Assessment of New Materials for Bio-compatible Microfluidic Chips
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biocompatible Microfluidics Background and Objectives
Microfluidic technology has evolved significantly over the past three decades, transforming from simple channel designs to sophisticated lab-on-a-chip systems capable of performing complex biological analyses. The integration of microfluidics with biological applications has created a distinct field known as biocompatible microfluidics, which focuses on developing platforms that can interact with biological materials without adverse effects.
The historical progression of biocompatible microfluidic materials began with silicon and glass substrates in the 1990s, followed by the introduction of polydimethylsiloxane (PDMS) in the early 2000s, which revolutionized the field due to its optical transparency, gas permeability, and ease of fabrication. Recent years have witnessed the emergence of alternative materials including thermoplastics, hydrogels, and paper-based substrates, each offering unique advantages for specific biological applications.
Current biocompatible microfluidic technologies face several limitations that hinder their widespread adoption in clinical and industrial settings. Traditional materials like PDMS exhibit challenges including protein adsorption, leaching of uncured oligomers, and absorption of small hydrophobic molecules, which can compromise experimental results and biocompatibility. Additionally, many existing materials require complex fabrication processes that limit scalability and increase production costs.
The primary objective of this assessment is to evaluate novel materials for biocompatible microfluidic chips that address these limitations while enhancing performance characteristics. Specifically, we aim to identify materials that demonstrate superior biocompatibility, reduced non-specific adsorption, improved chemical resistance, and compatibility with high-throughput manufacturing processes.
The technological trajectory indicates a growing demand for microfluidic platforms capable of supporting advanced biological applications, including organ-on-a-chip models, point-of-care diagnostics, and personalized medicine approaches. This necessitates materials that can maintain cellular viability, support tissue growth, and accurately replicate physiological conditions while remaining cost-effective and manufacturable at scale.
Recent advancements in material science, including the development of bioinspired materials, surface modification techniques, and composite substrates, present promising opportunities for addressing current challenges in biocompatible microfluidics. These innovations may enable the creation of next-generation microfluidic platforms with enhanced functionality, improved reliability, and broader applicability across various biological research and clinical diagnostic applications.
This assessment will provide a comprehensive evaluation of emerging materials for biocompatible microfluidic chips, considering factors such as mechanical properties, surface chemistry, optical characteristics, fabrication requirements, and long-term stability under biological conditions. The findings will inform strategic research directions and potential commercial applications in this rapidly evolving field.
The historical progression of biocompatible microfluidic materials began with silicon and glass substrates in the 1990s, followed by the introduction of polydimethylsiloxane (PDMS) in the early 2000s, which revolutionized the field due to its optical transparency, gas permeability, and ease of fabrication. Recent years have witnessed the emergence of alternative materials including thermoplastics, hydrogels, and paper-based substrates, each offering unique advantages for specific biological applications.
Current biocompatible microfluidic technologies face several limitations that hinder their widespread adoption in clinical and industrial settings. Traditional materials like PDMS exhibit challenges including protein adsorption, leaching of uncured oligomers, and absorption of small hydrophobic molecules, which can compromise experimental results and biocompatibility. Additionally, many existing materials require complex fabrication processes that limit scalability and increase production costs.
The primary objective of this assessment is to evaluate novel materials for biocompatible microfluidic chips that address these limitations while enhancing performance characteristics. Specifically, we aim to identify materials that demonstrate superior biocompatibility, reduced non-specific adsorption, improved chemical resistance, and compatibility with high-throughput manufacturing processes.
The technological trajectory indicates a growing demand for microfluidic platforms capable of supporting advanced biological applications, including organ-on-a-chip models, point-of-care diagnostics, and personalized medicine approaches. This necessitates materials that can maintain cellular viability, support tissue growth, and accurately replicate physiological conditions while remaining cost-effective and manufacturable at scale.
Recent advancements in material science, including the development of bioinspired materials, surface modification techniques, and composite substrates, present promising opportunities for addressing current challenges in biocompatible microfluidics. These innovations may enable the creation of next-generation microfluidic platforms with enhanced functionality, improved reliability, and broader applicability across various biological research and clinical diagnostic applications.
