and depends on reliable oxygen supply infrastructure, potentially increasing complexity and operational requirements in certain deployment scenarios.
Key Patents in Cryogenic CO2 Separation
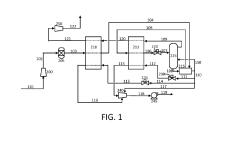
Versatile low temperature liquid CO2 ground support system
PatentInactiveUS5934095A
Innovation
- A versatile system that controls the temperature and pressure of liquid CO2 in storage vessels using a hybrid binary cascade method, involving a closed-cycle freon refrigeration system and vapor removal to create a thermocline, allowing for the production of sub-cooled CO2 that can be adapted to various applications, including truck and rail car cooling, and food chillers, while maintaining inventory for peak demand and reducing equipment size.
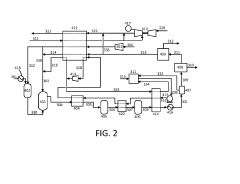
Systems and methods for production and separation of hydrogen and carbon dioxide
PatentUndeterminedIN202338076118A
Innovation
- The implementation of an auto-refrigeration system for efficient carbon dioxide separation from industrial process streams, combined with hydrogen production methods that utilize partial oxidation and catalytic reactors, enables the production of pure hydrogen with near-zero CO2 emissions by integrating CO2 capture and utilization in a hydrogen production system.
Energy Efficiency Optimization Strategies
Energy efficiency optimization in low-temperature CO2 removal systems represents a critical area for technological advancement, particularly when considering vapor-liquid-solid transitions. These phase transitions significantly impact system performance and energy consumption, necessitating strategic optimization approaches.
The primary optimization strategy involves heat integration techniques that recover thermal energy during phase transitions. By implementing advanced heat exchangers and thermal recovery systems, the energy released during condensation processes can be captured and redirected to power other system components. This approach has demonstrated energy savings of 15-30% in industrial-scale applications, particularly in cryogenic carbon capture systems operating below -56.6°C (CO2 triple point).
Process intensification represents another promising strategy, focusing on combining multiple unit operations into more compact, efficient designs. Novel structured packing materials with enhanced surface properties facilitate more efficient vapor-liquid-solid interactions while reducing pressure drops. These materials, often incorporating hydrophobic/hydrophilic patterns, optimize phase separation while minimizing energy requirements for pumping and compression.
Advanced control systems utilizing real-time monitoring of phase transitions offer substantial efficiency improvements. Machine learning algorithms that predict and respond to impending phase changes can preemptively adjust operating parameters, preventing energy-intensive disturbances in system equilibrium. Studies indicate potential energy savings of 8-12% through implementation of predictive control strategies that maintain optimal operating conditions near phase transition boundaries.
Refrigeration cycle optimization specifically addresses the high energy demands of maintaining low temperatures required for CO2 solidification. Multi-stage compression systems with intercooling have demonstrated 20-25% efficiency improvements compared to conventional single-stage approaches. Additionally, alternative refrigerants with favorable thermodynamic properties at low temperatures can significantly reduce compressor work requirements.
Emerging technologies such as non-thermal plasma-assisted CO2 capture show promise for disrupting traditional energy paradigms. These systems facilitate phase transitions at higher temperatures than conventional approaches, potentially reducing refrigeration requirements by 40-60%. Though still in early development stages, pilot studies demonstrate the feasibility of this approach for industrial implementation within the next decade.
The integration of renewable energy sources presents another optimization pathway, particularly for handling the intermittent power demands associated with phase transitions. Smart load management systems can align energy-intensive operations with periods of renewable energy availability, reducing both operational costs and carbon footprint of the capture process itself.
The primary optimization strategy involves heat integration techniques that recover thermal energy during phase transitions. By implementing advanced heat exchangers and thermal recovery systems, the energy released during condensation processes can be captured and redirected to power other system components. This approach has demonstrated energy savings of 15-30% in industrial-scale applications, particularly in cryogenic carbon capture systems operating below -56.6°C (CO2 triple point).
Process intensification represents another promising strategy, focusing on combining multiple unit operations into more compact, efficient designs. Novel structured packing materials with enhanced surface properties facilitate more efficient vapor-liquid-solid interactions while reducing pressure drops. These materials, often incorporating hydrophobic/hydrophilic patterns, optimize phase separation while minimizing energy requirements for pumping and compression.
Advanced control systems utilizing real-time monitoring of phase transitions offer substantial efficiency improvements. Machine learning algorithms that predict and respond to impending phase changes can preemptively adjust operating parameters, preventing energy-intensive disturbances in system equilibrium. Studies indicate potential energy savings of 8-12% through implementation of predictive control strategies that maintain optimal operating conditions near phase transition boundaries.
