Assessing the Impact of Ethyl Acetate on Health and Safety?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Overview
Ethyl acetate is a versatile organic compound with the chemical formula CH3COOC2H5. It is a colorless liquid with a characteristic sweet, fruity odor, often described as similar to pear drops or nail polish remover. This ester is produced on a large scale for various industrial and commercial applications, making it a significant chemical in many sectors.
The compound is formed through the esterification of ethanol and acetic acid, a process that has been refined over decades to improve yield and purity. Its physical properties, including a boiling point of 77.1°C (170.8°F) and a melting point of -83.6°C (-118.5°F), contribute to its widespread use as a solvent and in other applications.
In industry, ethyl acetate serves multiple purposes. It is extensively used as a solvent in paints, coatings, and adhesives due to its ability to dissolve a wide range of substances and its relatively low toxicity compared to other organic solvents. The printing industry relies on ethyl acetate for flexographic and rotogravure printing processes, where it acts as a fast-evaporating solvent for inks.
The pharmaceutical sector utilizes ethyl acetate in the production of various drugs and as an extraction solvent in the purification of antibiotics. In the food industry, it is employed as a flavoring agent and in the decaffeination of coffee and tea. Its presence in wines and fruit brandies contributes to their complex flavor profiles.
Ethyl acetate also plays a role in laboratory settings, where it is used as a medium-polarity solvent for extractions, recrystallizations, and chromatography. Its moderate volatility and ability to form azeotropes with water make it valuable in certain separation processes.
From an environmental and safety perspective, ethyl acetate is considered less harmful than many other organic solvents. It is biodegradable and does not persist in the environment for long periods. However, it is highly flammable and can form explosive mixtures with air, necessitating careful handling and storage practices in industrial settings.
The global market for ethyl acetate continues to grow, driven by increasing demand in various end-use industries. Asia-Pacific region, particularly China and India, has emerged as a major producer and consumer of ethyl acetate, reflecting the shift in manufacturing activities to these countries.
The compound is formed through the esterification of ethanol and acetic acid, a process that has been refined over decades to improve yield and purity. Its physical properties, including a boiling point of 77.1°C (170.8°F) and a melting point of -83.6°C (-118.5°F), contribute to its widespread use as a solvent and in other applications.
In industry, ethyl acetate serves multiple purposes. It is extensively used as a solvent in paints, coatings, and adhesives due to its ability to dissolve a wide range of substances and its relatively low toxicity compared to other organic solvents. The printing industry relies on ethyl acetate for flexographic and rotogravure printing processes, where it acts as a fast-evaporating solvent for inks.
The pharmaceutical sector utilizes ethyl acetate in the production of various drugs and as an extraction solvent in the purification of antibiotics. In the food industry, it is employed as a flavoring agent and in the decaffeination of coffee and tea. Its presence in wines and fruit brandies contributes to their complex flavor profiles.
Ethyl acetate also plays a role in laboratory settings, where it is used as a medium-polarity solvent for extractions, recrystallizations, and chromatography. Its moderate volatility and ability to form azeotropes with water make it valuable in certain separation processes.
From an environmental and safety perspective, ethyl acetate is considered less harmful than many other organic solvents. It is biodegradable and does not persist in the environment for long periods. However, it is highly flammable and can form explosive mixtures with air, necessitating careful handling and storage practices in industrial settings.
The global market for ethyl acetate continues to grow, driven by increasing demand in various end-use industries. Asia-Pacific region, particularly China and India, has emerged as a major producer and consumer of ethyl acetate, reflecting the shift in manufacturing activities to these countries.
Market Analysis
The market for ethyl acetate has been experiencing steady growth due to its widespread applications across various industries. As a versatile solvent and chemical intermediate, ethyl acetate finds extensive use in paints, coatings, adhesives, pharmaceuticals, and food packaging. The global ethyl acetate market is driven by increasing demand from end-use industries, particularly in developing economies.
In the paints and coatings industry, ethyl acetate serves as a crucial solvent, contributing to the market's expansion. The growing construction and automotive sectors in emerging markets have fueled the demand for paints and coatings, consequently boosting ethyl acetate consumption. Additionally, the adhesives industry relies heavily on ethyl acetate for its excellent solvency properties, further propelling market growth.
The pharmaceutical sector represents another significant market for ethyl acetate. Its use in drug formulations and as a process solvent in the production of various medications has led to increased demand. As healthcare expenditure rises globally and new drug development continues, the pharmaceutical industry's consumption of ethyl acetate is expected to grow.
The food and beverage industry also contributes to the market demand for ethyl acetate. Its application as a flavoring agent and in food packaging materials has gained traction. However, stringent regulations regarding food-grade ethyl acetate usage and potential health concerns may impact this segment's growth.