This assessment will provide a comprehensive evaluation of emerging materials for biocompatible microfluidic chips, considering factors such as mechanical properties, surface chemistry, optical characteristics, fabrication requirements, and long-term stability under biological conditions. The findings will inform strategic research directions and potential commercial applications in this rapidly evolving field.
Market Analysis for Biocompatible Microfluidic Applications
The global market for biocompatible microfluidic chips has experienced significant growth in recent years, driven by increasing applications in healthcare, pharmaceuticals, and life sciences. The market value reached approximately $2.5 billion in 2022 and is projected to grow at a CAGR of 18.7% through 2028, potentially reaching $7.1 billion by the end of the forecast period.
Healthcare applications currently dominate the biocompatible microfluidic chip market, accounting for nearly 45% of the total market share. Point-of-care diagnostics represents the fastest-growing segment within healthcare applications, with a projected growth rate of 22.3% annually. This growth is primarily attributed to the increasing demand for rapid, accurate, and cost-effective diagnostic solutions in both developed and developing regions.
Pharmaceutical and biotechnology companies constitute the second-largest end-user segment, utilizing biocompatible microfluidic chips for drug discovery, development, and testing processes. The adoption of organ-on-chip and lab-on-chip technologies has significantly reduced the time and cost associated with drug development, driving market expansion in this sector.
Geographically, North America holds the largest market share at approximately 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, primarily due to increasing healthcare expenditure, growing research activities, and favorable government initiatives in countries like China, Japan, and India.
The demand for biocompatible materials in microfluidic applications is increasingly driven by specific performance requirements. End-users are seeking materials that offer excellent biocompatibility, chemical resistance, optical transparency, and mechanical stability. Additionally, there is growing demand for materials that can be easily sterilized and are suitable for mass production techniques such as injection molding or 3D printing.
Market trends indicate a shift toward sustainable and environmentally friendly biocompatible materials. Biodegradable polymers and bio-based materials are gaining traction as alternatives to traditional petroleum-based polymers. This trend is particularly evident in single-use diagnostic applications, where environmental concerns are prompting manufacturers to explore greener material options.
Customer preferences are evolving toward integrated microfluidic solutions that combine multiple functionalities on a single chip. This trend is creating opportunities for materials that can accommodate various surface modifications and functionalities while maintaining biocompatibility. The market is also witnessing increased demand for customized solutions tailored to specific application requirements, indicating a potential shift from standardized products to application-specific microfluidic platforms.
Healthcare applications currently dominate the biocompatible microfluidic chip market, accounting for nearly 45% of the total market share. Point-of-care diagnostics represents the fastest-growing segment within healthcare applications, with a projected growth rate of 22.3% annually. This growth is primarily attributed to the increasing demand for rapid, accurate, and cost-effective diagnostic solutions in both developed and developing regions.
Pharmaceutical and biotechnology companies constitute the second-largest end-user segment, utilizing biocompatible microfluidic chips for drug discovery, development, and testing processes. The adoption of organ-on-chip and lab-on-chip technologies has significantly reduced the time and cost associated with drug development, driving market expansion in this sector.
Geographically, North America holds the largest market share at approximately 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, primarily due to increasing healthcare expenditure, growing research activities, and favorable government initiatives in countries like China, Japan, and India.
The demand for biocompatible materials in microfluidic applications is increasingly driven by specific performance requirements. End-users are seeking materials that offer excellent biocompatibility, chemical resistance, optical transparency, and mechanical stability. Additionally, there is growing demand for materials that can be easily sterilized and are suitable for mass production techniques such as injection molding or 3D printing.
Market trends indicate a shift toward sustainable and environmentally friendly biocompatible materials. Biodegradable polymers and bio-based materials are gaining traction as alternatives to traditional petroleum-based polymers. This trend is particularly evident in single-use diagnostic applications, where environmental concerns are prompting manufacturers to explore greener material options.
Customer preferences are evolving toward integrated microfluidic solutions that combine multiple functionalities on a single chip. This trend is creating opportunities for materials that can accommodate various surface modifications and functionalities while maintaining biocompatibility. The market is also witnessing increased demand for customized solutions tailored to specific application requirements, indicating a potential shift from standardized products to application-specific microfluidic platforms.