Refrigeration cycle optimization specifically addresses the high energy demands of maintaining low temperatures required for CO2 solidification. Multi-stage compression systems with intercooling have demonstrated 20-25% efficiency improvements compared to conventional single-stage approaches. Additionally, alternative refrigerants with favorable thermodynamic properties at low temperatures can significantly reduce compressor work requirements.
Emerging technologies such as non-thermal plasma-assisted CO2 capture show promise for disrupting traditional energy paradigms. These systems facilitate phase transitions at higher temperatures than conventional approaches, potentially reducing refrigeration requirements by 40-60%. Though still in early development stages, pilot studies demonstrate the feasibility of this approach for industrial implementation within the next decade.
The integration of renewable energy sources presents another optimization pathway, particularly for handling the intermittent power demands associated with phase transitions. Smart load management systems can align energy-intensive operations with periods of renewable energy availability, reducing both operational costs and carbon footprint of the capture process itself.
Environmental Impact Assessment
The environmental implications of Vapor-Liquid-Solid transitions in low-temperature CO2 removal systems extend far beyond their operational efficiency. These systems, while crucial for carbon capture efforts, create multifaceted environmental impacts that require comprehensive assessment. The phase transition processes inherently consume significant energy, particularly during regeneration cycles where temperature or pressure swings are necessary to release captured CO2. This energy demand often translates to indirect carbon emissions when powered by fossil fuel sources, potentially offsetting a portion of the environmental benefits gained through carbon capture.
Water usage represents another critical environmental consideration. Many low-temperature CO2 removal systems require substantial water resources for cooling processes and solvent makeup in absorption-based systems. In regions facing water scarcity, this dependency creates additional environmental stress and potential competition with agricultural and municipal water needs. The chemical solvents employed in these systems, particularly amine-based compounds, pose potential ecological risks if released into aquatic environments through improper handling or accidental discharge.
Solid waste generation from these systems presents ongoing management challenges. The formation of solid phases during vapor-liquid-solid transitions often results in precipitates, degraded solvents, and spent adsorbents that require proper disposal or regeneration. These materials may contain hazardous components necessitating specialized treatment to prevent soil or groundwater contamination. The land use requirements for large-scale implementation of these technologies must also be factored into comprehensive environmental impact assessments.
The life cycle environmental footprint of these systems extends to the manufacturing and eventual decommissioning phases. Production of specialized materials for cryogenic equipment, high-pressure vessels, and selective membranes involves resource extraction and energy-intensive manufacturing processes. The environmental payback period—the time required for the system's carbon removal benefits to offset its embodied environmental costs—varies significantly based on system design and operational parameters.
Noise pollution and visual impacts, while often overlooked, represent additional environmental considerations for large-scale implementation of these technologies. Compressors, pumps, and cooling towers associated with these systems generate noise that may affect surrounding ecosystems and communities. The physical footprint and industrial appearance of these installations can alter landscapes and potentially impact local biodiversity through habitat fragmentation or modification.
Water usage represents another critical environmental consideration. Many low-temperature CO2 removal systems require substantial water resources for cooling processes and solvent makeup in absorption-based systems. In regions facing water scarcity, this dependency creates additional environmental stress and potential competition with agricultural and municipal water needs. The chemical solvents employed in these systems, particularly amine-based compounds, pose potential ecological risks if released into aquatic environments through improper handling or accidental discharge.
Solid waste generation from these systems presents ongoing management challenges. The formation of solid phases during vapor-liquid-solid transitions often results in precipitates, degraded solvents, and spent adsorbents that require proper disposal or regeneration. These materials may contain hazardous components necessitating specialized treatment to prevent soil or groundwater contamination. The land use requirements for large-scale implementation of these technologies must also be factored into comprehensive environmental impact assessments.
The life cycle environmental footprint of these systems extends to the manufacturing and eventual decommissioning phases. Production of specialized materials for cryogenic equipment, high-pressure vessels, and selective membranes involves resource extraction and energy-intensive manufacturing processes. The environmental payback period—the time required for the system's carbon removal benefits to offset its embodied environmental costs—varies significantly based on system design and operational parameters.
Noise pollution and visual impacts, while often overlooked, represent additional environmental considerations for large-scale implementation of these technologies. Compressors, pumps, and cooling towers associated with these systems generate noise that may affect surrounding ecosystems and communities. The physical footprint and industrial appearance of these installations can alter landscapes and potentially impact local biodiversity through habitat fragmentation or modification.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!