PEEK Polymer Properties: Analysis of Chemical Resistance
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEEK Polymer Evolution and Research Objectives
Polyether ether ketone (PEEK) emerged in the late 1970s as a high-performance thermoplastic polymer, developed by Imperial Chemical Industries (ICI). Its exceptional chemical resistance properties were immediately recognized as revolutionary for industries requiring materials capable of withstanding harsh chemical environments. Over the past four decades, PEEK has evolved from a specialty material to a critical component in aerospace, automotive, medical, and chemical processing applications.
The evolution of PEEK polymer technology has been characterized by continuous improvements in processing techniques, resulting in enhanced chemical resistance profiles. Initial formulations demonstrated remarkable stability against common organic solvents, but showed vulnerability to concentrated sulfuric acid and certain halogenated compounds. Subsequent generations incorporated modified crystalline structures and reinforcement additives that significantly expanded the chemical resistance envelope.
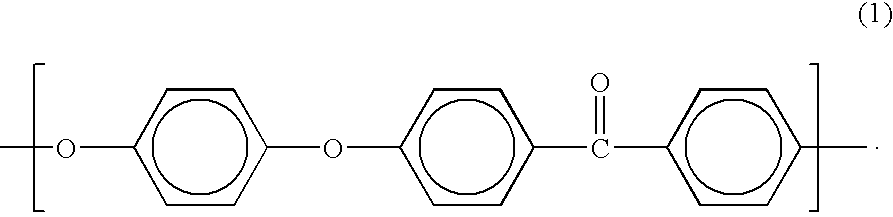
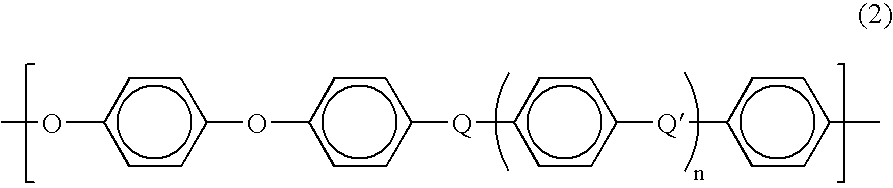
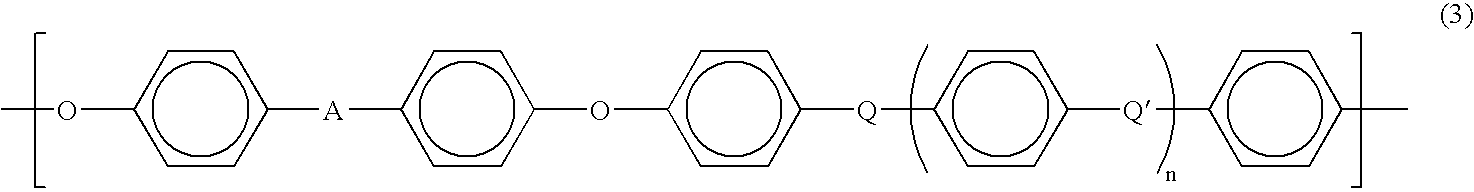
Current research indicates that PEEK's chemical resistance stems from its aromatic backbone structure, which provides exceptional stability through strong carbon-carbon bonds and aromatic rings. This molecular architecture creates an effective barrier against chemical attack, particularly in environments where other high-performance polymers would rapidly degrade. The semi-crystalline nature of PEEK further contributes to its resistance properties, with crystalline regions providing enhanced barrier properties against chemical permeation.
Industry trends suggest growing demand for PEEK materials with customized chemical resistance profiles tailored to specific application environments. This has driven research toward understanding the fundamental mechanisms of chemical interaction at the molecular level, particularly the relationship between crystallinity, molecular weight distribution, and chemical resistance performance.
The technical objectives of this research focus on quantifying and characterizing PEEK's resistance to a comprehensive range of chemical agents under varying conditions of temperature, pressure, and exposure duration. Specifically, we aim to develop predictive models correlating polymer structure with chemical resistance performance, enabling the design of optimized PEEK formulations for targeted applications.