Current Materials Landscape and Technical Barriers
The microfluidic chip materials landscape has evolved significantly over the past decade, with traditional materials like polydimethylsiloxane (PDMS), glass, and silicon still dominating commercial applications. PDMS remains the most widely used material due to its optical transparency, gas permeability, and ease of fabrication through soft lithography techniques. However, its hydrophobic nature and tendency to absorb small molecules present significant limitations for certain biological applications.
Glass and silicon offer excellent chemical resistance and well-established microfabrication protocols, but their rigid nature and higher manufacturing costs restrict their application in flexible or disposable devices. Thermoplastics such as polymethyl methacrylate (PMMA), polycarbonate (PC), and cyclic olefin copolymer (COC) have gained traction as alternatives, offering better mass production capabilities through injection molding or hot embossing.
Recent advances have introduced paper-based microfluidic devices as low-cost alternatives for point-of-care diagnostics, though their resolution limitations and structural integrity issues prevent broader adoption. Hydrogel-based materials show promise for tissue engineering applications but face challenges in standardization and long-term stability.
The primary technical barriers in current microfluidic materials center around biocompatibility issues. Many materials exhibit surface properties that can trigger protein adsorption, cell adhesion, or immune responses when in contact with biological samples. Surface modification techniques like plasma treatment or chemical functionalization offer partial solutions but often provide only temporary improvements.
Manufacturing scalability presents another significant challenge. Laboratory-scale fabrication methods frequently fail to translate effectively to mass production, creating a substantial gap between research prototypes and commercially viable products. This is particularly evident with complex multi-material chips that require precise alignment and bonding.
Long-term stability remains problematic for many biocompatible materials, with issues including leaching of additives, surface degradation upon exposure to biological fluids, and changes in mechanical properties over time. These factors can compromise experimental reproducibility and device reliability in clinical settings.
Integration capabilities also pose technical barriers, as many biocompatible materials are difficult to interface with electronic components, sensors, or external equipment. This limitation hinders the development of fully integrated lab-on-chip systems capable of sample preparation, analysis, and detection within a single platform.
Regulatory hurdles further complicate material selection, with stringent requirements for materials used in diagnostic or therapeutic applications. The lack of standardized testing protocols specifically designed for microfluidic materials creates additional uncertainty in the development pipeline.
Glass and silicon offer excellent chemical resistance and well-established microfabrication protocols, but their rigid nature and higher manufacturing costs restrict their application in flexible or disposable devices. Thermoplastics such as polymethyl methacrylate (PMMA), polycarbonate (PC), and cyclic olefin copolymer (COC) have gained traction as alternatives, offering better mass production capabilities through injection molding or hot embossing.
Recent advances have introduced paper-based microfluidic devices as low-cost alternatives for point-of-care diagnostics, though their resolution limitations and structural integrity issues prevent broader adoption. Hydrogel-based materials show promise for tissue engineering applications but face challenges in standardization and long-term stability.
The primary technical barriers in current microfluidic materials center around biocompatibility issues. Many materials exhibit surface properties that can trigger protein adsorption, cell adhesion, or immune responses when in contact with biological samples. Surface modification techniques like plasma treatment or chemical functionalization offer partial solutions but often provide only temporary improvements.
Manufacturing scalability presents another significant challenge. Laboratory-scale fabrication methods frequently fail to translate effectively to mass production, creating a substantial gap between research prototypes and commercially viable products. This is particularly evident with complex multi-material chips that require precise alignment and bonding.
Long-term stability remains problematic for many biocompatible materials, with issues including leaching of additives, surface degradation upon exposure to biological fluids, and changes in mechanical properties over time. These factors can compromise experimental reproducibility and device reliability in clinical settings.
Integration capabilities also pose technical barriers, as many biocompatible materials are difficult to interface with electronic components, sensors, or external equipment. This limitation hinders the development of fully integrated lab-on-chip systems capable of sample preparation, analysis, and detection within a single platform.
Regulatory hurdles further complicate material selection, with stringent requirements for materials used in diagnostic or therapeutic applications. The lack of standardized testing protocols specifically designed for microfluidic materials creates additional uncertainty in the development pipeline.