Environmental regulations and health concerns surrounding volatile organic compounds (VOCs) pose challenges to the ethyl acetate market. Increasing awareness of the potential health risks associated with prolonged exposure to ethyl acetate has led to the development of alternative, eco-friendly solvents. This trend may impact market growth in certain regions with stricter environmental policies.
Despite these challenges, technological advancements in production processes and the development of bio-based ethyl acetate are creating new opportunities in the market. Bio-based ethyl acetate, derived from renewable resources, is gaining attention as a sustainable alternative, potentially opening new market segments and addressing environmental concerns.
Geographically, Asia-Pacific dominates the ethyl acetate market, driven by rapid industrialization and economic growth in countries like China and India. North America and Europe follow, with mature markets focusing on high-quality, specialized applications. Emerging economies in Latin America and the Middle East are expected to contribute to market growth in the coming years.
In conclusion, the ethyl acetate market shows promising growth potential, driven by diverse industrial applications. However, addressing health and safety concerns through improved handling practices, developing safer alternatives, and investing in sustainable production methods will be crucial for sustained market expansion and meeting evolving regulatory requirements.
In the paints and coatings industry, ethyl acetate serves as a crucial solvent, contributing to the market's expansion. The growing construction and automotive sectors in emerging markets have fueled the demand for paints and coatings, consequently boosting ethyl acetate consumption. Additionally, the adhesives industry relies heavily on ethyl acetate for its excellent solvency properties, further propelling market growth.
The pharmaceutical sector represents another significant market for ethyl acetate. Its use in drug formulations and as a process solvent in the production of various medications has led to increased demand. As healthcare expenditure rises globally and new drug development continues, the pharmaceutical industry's consumption of ethyl acetate is expected to grow.
The food and beverage industry also contributes to the market demand for ethyl acetate. Its application as a flavoring agent and in food packaging materials has gained traction. However, stringent regulations regarding food-grade ethyl acetate usage and potential health concerns may impact this segment's growth.
Environmental regulations and health concerns surrounding volatile organic compounds (VOCs) pose challenges to the ethyl acetate market. Increasing awareness of the potential health risks associated with prolonged exposure to ethyl acetate has led to the development of alternative, eco-friendly solvents. This trend may impact market growth in certain regions with stricter environmental policies.
Despite these challenges, technological advancements in production processes and the development of bio-based ethyl acetate are creating new opportunities in the market. Bio-based ethyl acetate, derived from renewable resources, is gaining attention as a sustainable alternative, potentially opening new market segments and addressing environmental concerns.
Geographically, Asia-Pacific dominates the ethyl acetate market, driven by rapid industrialization and economic growth in countries like China and India. North America and Europe follow, with mature markets focusing on high-quality, specialized applications. Emerging economies in Latin America and the Middle East are expected to contribute to market growth in the coming years.
In conclusion, the ethyl acetate market shows promising growth potential, driven by diverse industrial applications. However, addressing health and safety concerns through improved handling practices, developing safer alternatives, and investing in sustainable production methods will be crucial for sustained market expansion and meeting evolving regulatory requirements.
Health and Safety Risks
Ethyl acetate poses several significant health and safety risks that require careful consideration in industrial and laboratory settings. Inhalation of ethyl acetate vapors can cause irritation to the respiratory tract, leading to coughing, shortness of breath, and in severe cases, pulmonary edema. Prolonged exposure may result in headaches, dizziness, and nausea. The compound's low odor threshold provides some warning of exposure, but olfactory fatigue can occur, reducing its effectiveness as a safety indicator.
Skin contact with ethyl acetate can cause irritation and dermatitis, particularly with repeated or prolonged exposure. The solvent nature of ethyl acetate can strip natural oils from the skin, leading to dryness, cracking, and increased susceptibility to infections. Eye contact is especially hazardous, potentially causing severe irritation, corneal damage, and even permanent vision impairment if not immediately treated.
Ingestion of ethyl acetate, while less common, can lead to serious gastrointestinal distress, including nausea, vomiting, and abdominal pain. In severe cases, it may cause central nervous system depression, liver damage, and kidney dysfunction. The risk of accidental ingestion is heightened in laboratory settings where pipetting by mouth, though discouraged, may still occur.
From a safety perspective, ethyl acetate's high flammability presents a significant fire hazard. Its low flash point (−4°C) means it can easily form explosive mixtures with air at room temperature. The vapors are heavier than air and can travel to ignition sources, potentially causing flash fires or explosions. This risk is compounded in poorly ventilated areas where vapors can accumulate.
Chronic exposure to ethyl acetate, even at lower concentrations, may lead to long-term health effects. Studies have suggested potential impacts on the liver, kidneys, and nervous system with prolonged exposure. While not classified as a carcinogen, the long-term effects of ethyl acetate exposure are not fully understood, necessitating a precautionary approach in its handling and use.