Additionally, this research seeks to establish standardized testing methodologies for evaluating chemical resistance properties, addressing the current inconsistencies in testing protocols across the industry. By developing more accurate accelerated aging tests, we can better predict long-term chemical resistance behavior, a critical factor for applications requiring decades of service life in chemically aggressive environments.
The ultimate goal is to expand PEEK's application potential by overcoming current limitations in chemical resistance, particularly against strong oxidizing acids and certain polar organic compounds, thereby opening new market opportunities in previously inaccessible industrial sectors.
The evolution of PEEK polymer technology has been characterized by continuous improvements in processing techniques, resulting in enhanced chemical resistance profiles. Initial formulations demonstrated remarkable stability against common organic solvents, but showed vulnerability to concentrated sulfuric acid and certain halogenated compounds. Subsequent generations incorporated modified crystalline structures and reinforcement additives that significantly expanded the chemical resistance envelope.
Current research indicates that PEEK's chemical resistance stems from its aromatic backbone structure, which provides exceptional stability through strong carbon-carbon bonds and aromatic rings. This molecular architecture creates an effective barrier against chemical attack, particularly in environments where other high-performance polymers would rapidly degrade. The semi-crystalline nature of PEEK further contributes to its resistance properties, with crystalline regions providing enhanced barrier properties against chemical permeation.
Industry trends suggest growing demand for PEEK materials with customized chemical resistance profiles tailored to specific application environments. This has driven research toward understanding the fundamental mechanisms of chemical interaction at the molecular level, particularly the relationship between crystallinity, molecular weight distribution, and chemical resistance performance.
The technical objectives of this research focus on quantifying and characterizing PEEK's resistance to a comprehensive range of chemical agents under varying conditions of temperature, pressure, and exposure duration. Specifically, we aim to develop predictive models correlating polymer structure with chemical resistance performance, enabling the design of optimized PEEK formulations for targeted applications.
Additionally, this research seeks to establish standardized testing methodologies for evaluating chemical resistance properties, addressing the current inconsistencies in testing protocols across the industry. By developing more accurate accelerated aging tests, we can better predict long-term chemical resistance behavior, a critical factor for applications requiring decades of service life in chemically aggressive environments.
The ultimate goal is to expand PEEK's application potential by overcoming current limitations in chemical resistance, particularly against strong oxidizing acids and certain polar organic compounds, thereby opening new market opportunities in previously inaccessible industrial sectors.
Market Demand Analysis for Chemical-Resistant Polymers
The global market for chemical-resistant polymers has witnessed substantial growth over the past decade, driven primarily by increasing demands from industries such as oil and gas, chemical processing, healthcare, automotive, and aerospace. Among these high-performance polymers, PEEK (Polyether Ether Ketone) has emerged as a standout material due to its exceptional resistance to a wide range of chemicals, including acids, bases, hydrocarbons, and organic solvents.
Market research indicates that the global PEEK market was valued at approximately 680 million USD in 2020 and is projected to reach 1.2 billion USD by 2027, growing at a CAGR of 8.7% during the forecast period. The chemical processing industry accounts for nearly 25% of this market, highlighting the critical importance of chemical resistance properties in material selection.
The oil and gas sector represents another significant market for chemical-resistant PEEK polymers, particularly in downhole applications where exposure to aggressive chemicals and extreme conditions is common. The increasing exploration activities in harsh environments, including deep-sea drilling and hydraulic fracturing operations, have amplified the demand for materials that can withstand corrosive fluids while maintaining structural integrity.
Healthcare applications constitute a rapidly growing segment for PEEK polymers, with the material being increasingly adopted in medical implants, surgical instruments, and pharmaceutical processing equipment. The biocompatibility of PEEK, combined with its resistance to sterilization chemicals and bodily fluids, positions it as an ideal candidate for long-term medical applications, driving a market growth rate of approximately 10% annually in this sector.