Existing Material Solutions for Microfluidic Chips
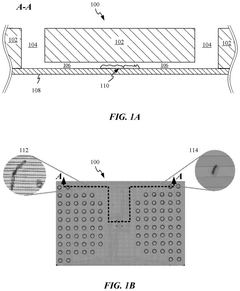
01 Materials for bio-compatible microfluidic chips
Various materials can be used to create bio-compatible microfluidic chips, including polymers, glass, and silicon. These materials are selected for their biocompatibility, optical transparency, and chemical resistance. Polymer-based materials like PDMS (polydimethylsiloxane) are particularly popular due to their flexibility, gas permeability, and ease of fabrication. Surface modifications can be applied to enhance biocompatibility and prevent non-specific protein adsorption, making these chips suitable for biological applications.- Materials for bio-compatible microfluidic chips: Various materials can be used to create bio-compatible microfluidic chips, including polymers, glass, and silicon. These materials are selected for their biocompatibility, optical transparency, and chemical resistance. Polymer-based materials like PDMS (polydimethylsiloxane) are particularly popular due to their flexibility, gas permeability, and ease of fabrication. Surface modifications can be applied to enhance biocompatibility and prevent non-specific protein adsorption, which is crucial for biological applications.
- Cell culture and analysis in microfluidic devices: Microfluidic chips can be designed for cell culture and analysis applications, providing controlled microenvironments that mimic in vivo conditions. These devices allow for precise control of nutrient delivery, waste removal, and environmental parameters. They can incorporate features for cell trapping, sorting, and long-term culture. Advanced designs may include integrated sensors for real-time monitoring of cellular responses, enabling high-throughput screening and personalized medicine applications.
- Organ-on-chip and tissue engineering applications: Bio-compatible microfluidic chips can be engineered to recreate the structure and function of human organs, known as organ-on-chip technology. These systems incorporate multiple cell types arranged in physiologically relevant architectures with appropriate mechanical and biochemical cues. They enable the study of organ-specific functions, disease models, and drug responses in a controlled environment. Such platforms reduce the need for animal testing and provide more relevant human data for pharmaceutical development.
- Diagnostic and point-of-care applications: Microfluidic chips are being developed for diagnostic and point-of-care testing applications, offering rapid, sensitive, and portable analysis of biological samples. These devices can integrate sample preparation, reaction, and detection steps into a single platform. They utilize various detection methods including optical, electrochemical, and mechanical sensing. The bio-compatible nature of these chips ensures accurate results when handling blood, saliva, urine, or other biological fluids, making them valuable tools for healthcare in resource-limited settings.
- Fabrication techniques for bio-compatible microfluidic chips: Various fabrication techniques are employed to create bio-compatible microfluidic chips, including soft lithography, injection molding, hot embossing, and 3D printing. These methods allow for precise control over channel dimensions, surface properties, and integration of functional elements. Advanced fabrication approaches enable the creation of complex 3D structures, gradient generators, and integrated valves and pumps. The selection of appropriate fabrication techniques is crucial for ensuring biocompatibility while maintaining the desired functionality and cost-effectiveness of the final device.
02 Cell culture and tissue engineering applications
Bio-compatible microfluidic chips enable the culture of cells in controlled microenvironments that better mimic in vivo conditions. These platforms support the growth of various cell types, including stem cells, and can be used to create organ-on-chip models. The chips provide precise control over nutrient delivery, waste removal, and mechanical stimuli, allowing for the study of cell behavior, tissue development, and disease progression. They also facilitate high-throughput screening of drugs and toxicity testing with reduced sample volumes.Expand Specific Solutions03 Diagnostic and analytical applications
Microfluidic chips are employed in various diagnostic and analytical applications, including point-of-care testing, disease detection, and biomarker analysis. These chips enable rapid and sensitive detection of pathogens, proteins, nucleic acids, and other biomarkers using minimal sample volumes. Integration with detection systems such as optical, electrochemical, or mass spectrometry methods enhances their analytical capabilities. The miniaturized format allows for portable diagnostic devices suitable for resource-limited settings.Expand Specific Solutions04 Fabrication techniques for microfluidic devices
Various fabrication techniques are employed to create bio-compatible microfluidic chips, including soft lithography, hot embossing, injection molding, and 3D printing. These methods allow for the creation of complex channel geometries, valves, mixers, and other functional elements at the microscale. Advanced manufacturing approaches enable multi-layer integration, incorporation of sensors, and creation of gradient generators. Surface treatment processes can be applied to modify the chemical properties of channel surfaces to enhance biocompatibility.Expand Specific Solutions05 Integration with sensing and control systems
Bio-compatible microfluidic chips can be integrated with various sensing and control systems to monitor and regulate the microenvironment. These include temperature controllers, pH sensors, oxygen sensors, and pressure regulators. Integration with optical systems enables real-time imaging and fluorescence-based detection. Electrical components such as electrodes can be incorporated for electrochemical sensing or cell stimulation. These integrated systems provide comprehensive platforms for complex biological studies and automated analysis.Expand Specific Solutions
Industry Leaders in Biocompatible Microfluidics
The microfluidic chip materials market is currently in a growth phase, with increasing demand for biocompatible materials driving innovation. The global market size for microfluidic devices is expanding rapidly, projected to reach significant value as applications in healthcare, diagnostics, and research proliferate. Technical maturity varies across material types, with established players like ANDE Corp. and MGI Tech leading commercial applications, while academic institutions including Tsinghua University, Fudan University, and Cornell University focus on fundamental research. Research organizations such as Agency for Science, Technology & Research and Korea Research Institute of Bioscience & Biotechnology bridge the gap between academic innovation and industrial implementation. The competitive landscape features collaboration between commercial entities developing proprietary materials and academic institutions advancing fundamental understanding of biocompatibility challenges.