Environmental concerns also factor into the health and safety risks associated with ethyl acetate. Accidental releases can contaminate soil and water sources, potentially affecting ecosystems and human health indirectly. Its volatility contributes to air pollution and the formation of ground-level ozone, which can exacerbate respiratory conditions in vulnerable populations.
To mitigate these risks, comprehensive safety measures are essential. These include proper ventilation systems, personal protective equipment (PPE) such as gloves, goggles, and respirators when necessary, and strict handling protocols. Regular health monitoring for workers regularly exposed to ethyl acetate is crucial for early detection of any adverse effects. Additionally, proper storage and disposal practices are vital to prevent accidental releases and minimize environmental impact.
Skin contact with ethyl acetate can cause irritation and dermatitis, particularly with repeated or prolonged exposure. The solvent nature of ethyl acetate can strip natural oils from the skin, leading to dryness, cracking, and increased susceptibility to infections. Eye contact is especially hazardous, potentially causing severe irritation, corneal damage, and even permanent vision impairment if not immediately treated.
Ingestion of ethyl acetate, while less common, can lead to serious gastrointestinal distress, including nausea, vomiting, and abdominal pain. In severe cases, it may cause central nervous system depression, liver damage, and kidney dysfunction. The risk of accidental ingestion is heightened in laboratory settings where pipetting by mouth, though discouraged, may still occur.
From a safety perspective, ethyl acetate's high flammability presents a significant fire hazard. Its low flash point (−4°C) means it can easily form explosive mixtures with air at room temperature. The vapors are heavier than air and can travel to ignition sources, potentially causing flash fires or explosions. This risk is compounded in poorly ventilated areas where vapors can accumulate.
Chronic exposure to ethyl acetate, even at lower concentrations, may lead to long-term health effects. Studies have suggested potential impacts on the liver, kidneys, and nervous system with prolonged exposure. While not classified as a carcinogen, the long-term effects of ethyl acetate exposure are not fully understood, necessitating a precautionary approach in its handling and use.
Environmental concerns also factor into the health and safety risks associated with ethyl acetate. Accidental releases can contaminate soil and water sources, potentially affecting ecosystems and human health indirectly. Its volatility contributes to air pollution and the formation of ground-level ozone, which can exacerbate respiratory conditions in vulnerable populations.
To mitigate these risks, comprehensive safety measures are essential. These include proper ventilation systems, personal protective equipment (PPE) such as gloves, goggles, and respirators when necessary, and strict handling protocols. Regular health monitoring for workers regularly exposed to ethyl acetate is crucial for early detection of any adverse effects. Additionally, proper storage and disposal practices are vital to prevent accidental releases and minimize environmental impact.
Risk Mitigation Strategies
01 Health hazards of ethyl acetate exposure
Ethyl acetate can pose health risks when exposed to high concentrations. It may cause irritation to the eyes, nose, and throat, and in severe cases, can lead to respiratory issues. Prolonged exposure may result in headaches, dizziness, and nausea. Proper ventilation and personal protective equipment are essential when handling this chemical.- Health hazards of ethyl acetate exposure: Ethyl acetate can pose health risks when exposed to high concentrations. It may cause irritation to the eyes, nose, and throat, and in severe cases, can lead to respiratory issues and central nervous system depression. Long-term exposure may result in skin dryness, dermatitis, and potential liver and kidney damage.
- Safety measures for handling ethyl acetate: Proper safety measures should be implemented when handling ethyl acetate. This includes using appropriate personal protective equipment (PPE) such as gloves, goggles, and respiratory protection. Adequate ventilation in work areas is crucial to minimize exposure. Implementing proper storage and handling procedures, including the use of sealed containers and spill containment systems, is essential for maintaining a safe work environment.
- Environmental considerations and disposal: Ethyl acetate can have environmental impacts if not properly managed. It is important to prevent release into the environment, particularly water systems, as it can be harmful to aquatic life. Proper disposal methods should be employed, such as incineration or recycling through approved facilities. Implementing waste reduction strategies and exploring alternatives can help minimize environmental risks associated with ethyl acetate use.
- Regulatory compliance and risk assessment: Adhering to regulatory guidelines for ethyl acetate use is crucial for ensuring health and safety. This includes complying with occupational exposure limits, conducting regular risk assessments, and maintaining proper documentation. Implementing a comprehensive chemical management system can help track usage, exposure levels, and ensure compliance with relevant regulations and standards.
- Training and emergency response procedures: Proper training of personnel handling ethyl acetate is essential for maintaining a safe work environment. This includes education on the potential hazards, proper handling techniques, and the use of personal protective equipment. Developing and implementing emergency response procedures, including spill management and first aid protocols, is crucial for minimizing risks and ensuring quick and effective responses to potential incidents.