The semiconductor and electronics manufacturing industries have also emerged as significant consumers of chemical-resistant polymers, particularly in wafer processing equipment where exposure to aggressive etching chemicals is routine. PEEK components in these applications have demonstrated superior performance compared to traditional materials, leading to market penetration rates increasing by 15% year-over-year in this segment.
Regional analysis reveals that North America and Europe currently dominate the chemical-resistant polymer market, collectively accounting for over 60% of global consumption. However, the Asia-Pacific region, particularly China, Japan, and South Korea, is experiencing the fastest growth rate at approximately 12% annually, driven by rapid industrialization and increasing adoption of high-performance materials in manufacturing processes.
Customer surveys indicate that while chemical resistance remains the primary selection criterion, manufacturers are increasingly seeking materials that combine this property with other performance characteristics such as high-temperature stability, mechanical strength, and dimensional stability. This trend is reshaping the competitive landscape, with material suppliers focusing on developing enhanced grades of PEEK that offer optimized performance across multiple parameters.
Market research indicates that the global PEEK market was valued at approximately 680 million USD in 2020 and is projected to reach 1.2 billion USD by 2027, growing at a CAGR of 8.7% during the forecast period. The chemical processing industry accounts for nearly 25% of this market, highlighting the critical importance of chemical resistance properties in material selection.
The oil and gas sector represents another significant market for chemical-resistant PEEK polymers, particularly in downhole applications where exposure to aggressive chemicals and extreme conditions is common. The increasing exploration activities in harsh environments, including deep-sea drilling and hydraulic fracturing operations, have amplified the demand for materials that can withstand corrosive fluids while maintaining structural integrity.
Healthcare applications constitute a rapidly growing segment for PEEK polymers, with the material being increasingly adopted in medical implants, surgical instruments, and pharmaceutical processing equipment. The biocompatibility of PEEK, combined with its resistance to sterilization chemicals and bodily fluids, positions it as an ideal candidate for long-term medical applications, driving a market growth rate of approximately 10% annually in this sector.
The semiconductor and electronics manufacturing industries have also emerged as significant consumers of chemical-resistant polymers, particularly in wafer processing equipment where exposure to aggressive etching chemicals is routine. PEEK components in these applications have demonstrated superior performance compared to traditional materials, leading to market penetration rates increasing by 15% year-over-year in this segment.
Regional analysis reveals that North America and Europe currently dominate the chemical-resistant polymer market, collectively accounting for over 60% of global consumption. However, the Asia-Pacific region, particularly China, Japan, and South Korea, is experiencing the fastest growth rate at approximately 12% annually, driven by rapid industrialization and increasing adoption of high-performance materials in manufacturing processes.
Customer surveys indicate that while chemical resistance remains the primary selection criterion, manufacturers are increasingly seeking materials that combine this property with other performance characteristics such as high-temperature stability, mechanical strength, and dimensional stability. This trend is reshaping the competitive landscape, with material suppliers focusing on developing enhanced grades of PEEK that offer optimized performance across multiple parameters.
Current Chemical Resistance Capabilities and Limitations of PEEK
PEEK (Polyetheretherketone) demonstrates exceptional chemical resistance across a wide range of environments, making it a preferred material for applications requiring durability under harsh chemical conditions. The polymer exhibits outstanding resistance to most organic and inorganic chemicals, including acids, bases, hydrocarbons, and solvents at room temperature. Specifically, PEEK maintains structural integrity when exposed to common industrial chemicals such as sulfuric acid (up to 50% concentration), hydrochloric acid (up to 10%), sodium hydroxide solutions, and various aliphatic and aromatic hydrocarbons.
In hydrocarbon environments, PEEK shows remarkable stability, with minimal swelling or degradation when exposed to crude oil, gasoline, diesel, and various lubricants. This property has positioned PEEK as a material of choice for oil and gas industry applications, where components must withstand continuous exposure to petroleum products and drilling fluids.