Fudan University
Technical Solution: Fudan University has developed cutting-edge biocompatible materials for microfluidic applications through their State Key Laboratory of Molecular Engineering of Polymers. Their research centers on photopatternable hydrogel systems that enable precise 3D microstructuring while maintaining excellent biocompatibility. Fudan's approach incorporates biomimetic phospholipid polymers that create ultra-low fouling surfaces, dramatically reducing protein adsorption and cell adhesion in long-term applications. Their materials innovation includes stimuli-responsive block copolymers that can dynamically alter surface properties in response to pH, temperature, or electrical signals, enabling smart microfluidic functions. Fudan researchers have pioneered nanocomposite materials combining biocompatible polymers with functionalized nanoparticles that provide enhanced mechanical properties and introduce novel functionalities like magnetic manipulation or localized heating. A significant advancement is their development of self-assembling peptide-based microfluidic materials that can form complex channel architectures under physiological conditions without harsh fabrication processes.
Strengths: Exceptional resistance to biofouling; excellent optical transparency for real-time imaging; sophisticated stimuli-responsive capabilities for dynamic applications. Weaknesses: Higher material costs compared to traditional polymers; some formulations have limited shelf stability; complex fabrication protocols may limit widespread adoption.
Tsinghua University
Technical Solution: Tsinghua University has developed innovative biocompatible materials for microfluidic applications through their Department of Biomedical Engineering. Their research focuses on cellulose nanocrystal-reinforced hydrogel composites that combine excellent mechanical properties with superior biocompatibility. Tsinghua's approach incorporates graphene oxide-modified polymers that enhance electrical conductivity while maintaining biocompatibility, enabling integrated sensing capabilities within microfluidic channels. Their materials platform features temperature-responsive copolymers that undergo reversible phase transitions, allowing for dynamic control of surface properties and flow characteristics. Tsinghua researchers have pioneered antimicrobial microfluidic materials incorporating silver nanoparticles in a controlled-release matrix, preventing bacterial colonization without compromising mammalian cell compatibility. A significant innovation is their development of biodegradable silk fibroin-based microfluidic chips with tunable degradation rates, allowing for implantable diagnostic applications with predetermined functional lifespans.
Strengths: Excellent mechanical stability under physiological conditions; superior antimicrobial properties without cytotoxicity; innovative biodegradable options for implantable applications. Weaknesses: Some materials require specialized fabrication equipment; higher production costs compared to traditional polymers; limited long-term stability data for newer material formulations.
Key Innovations in Biocompatible Material Science
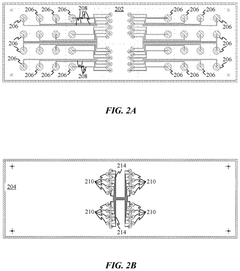
Active composite materials for micro-fluidic and NANO- fluidic devices
PatentPendingCA3098046A1
Innovation
- Development of composite materials combining an active moiety with a structural material, such as polydimethylsiloxane (PDMS), where the active material is dispersed within the passive material, incorporating electrically conductive and non-conductive polymer additives, microparticles, or nanoparticles to enhance sensitivity and interaction with analytes.