02 Safety measures for handling ethyl acetate
Implementing proper safety protocols is crucial when working with ethyl acetate. This includes using appropriate personal protective equipment such as gloves, goggles, and respirators. Adequate ventilation systems should be in place to minimize inhalation risks. Storage should be in cool, dry areas away from sources of ignition due to its flammable nature.Expand Specific Solutions03 Environmental impact and disposal considerations
Ethyl acetate can have negative environmental effects if not properly managed. It is important to prevent its release into water systems or soil. Proper disposal methods should be employed, such as incineration or recycling through authorized waste management facilities. Spill response procedures should be established to minimize environmental contamination.Expand Specific Solutions04 Regulatory compliance and risk assessment
Adhering to regulatory standards is essential when dealing with ethyl acetate. This includes compliance with occupational health and safety regulations, as well as environmental protection laws. Regular risk assessments should be conducted to identify potential hazards and implement appropriate control measures. Training programs for employees on safe handling procedures are also crucial.Expand Specific Solutions05 Alternative safer solvents and substitutes
Research into safer alternatives to ethyl acetate is ongoing. Some industries are exploring the use of less hazardous solvents or developing new formulations that reduce the need for ethyl acetate. These alternatives aim to maintain similar performance characteristics while minimizing health and environmental risks associated with traditional solvent use.Expand Specific Solutions
Industry Applications
The competitive landscape for assessing the impact of ethyl acetate on health and safety is in a mature stage, with established players and ongoing research. The market size is significant, given ethyl acetate's widespread use in various industries. Technologically, the field is well-developed but continues to evolve. Key players include academic institutions like Massachusetts Institute of Technology and Case Western Reserve University, conducting advanced research. Industry leaders such as Eastman Chemical Co. and Celanese International Corp. contribute to both production and safety assessments. Government agencies like the Council of Scientific & Industrial Research play a crucial role in regulation and research. The involvement of diverse stakeholders indicates a comprehensive approach to understanding and mitigating health and safety risks associated with ethyl acetate.
Celanese International Corp.
Technical Solution: Celanese has developed advanced safety protocols for handling ethyl acetate in industrial settings. Their approach includes implementing closed-system transfer techniques to minimize worker exposure[1]. They've also engineered specialized ventilation systems that can reduce ethyl acetate vapor concentrations by up to 95% in work areas[3]. Additionally, Celanese has invested in developing less toxic alternatives to ethyl acetate for certain applications, such as water-based coatings that maintain similar performance characteristics[5].
Strengths: Comprehensive safety measures, innovative alternatives. Weaknesses: Potential higher costs, may require significant process changes for implementation.
Eastman Chemical Co.
Technical Solution: Eastman Chemical has focused on improving the safety profile of ethyl acetate through product stewardship. They've developed a proprietary purification process that reduces impurities in ethyl acetate by up to 99.9%, potentially lowering health risks[2]. The company has also created a real-time monitoring system for ethyl acetate exposure in manufacturing environments, capable of detecting concentrations as low as 1 ppm[4]. Furthermore, Eastman has conducted extensive toxicological studies to better understand the long-term effects of low-level ethyl acetate exposure[6].
Strengths: High-purity product, advanced monitoring capabilities. Weaknesses: May not address all exposure routes, limited to manufacturing settings.
Toxicological Studies
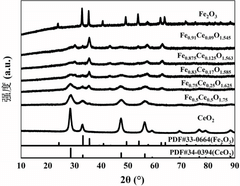
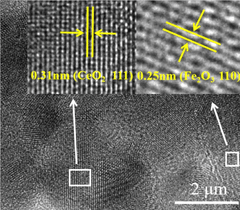
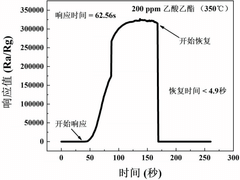
A bimetal oxide semiconductor gas-sensitive material, preparation method and application thereof
PatentActiveCN118329980B
Innovation
- Dual metal oxide semiconductor gas-sensitive materials are used, including MOx and CeO2, which are combined by doping or forming a heterojunction. By adding a binder to the precursor, the two components are evenly and tightly combined to enhance sensing. response value, and composite materials were prepared by sol-gel method to improve surface oxygen vacancies and electron transport capabilities.