However, PEEK does exhibit limitations in certain chemical environments. Strong oxidizing acids such as concentrated nitric acid and sulfuric acid at high concentrations can cause degradation of the polymer structure. Similarly, certain halogenated compounds like methylene chloride and chloroform can induce swelling and potentially compromise mechanical properties when exposure is prolonged.
Temperature significantly influences PEEK's chemical resistance profile. While the material maintains its chemical resistance properties up to approximately 260°C in most environments, this threshold decreases substantially in the presence of certain aggressive chemicals. For instance, in concentrated sulfuric acid, the maximum service temperature may drop to below 100°C before noticeable degradation occurs.
Stress state also plays a crucial role in determining PEEK's chemical resistance. Components under mechanical stress while simultaneously exposed to aggressive chemicals may experience environmental stress cracking (ESC), particularly at elevated temperatures. This phenomenon has been documented in applications involving continuous exposure to certain aromatic compounds under load.
The crystallinity of PEEK significantly impacts its chemical resistance properties. Higher crystallinity generally correlates with improved chemical resistance, as the ordered molecular structure presents fewer pathways for chemical attack. Manufacturing processes that enhance crystallinity, such as annealing, can therefore improve chemical resistance performance.
Recent research has identified that surface modifications, including plasma treatment and chemical grafting, can alter PEEK's chemical resistance profile. While some treatments enhance resistance to specific chemicals, they may simultaneously reduce resistance to others, creating a complex optimization challenge for specialized applications.
In hydrocarbon environments, PEEK shows remarkable stability, with minimal swelling or degradation when exposed to crude oil, gasoline, diesel, and various lubricants. This property has positioned PEEK as a material of choice for oil and gas industry applications, where components must withstand continuous exposure to petroleum products and drilling fluids.
However, PEEK does exhibit limitations in certain chemical environments. Strong oxidizing acids such as concentrated nitric acid and sulfuric acid at high concentrations can cause degradation of the polymer structure. Similarly, certain halogenated compounds like methylene chloride and chloroform can induce swelling and potentially compromise mechanical properties when exposure is prolonged.
Temperature significantly influences PEEK's chemical resistance profile. While the material maintains its chemical resistance properties up to approximately 260°C in most environments, this threshold decreases substantially in the presence of certain aggressive chemicals. For instance, in concentrated sulfuric acid, the maximum service temperature may drop to below 100°C before noticeable degradation occurs.
Stress state also plays a crucial role in determining PEEK's chemical resistance. Components under mechanical stress while simultaneously exposed to aggressive chemicals may experience environmental stress cracking (ESC), particularly at elevated temperatures. This phenomenon has been documented in applications involving continuous exposure to certain aromatic compounds under load.
The crystallinity of PEEK significantly impacts its chemical resistance properties. Higher crystallinity generally correlates with improved chemical resistance, as the ordered molecular structure presents fewer pathways for chemical attack. Manufacturing processes that enhance crystallinity, such as annealing, can therefore improve chemical resistance performance.
Recent research has identified that surface modifications, including plasma treatment and chemical grafting, can alter PEEK's chemical resistance profile. While some treatments enhance resistance to specific chemicals, they may simultaneously reduce resistance to others, creating a complex optimization challenge for specialized applications.
Current Testing Methodologies for PEEK Chemical Resistance
01 Chemical resistance properties of PEEK polymer
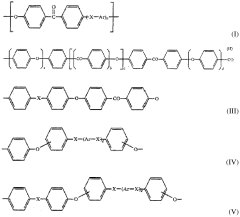
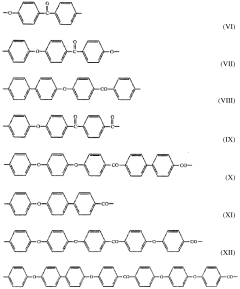
PEEK (Polyetheretherketone) polymer exhibits exceptional chemical resistance to a wide range of substances including acids, bases, hydrocarbons, and organic solvents. This resistance is attributed to its semi-crystalline structure and strong carbon-carbon bonds in the polymer backbone. The chemical stability makes PEEK suitable for applications in harsh chemical environments where other polymers would degrade.- Chemical resistance properties of PEEK polymer: PEEK (Polyetheretherketone) polymer exhibits exceptional resistance to a wide range of chemicals including acids, bases, hydrocarbons, and organic solvents. This inherent chemical stability makes it suitable for applications in harsh chemical environments. The polymer's aromatic backbone structure contributes to its outstanding chemical resistance, allowing it to maintain structural integrity and mechanical properties even after prolonged exposure to aggressive chemicals.