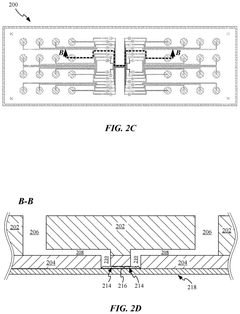
Microfluidic chip
PatentPendingUS20250276313A1
Innovation
- A microfluidic chip design with a separation between a loading/unloading layer and a sample interface layer minimizes exposure area, using a vacuum or pressure gradient to deliver reagents only to the sample region, reducing contact with non-sample areas and substrate coatings.
Regulatory Framework for Medical-Grade Materials
The regulatory landscape for medical-grade materials used in microfluidic chips is complex and constantly evolving, requiring manufacturers to navigate multiple jurisdictional frameworks. In the United States, the FDA's Center for Devices and Radiological Health (CDRH) oversees the approval process for medical devices incorporating microfluidic technology, with specific requirements outlined in the 21 CFR Part 820 Quality System Regulation. Materials must demonstrate biocompatibility according to ISO 10993 standards, which include cytotoxicity, sensitization, and hemocompatibility testing when blood contact is involved.
The European Union has implemented the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), which impose stricter requirements for clinical evidence and post-market surveillance. These regulations specifically address the chemical composition and leaching potential of materials used in medical applications, with particular attention to microfluidic devices that may contain novel polymers or surface modifications.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory pathways for biocompatible materials, often requiring additional local testing despite international harmonization efforts. This creates significant challenges for global manufacturers seeking multi-market approvals for innovative microfluidic materials.
Regulatory bodies are increasingly focusing on endocrine-disrupting chemicals (EDCs) and per- and polyfluoroalkyl substances (PFAS) that may be present in certain polymers used for microfluidic applications. The EPA and European Chemicals Agency (ECHA) have implemented restrictions that impact material selection for biomedical devices, requiring manufacturers to develop alternative formulations that maintain performance while eliminating substances of concern.
Emerging regulatory trends include the implementation of Unique Device Identification (UDI) systems that trace materials throughout the supply chain, and the development of regulatory science initiatives focused specifically on nanotechnology and advanced materials. The FDA's Emerging Technology Program and the EU's Innovation Network provide pathways for early regulatory engagement when developing novel biocompatible materials for microfluidic applications.
International standards organizations, including ASTM International and the International Organization for Standardization (ISO), continue to develop specialized testing protocols for microfluidic materials. The recently updated ISO 18562 series addresses the biocompatibility evaluation of breathing gas pathways in healthcare applications, providing valuable frameworks that can be adapted for microfluidic contexts where gas exchange may occur.
The European Union has implemented the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746), which impose stricter requirements for clinical evidence and post-market surveillance. These regulations specifically address the chemical composition and leaching potential of materials used in medical applications, with particular attention to microfluidic devices that may contain novel polymers or surface modifications.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory pathways for biocompatible materials, often requiring additional local testing despite international harmonization efforts. This creates significant challenges for global manufacturers seeking multi-market approvals for innovative microfluidic materials.
Regulatory bodies are increasingly focusing on endocrine-disrupting chemicals (EDCs) and per- and polyfluoroalkyl substances (PFAS) that may be present in certain polymers used for microfluidic applications. The EPA and European Chemicals Agency (ECHA) have implemented restrictions that impact material selection for biomedical devices, requiring manufacturers to develop alternative formulations that maintain performance while eliminating substances of concern.
Emerging regulatory trends include the implementation of Unique Device Identification (UDI) systems that trace materials throughout the supply chain, and the development of regulatory science initiatives focused specifically on nanotechnology and advanced materials. The FDA's Emerging Technology Program and the EU's Innovation Network provide pathways for early regulatory engagement when developing novel biocompatible materials for microfluidic applications.
International standards organizations, including ASTM International and the International Organization for Standardization (ISO), continue to develop specialized testing protocols for microfluidic materials. The recently updated ISO 18562 series addresses the biocompatibility evaluation of breathing gas pathways in healthcare applications, providing valuable frameworks that can be adapted for microfluidic contexts where gas exchange may occur.