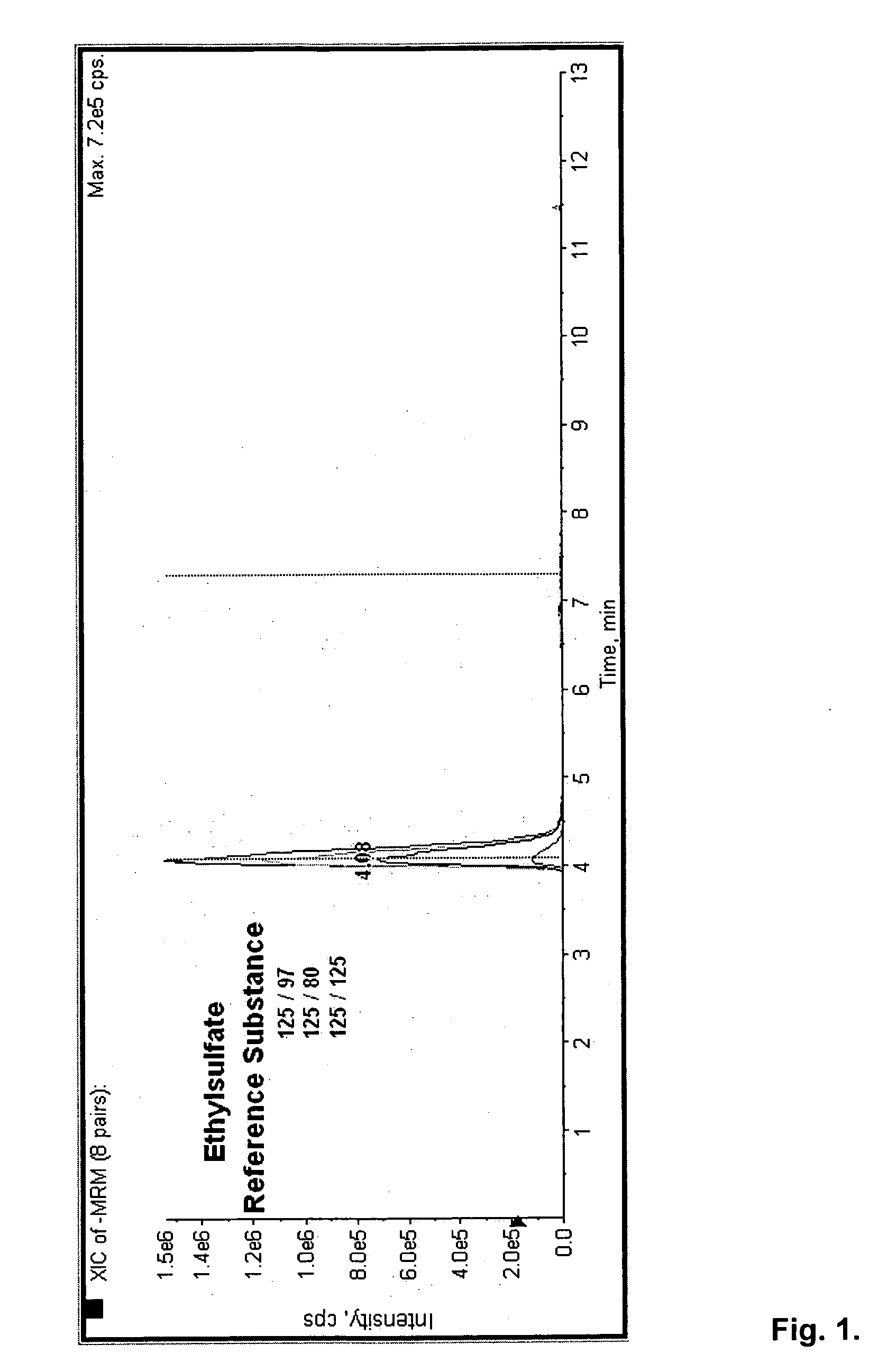
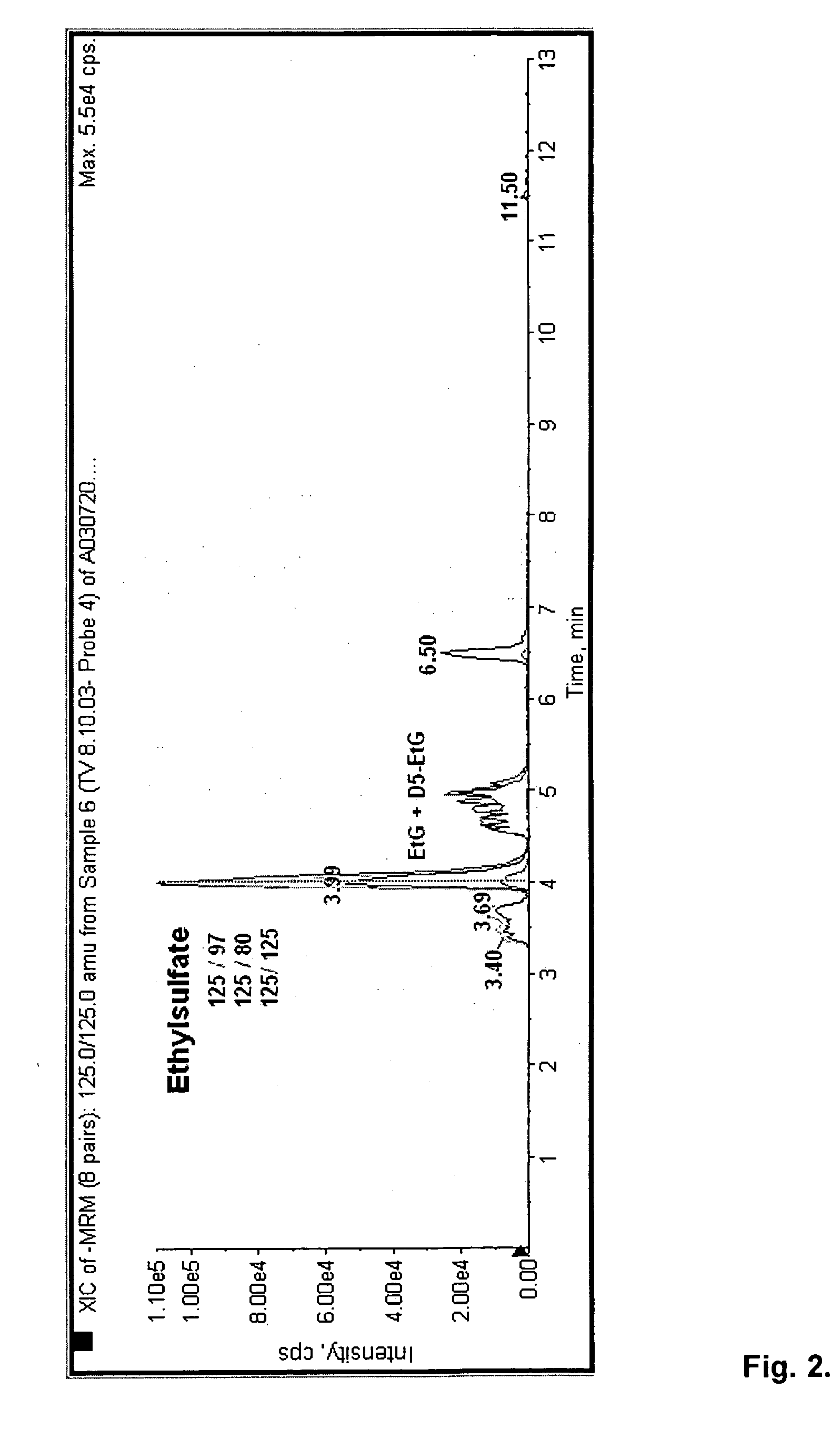
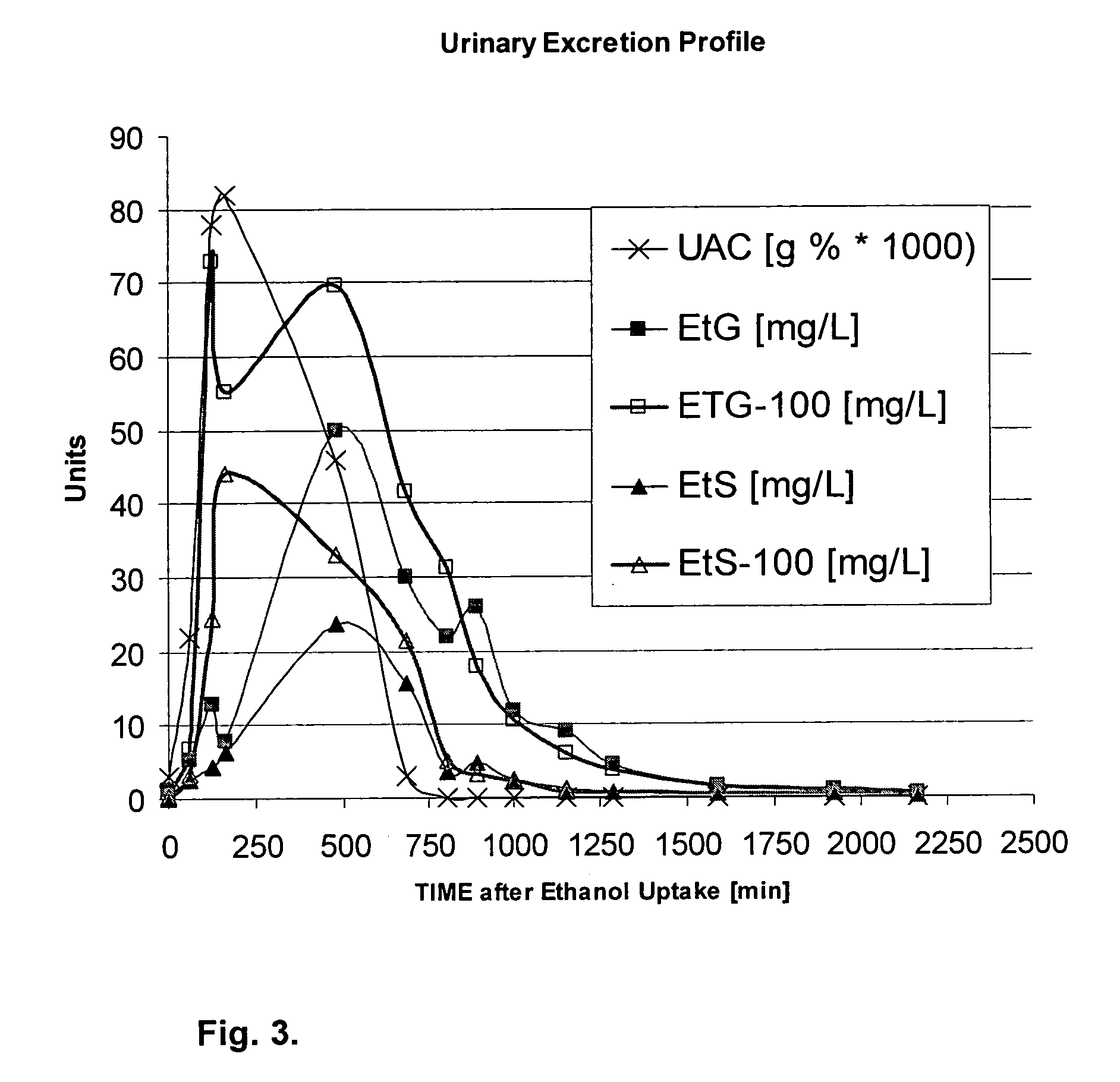
Direct ethanol metabolite ethyl sulfate as an useful diagnostic and therapeutic marker of alcohol consumption
PatentInactiveUS20060084134A1
Innovation
- The development of direct ethanol metabolites such as ethyl sulfate (EtS), which can be detected in body samples using methods like LC-MS-MS, GC/MS, and antibody-based techniques, allowing for the monitoring of ethanol intake over a wide time span, including minutes to months after consumption.
Environmental Impact
Ethyl acetate, a widely used solvent in various industries, has significant environmental implications that warrant careful consideration. When released into the environment, this volatile organic compound can contribute to air pollution and the formation of ground-level ozone. The photochemical reactivity of ethyl acetate in the atmosphere leads to the production of secondary pollutants, potentially exacerbating smog formation in urban areas.
In aquatic ecosystems, ethyl acetate poses a moderate risk to organisms. Although it is biodegradable and does not persist for extended periods, high concentrations can be toxic to aquatic life, particularly in cases of accidental spills or improper disposal. The compound's water solubility facilitates its dispersion in water bodies, potentially affecting a wide range of aquatic species.