- PEEK polymer composites with enhanced chemical resistance: PEEK polymer can be formulated into composites with various fillers and reinforcements to further enhance its chemical resistance properties. These composites often incorporate materials such as carbon fibers, glass fibers, or ceramic particles that improve the overall performance in chemically aggressive environments. The resulting materials demonstrate superior resistance to chemical degradation while maintaining or improving mechanical properties, making them suitable for demanding industrial applications.
- PEEK polymer applications in corrosive environments: Due to its exceptional chemical resistance, PEEK polymer is widely used in applications exposed to corrosive environments. These applications include chemical processing equipment, oil and gas components, semiconductor manufacturing parts, and medical implants. The polymer's ability to withstand aggressive chemicals while maintaining dimensional stability and mechanical strength makes it an ideal material for components that must function reliably in the presence of corrosive substances.
- Surface modification of PEEK for improved chemical resistance: Various surface modification techniques can be applied to PEEK polymer to further enhance its chemical resistance properties. These methods include plasma treatment, chemical etching, coating applications, and surface functionalization. Modified PEEK surfaces can provide additional protection against specific chemicals or improve the polymer's compatibility with other materials in composite structures, extending the range of environments in which PEEK-based components can operate effectively.
- Testing and characterization of PEEK chemical resistance: Standardized testing methods are employed to evaluate and characterize the chemical resistance of PEEK polymer and its composites. These tests typically involve exposing PEEK samples to various chemicals under controlled conditions and measuring changes in physical properties, weight, appearance, and mechanical performance. Advanced analytical techniques such as spectroscopy and microscopy are used to assess the extent of chemical interaction and potential degradation mechanisms, providing valuable data for material selection in specific chemical environments.
02 PEEK polymer composites with enhanced chemical resistance
PEEK polymer can be formulated into composites with various fillers and reinforcements to further enhance its chemical resistance properties. These composites often incorporate materials such as carbon fibers, glass fibers, or ceramic particles. The resulting materials demonstrate improved resistance to chemical attack while maintaining or enhancing mechanical properties, making them suitable for applications in aggressive chemical environments.Expand Specific Solutions03 Surface treatments for improving PEEK chemical resistance
Various surface modification techniques can be applied to PEEK polymer to enhance its chemical resistance. These include plasma treatment, chemical etching, coating applications, and surface functionalization. Such treatments can create protective barriers or alter the surface chemistry of PEEK to provide additional protection against specific chemicals or environments without compromising the bulk properties of the material.Expand Specific Solutions04 PEEK polymer in filtration and separation applications
PEEK polymer is utilized in filtration and separation systems due to its excellent chemical resistance. PEEK-based membranes, filters, and separation media can withstand aggressive chemical environments during filtration processes. These materials maintain structural integrity and separation efficiency when exposed to harsh chemicals, making them valuable in pharmaceutical processing, chemical manufacturing, and water treatment applications.Expand Specific Solutions05 High-temperature chemical resistance of PEEK polymer
PEEK polymer maintains its chemical resistance properties even at elevated temperatures, which distinguishes it from many other engineering plastics. This combination of thermal stability and chemical resistance allows PEEK to be used in applications involving hot aggressive chemicals or in processes requiring chemical resistance during high-temperature operations. The material retains its structural integrity and resistance to chemical attack at temperatures up to 250°C in many environments.Expand Specific Solutions
Key Manufacturers and Competitors in High-Performance Polymer Industry
The chemical resistance properties of PEEK polymer market is currently in a growth phase, with an estimated global market size exceeding $1 billion. The technology has reached commercial maturity, with established players like Solvay Specialty Polymers and Victrex Manufacturing dominating the landscape through extensive product portfolios and advanced R&D capabilities. Emerging competitors such as Jilin Joinature Polymer and Guangdong Silver Age Sci & Tech are expanding market presence, particularly in Asia. Academic institutions including Changsha University of Science & Technology and Jilin University are contributing to technological advancements through research collaborations with industry. The market shows increasing diversification across applications in aerospace, automotive, medical devices, and electronics, with companies like Arthrex GmbH and NOK Corp developing specialized PEEK formulations for sector-specific chemical resistance requirements.