Sustainability Aspects of New Microfluidic Materials
The environmental impact of microfluidic chip materials has become increasingly important as the field expands. Traditional microfluidic devices often rely on materials like polydimethylsiloxane (PDMS) and other polymers that present significant end-of-life challenges. Recent sustainability assessments reveal that many conventional materials used in microfluidic applications have poor biodegradability profiles and contribute to persistent waste streams in laboratory and clinical settings.
Emerging bio-compatible materials for microfluidic applications are being developed with sustainability as a core design principle. Paper-based microfluidic platforms represent a significant advancement, offering biodegradable alternatives that maintain necessary biocompatibility while dramatically reducing environmental footprint. Similarly, cellulose-derived materials and other plant-based polymers are showing promise as renewable alternatives to petroleum-based polymers traditionally used in chip fabrication.
Life cycle assessment (LCA) studies of new microfluidic materials indicate potential for substantial reductions in carbon footprint. Bio-derived polymers like polylactic acid (PLA) and polyhydroxyalkanoates (PHAs) demonstrate up to 65% lower greenhouse gas emissions during production compared to conventional materials, while maintaining the necessary mechanical and surface properties for microfluidic applications.
Water consumption represents another critical sustainability metric for microfluidic materials. Manufacturing processes for traditional chip materials can be water-intensive, whereas some newer bio-compatible alternatives require significantly less water during production. Additionally, certain hydrogel-based materials are being engineered to be reprocessed with minimal water requirements, further enhancing their sustainability profile.
Chemical safety considerations extend beyond the use phase to end-of-life management. New materials are being designed to decompose into non-toxic components, eliminating the leaching of potentially harmful substances that occurs with some conventional microfluidic materials. This represents a dual benefit for both environmental sustainability and long-term biocompatibility.
Resource efficiency is being addressed through the development of microfluidic materials that can be produced using less energy-intensive processes. Ambient temperature curing systems and UV-initiated polymerization methods reduce the energy requirements compared to traditional thermal curing approaches, while maintaining or even enhancing the bio-compatibility of the resulting materials.
Circular economy principles are increasingly being applied to microfluidic material development. Research into recyclable and reusable bio-compatible materials is advancing, with some promising candidates allowing for the recovery and reprocessing of materials after their initial use cycle. This approach significantly extends the useful life of the materials and reduces waste generation in research and clinical applications.
Emerging bio-compatible materials for microfluidic applications are being developed with sustainability as a core design principle. Paper-based microfluidic platforms represent a significant advancement, offering biodegradable alternatives that maintain necessary biocompatibility while dramatically reducing environmental footprint. Similarly, cellulose-derived materials and other plant-based polymers are showing promise as renewable alternatives to petroleum-based polymers traditionally used in chip fabrication.
Life cycle assessment (LCA) studies of new microfluidic materials indicate potential for substantial reductions in carbon footprint. Bio-derived polymers like polylactic acid (PLA) and polyhydroxyalkanoates (PHAs) demonstrate up to 65% lower greenhouse gas emissions during production compared to conventional materials, while maintaining the necessary mechanical and surface properties for microfluidic applications.
Water consumption represents another critical sustainability metric for microfluidic materials. Manufacturing processes for traditional chip materials can be water-intensive, whereas some newer bio-compatible alternatives require significantly less water during production. Additionally, certain hydrogel-based materials are being engineered to be reprocessed with minimal water requirements, further enhancing their sustainability profile.
Chemical safety considerations extend beyond the use phase to end-of-life management. New materials are being designed to decompose into non-toxic components, eliminating the leaching of potentially harmful substances that occurs with some conventional microfluidic materials. This represents a dual benefit for both environmental sustainability and long-term biocompatibility.
Resource efficiency is being addressed through the development of microfluidic materials that can be produced using less energy-intensive processes. Ambient temperature curing systems and UV-initiated polymerization methods reduce the energy requirements compared to traditional thermal curing approaches, while maintaining or even enhancing the bio-compatibility of the resulting materials.
Circular economy principles are increasingly being applied to microfluidic material development. Research into recyclable and reusable bio-compatible materials is advancing, with some promising candidates allowing for the recovery and reprocessing of materials after their initial use cycle. This approach significantly extends the useful life of the materials and reduces waste generation in research and clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!