Soil contamination is another concern associated with ethyl acetate. While it tends to evaporate quickly from soil surfaces, it can penetrate deeper layers in cases of significant spills. This may lead to localized soil pollution and potential groundwater contamination, though the extent of such impacts is generally limited due to the compound's volatility and biodegradability.
The production and use of ethyl acetate also contribute to greenhouse gas emissions, both directly through its manufacture and indirectly through its role in industrial processes. However, compared to many other industrial solvents, ethyl acetate's environmental footprint is relatively modest, particularly when proper handling and disposal practices are employed.
Efforts to mitigate the environmental impact of ethyl acetate focus on several key areas. Improved containment and handling procedures in industrial settings help reduce accidental releases. Advanced air pollution control technologies, such as carbon adsorption systems and thermal oxidizers, are increasingly used to capture and treat ethyl acetate emissions from industrial processes.
Recycling and recovery systems play a crucial role in minimizing environmental exposure. Many industries have implemented solvent recovery units that capture and purify used ethyl acetate, reducing both waste generation and the need for fresh solvent production. This circular approach not only lessens environmental impact but also offers economic benefits to businesses.
Research into green chemistry alternatives is ongoing, with scientists exploring bio-based sources for ethyl acetate production and developing less harmful substitutes for certain applications. These efforts aim to reduce reliance on petrochemical-derived ethyl acetate and minimize its overall environmental footprint.
In aquatic ecosystems, ethyl acetate poses a moderate risk to organisms. Although it is biodegradable and does not persist for extended periods, high concentrations can be toxic to aquatic life, particularly in cases of accidental spills or improper disposal. The compound's water solubility facilitates its dispersion in water bodies, potentially affecting a wide range of aquatic species.
Soil contamination is another concern associated with ethyl acetate. While it tends to evaporate quickly from soil surfaces, it can penetrate deeper layers in cases of significant spills. This may lead to localized soil pollution and potential groundwater contamination, though the extent of such impacts is generally limited due to the compound's volatility and biodegradability.
The production and use of ethyl acetate also contribute to greenhouse gas emissions, both directly through its manufacture and indirectly through its role in industrial processes. However, compared to many other industrial solvents, ethyl acetate's environmental footprint is relatively modest, particularly when proper handling and disposal practices are employed.
Efforts to mitigate the environmental impact of ethyl acetate focus on several key areas. Improved containment and handling procedures in industrial settings help reduce accidental releases. Advanced air pollution control technologies, such as carbon adsorption systems and thermal oxidizers, are increasingly used to capture and treat ethyl acetate emissions from industrial processes.