Victrex Manufacturing Ltd.
Technical Solution: Victrex employs a multi-faceted approach to analyzing PEEK chemical resistance properties, focusing on their VICTREX™ PEEK polymer portfolio. Their methodology includes immersion testing according to ASTM D543 standards, with extended exposure periods up to 10,000 hours for critical applications. They utilize advanced analytical techniques including FTIR spectroscopy and DSC thermal analysis to detect subtle chemical interactions at the molecular level. Victrex has developed proprietary test protocols that simulate real-world conditions, including multi-phase chemical environments under dynamic stress and temperature cycling. Their research has demonstrated exceptional resistance to over 250 chemicals, including strong acids (except concentrated sulfuric acid), bases, hydrocarbons, and aggressive solvents. Their data shows less than 0.1% weight change after prolonged exposure to most industrial chemicals at temperatures up to 260°C, and they've documented retention of mechanical properties exceeding 90% after chemical aging in harsh environments.
Strengths: Industry-leading expertise in PEEK formulation with extensive historical performance data across numerous chemical environments. Their testing protocols closely simulate real-world application conditions, providing highly reliable predictive data. Weaknesses: Their premium-grade materials with enhanced chemical resistance come at a significant cost premium, and some specialized formulations may have limited global availability or longer lead times.
Jilin Joinature Polymer Co., Ltd.
Technical Solution: Jilin Joinature Polymer has developed a systematic approach to analyzing PEEK chemical resistance, focusing on applications in harsh industrial environments. Their methodology combines standardized testing (ASTM D543, ISO 175) with application-specific protocols developed for Chinese industrial standards. Their PEEK materials undergo chemical resistance evaluation through weight change measurement, visual inspection for surface degradation, and mechanical property retention testing after chemical exposure. The company has established a comprehensive database of chemical resistance data for their PEEK grades across over 150 chemicals commonly encountered in industrial applications. Their research has shown particular strength in developing PEEK formulations with enhanced resistance to strong acids and oxidizing environments, achieving less than 2% weight change after 1000 hours of exposure to 30% sulfuric acid at 80°C. They've also focused on cost-effective PEEK formulations that maintain critical chemical resistance properties while reducing overall material costs through proprietary processing techniques.
Strengths: Strong focus on cost-effective PEEK formulations that maintain essential chemical resistance properties, making advanced polymer solutions more accessible to broader markets. Their materials show particularly good resistance to acidic environments common in Chinese industrial applications. Weaknesses: Their testing protocols may be less comprehensive than larger global competitors, and their materials may have less extensive third-party validation in certain specialized applications.
Critical Patents and Research on PEEK Chemical Resistance Properties
Cross-linkable poly(aryl ether ketone)s and articles made therefrom
PatentWO2009021999A1
Innovation
- Development of cross-linkable poly(aryl ether ketone)s with more than 50 wt.% of recurring units featuring carbonyl groups between arylene groups, which can be prepared through nucleophilic or electrophilic polymerization methods, allowing for the creation of thermally stable, two-dimensional shaped articles with improved mechanical and chemical resistance.
Semiconductive film, electric charge control member and process for production the semiconductive film
PatentInactiveUS20070020450A1
Innovation
- A production process involving extrusion of a resin composition comprising PEEK and a conductive filler through a T-die or ring die with controlled lip clearance and temperature, followed by cooling and solidification using a cooling roll or mandrel, to produce a semiconductive film with balanced properties including narrow thickness and volume resistivity scatter, high folding endurance, and excellent mechanical strength.
Environmental Impact and Sustainability of PEEK Applications
The environmental profile of PEEK (Polyetheretherketone) polymer represents a significant consideration in its growing industrial adoption. PEEK's exceptional chemical resistance properties contribute substantially to its environmental sustainability credentials. Unlike many conventional polymers, PEEK components demonstrate remarkable longevity in harsh chemical environments, significantly extending product lifecycles and reducing replacement frequency. This durability directly translates to decreased waste generation and resource consumption over time, aligning with circular economy principles.
From a manufacturing perspective, PEEK processing requires higher energy inputs compared to commodity plastics due to its elevated melting temperature (approximately 343°C). However, this energy investment is offset by the polymer's extended service life and reduced maintenance requirements. Life cycle assessments indicate that PEEK components typically demonstrate lower cumulative environmental impacts when evaluated across their entire operational lifespan, particularly in applications where chemical exposure would rapidly degrade alternative materials.
PEEK's recyclability presents both challenges and opportunities. While technically recyclable, its high-performance characteristics can degrade after multiple processing cycles. Advanced recycling technologies, including solvent-based recovery methods and pyrolysis techniques, are emerging to address this limitation. These processes enable the recovery of valuable monomers and oligomers from end-of-life PEEK products, potentially closing the material loop.
The chemical resistance of PEEK also contributes to environmental protection in critical applications. In chemical processing equipment, PEEK components minimize the risk of leakage and contamination events that could result in environmental damage. Similarly, in oil and gas extraction, PEEK-based solutions withstand aggressive chemicals while providing reliable containment, reducing the probability of environmentally harmful spills.
Regulatory frameworks increasingly recognize PEEK's environmental advantages. The polymer complies with stringent environmental regulations, including REACH and RoHS directives, containing no halogenated compounds or other substances of very high concern. Furthermore, PEEK's inertness means it does not leach harmful chemicals into surrounding environments during use, an important consideration for applications in water treatment systems and food processing equipment.
Future sustainability improvements for PEEK applications focus on developing bio-based precursors for its synthesis and implementing energy-efficient manufacturing processes. Research into catalytic methods that lower processing temperatures shows promise for reducing the carbon footprint of PEEK production while maintaining its exceptional chemical resistance properties that make it environmentally advantageous in the first place.
From a manufacturing perspective, PEEK processing requires higher energy inputs compared to commodity plastics due to its elevated melting temperature (approximately 343°C). However, this energy investment is offset by the polymer's extended service life and reduced maintenance requirements. Life cycle assessments indicate that PEEK components typically demonstrate lower cumulative environmental impacts when evaluated across their entire operational lifespan, particularly in applications where chemical exposure would rapidly degrade alternative materials.
PEEK's recyclability presents both challenges and opportunities. While technically recyclable, its high-performance characteristics can degrade after multiple processing cycles. Advanced recycling technologies, including solvent-based recovery methods and pyrolysis techniques, are emerging to address this limitation. These processes enable the recovery of valuable monomers and oligomers from end-of-life PEEK products, potentially closing the material loop.
The chemical resistance of PEEK also contributes to environmental protection in critical applications. In chemical processing equipment, PEEK components minimize the risk of leakage and contamination events that could result in environmental damage. Similarly, in oil and gas extraction, PEEK-based solutions withstand aggressive chemicals while providing reliable containment, reducing the probability of environmentally harmful spills.
Regulatory frameworks increasingly recognize PEEK's environmental advantages. The polymer complies with stringent environmental regulations, including REACH and RoHS directives, containing no halogenated compounds or other substances of very high concern. Furthermore, PEEK's inertness means it does not leach harmful chemicals into surrounding environments during use, an important consideration for applications in water treatment systems and food processing equipment.
Future sustainability improvements for PEEK applications focus on developing bio-based precursors for its synthesis and implementing energy-efficient manufacturing processes. Research into catalytic methods that lower processing temperatures shows promise for reducing the carbon footprint of PEEK production while maintaining its exceptional chemical resistance properties that make it environmentally advantageous in the first place.