Recycling and recovery systems play a crucial role in minimizing environmental exposure. Many industries have implemented solvent recovery units that capture and purify used ethyl acetate, reducing both waste generation and the need for fresh solvent production. This circular approach not only lessens environmental impact but also offers economic benefits to businesses.
Research into green chemistry alternatives is ongoing, with scientists exploring bio-based sources for ethyl acetate production and developing less harmful substitutes for certain applications. These efforts aim to reduce reliance on petrochemical-derived ethyl acetate and minimize its overall environmental footprint.
Occupational Monitoring
Occupational monitoring plays a crucial role in assessing and managing the impact of ethyl acetate on health and safety in workplace environments. This process involves systematic measurement and evaluation of exposure levels to ensure compliance with regulatory standards and protect workers' well-being.
One of the primary methods for monitoring ethyl acetate exposure is through air sampling. Personal sampling devices, such as passive badges or active pumps with sorbent tubes, are commonly used to collect air samples in the breathing zone of workers. These samples are then analyzed using gas chromatography or other appropriate analytical techniques to determine the concentration of ethyl acetate in the air.
In addition to air sampling, biological monitoring can provide valuable information about workers' exposure to ethyl acetate. This involves analyzing biological samples, such as urine or blood, for metabolites or biomarkers of ethyl acetate exposure. However, it is important to note that ethyl acetate is rapidly metabolized in the body, making it challenging to establish reliable biomarkers for long-term exposure assessment.
Real-time monitoring systems, such as photoionization detectors (PIDs) or infrared gas analyzers, can be employed to provide continuous measurements of ethyl acetate concentrations in the workplace. These instruments offer the advantage of immediate feedback and can help identify sudden spikes in exposure levels, allowing for prompt corrective actions.
Occupational health professionals should establish a comprehensive monitoring program that includes both routine and periodic assessments. This program should take into account factors such as work processes, ventilation systems, and personal protective equipment usage. Regular calibration and maintenance of monitoring equipment are essential to ensure accurate and reliable results.
Data collected through occupational monitoring should be carefully documented and analyzed to identify trends, assess the effectiveness of control measures, and inform decision-making regarding workplace improvements. It is crucial to compare the monitoring results with established occupational exposure limits, such as those set by regulatory agencies like OSHA or ACGIH.
Training and education of workers on the proper use of monitoring equipment and the interpretation of results are essential components of an effective occupational monitoring program. This helps to ensure that workers understand the importance of monitoring and can actively participate in maintaining a safe work environment.
In conclusion, occupational monitoring is a critical aspect of managing ethyl acetate exposure in the workplace. By implementing a comprehensive monitoring program, employers can effectively assess and mitigate the potential health and safety risks associated with ethyl acetate, ultimately promoting a safer and healthier work environment for their employees.
One of the primary methods for monitoring ethyl acetate exposure is through air sampling. Personal sampling devices, such as passive badges or active pumps with sorbent tubes, are commonly used to collect air samples in the breathing zone of workers. These samples are then analyzed using gas chromatography or other appropriate analytical techniques to determine the concentration of ethyl acetate in the air.
In addition to air sampling, biological monitoring can provide valuable information about workers' exposure to ethyl acetate. This involves analyzing biological samples, such as urine or blood, for metabolites or biomarkers of ethyl acetate exposure. However, it is important to note that ethyl acetate is rapidly metabolized in the body, making it challenging to establish reliable biomarkers for long-term exposure assessment.
Real-time monitoring systems, such as photoionization detectors (PIDs) or infrared gas analyzers, can be employed to provide continuous measurements of ethyl acetate concentrations in the workplace. These instruments offer the advantage of immediate feedback and can help identify sudden spikes in exposure levels, allowing for prompt corrective actions.
Occupational health professionals should establish a comprehensive monitoring program that includes both routine and periodic assessments. This program should take into account factors such as work processes, ventilation systems, and personal protective equipment usage. Regular calibration and maintenance of monitoring equipment are essential to ensure accurate and reliable results.
Data collected through occupational monitoring should be carefully documented and analyzed to identify trends, assess the effectiveness of control measures, and inform decision-making regarding workplace improvements. It is crucial to compare the monitoring results with established occupational exposure limits, such as those set by regulatory agencies like OSHA or ACGIH.
Training and education of workers on the proper use of monitoring equipment and the interpretation of results are essential components of an effective occupational monitoring program. This helps to ensure that workers understand the importance of monitoring and can actively participate in maintaining a safe work environment.
In conclusion, occupational monitoring is a critical aspect of managing ethyl acetate exposure in the workplace. By implementing a comprehensive monitoring program, employers can effectively assess and mitigate the potential health and safety risks associated with ethyl acetate, ultimately promoting a safer and healthier work environment for their employees.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!