Comparative Analysis with Alternative Chemical-Resistant Materials
When comparing PEEK polymer with alternative chemical-resistant materials, several key competitors emerge in high-performance applications. Fluoropolymers, particularly PTFE (polytetrafluoroethylene), offer superior chemical resistance across a broader pH spectrum than PEEK, especially against strong oxidizing acids. However, PTFE's mechanical properties are significantly inferior, with lower tensile strength and wear resistance, making it unsuitable for structural applications where PEEK excels.
PPS (polyphenylene sulfide) presents a cost-effective alternative to PEEK with good chemical resistance, particularly to organic solvents and bases. Nevertheless, PPS demonstrates lower temperature resistance (maximum continuous use temperature of 200°C versus PEEK's 250°C) and inferior mechanical properties under prolonged chemical exposure.
PVDF (polyvinylidene fluoride) offers excellent resistance to halogens and oxidizing environments where PEEK may be vulnerable. Its lower cost makes it attractive for certain applications, though its maximum service temperature (150°C) and mechanical strength limitations restrict its use in high-temperature chemical environments where PEEK remains dominant.
Ultra-high-molecular-weight polyethylene (UHMWPE) provides outstanding abrasion resistance and self-lubricating properties with good chemical resistance to many acids and bases. However, its temperature ceiling of approximately 80-100°C severely limits applications compared to PEEK's high-temperature capabilities.
Ceramic materials like silicon carbide and alumina offer exceptional chemical resistance across virtually all environments, even surpassing PEEK in extreme conditions. Their brittleness, difficult machinability, and higher weight create significant design and implementation challenges that PEEK avoids.
Metal alloys, particularly titanium, Hastelloy, and certain stainless steels, compete with PEEK in highly corrosive environments. While these alloys provide superior mechanical properties and temperature resistance, they are substantially heavier, more expensive to process, and vulnerable to specific corrosion mechanisms that polymers like PEEK avoid.
The comparative analysis reveals PEEK's unique position in the materials spectrum—offering a balanced combination of chemical resistance, mechanical properties, and processability that alternative materials cannot match across all parameters. This explains PEEK's growing adoption in critical applications despite its premium cost position relative to most alternatives.
PPS (polyphenylene sulfide) presents a cost-effective alternative to PEEK with good chemical resistance, particularly to organic solvents and bases. Nevertheless, PPS demonstrates lower temperature resistance (maximum continuous use temperature of 200°C versus PEEK's 250°C) and inferior mechanical properties under prolonged chemical exposure.
PVDF (polyvinylidene fluoride) offers excellent resistance to halogens and oxidizing environments where PEEK may be vulnerable. Its lower cost makes it attractive for certain applications, though its maximum service temperature (150°C) and mechanical strength limitations restrict its use in high-temperature chemical environments where PEEK remains dominant.
Ultra-high-molecular-weight polyethylene (UHMWPE) provides outstanding abrasion resistance and self-lubricating properties with good chemical resistance to many acids and bases. However, its temperature ceiling of approximately 80-100°C severely limits applications compared to PEEK's high-temperature capabilities.
Ceramic materials like silicon carbide and alumina offer exceptional chemical resistance across virtually all environments, even surpassing PEEK in extreme conditions. Their brittleness, difficult machinability, and higher weight create significant design and implementation challenges that PEEK avoids.
Metal alloys, particularly titanium, Hastelloy, and certain stainless steels, compete with PEEK in highly corrosive environments. While these alloys provide superior mechanical properties and temperature resistance, they are substantially heavier, more expensive to process, and vulnerable to specific corrosion mechanisms that polymers like PEEK avoid.
The comparative analysis reveals PEEK's unique position in the materials spectrum—offering a balanced combination of chemical resistance, mechanical properties, and processability that alternative materials cannot match across all parameters. This explains PEEK's growing adoption in critical applications despite its premium cost position relative to most alternatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!