How Are Microfluidic Chips Implemented in Microbial Testing?
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Technology Evolution and Testing Objectives
Microfluidic technology has evolved significantly over the past three decades, transforming from simple capillary-based systems to sophisticated lab-on-a-chip platforms. The journey began in the 1990s with the development of basic microfluidic channels etched in glass and silicon substrates, primarily for analytical chemistry applications. By the early 2000s, the introduction of polydimethylsiloxane (PDMS) as a fabrication material revolutionized the field, enabling rapid prototyping and more complex designs at reduced costs.
The evolution accelerated with the integration of various functional components such as micropumps, microvalves, and micromixers, allowing for precise fluid manipulation at the microscale. Recent advancements have seen the incorporation of biosensors, electrochemical detectors, and optical systems directly into microfluidic platforms, creating truly integrated analytical devices capable of sample-to-answer testing.
In the context of microbial testing, microfluidic technology has progressed from simple bacterial culture chambers to comprehensive systems capable of automated sample preparation, DNA amplification, and pathogen detection. The miniaturization of conventional laboratory processes has enabled faster analysis times, reduced reagent consumption, and enhanced sensitivity compared to traditional microbiology methods.
The primary objectives of implementing microfluidic chips in microbial testing include achieving rapid detection of pathogens in various matrices, from clinical samples to food and environmental specimens. Current targets aim for detection limits below 100 CFU/mL with analysis times under 60 minutes, representing significant improvements over conventional culture-based methods that typically require 24-72 hours.
Another critical objective is the development of point-of-care testing capabilities, allowing for decentralized microbial analysis in resource-limited settings. This includes creating robust, user-friendly devices that maintain analytical performance without requiring specialized laboratory infrastructure or trained personnel.
Automation represents a key technological goal, with efforts focused on integrating all testing steps—from sample introduction to result reporting—into a single microfluidic platform. This "sample-in, answer-out" paradigm aims to minimize human intervention and reduce the risk of contamination or operator error.
Multiplexing capabilities constitute another important objective, with current research targeting simultaneous detection of multiple pathogens or antimicrobial resistance markers from a single sample. Advanced microfluidic designs incorporating multiple detection chambers, reagent reservoirs, and detection modalities are being developed to address this need.
The trend toward digital microfluidics and droplet-based systems represents the cutting edge of the field, offering unprecedented control over sample partitioning and enabling absolute quantification of microbial loads through digital PCR and similar techniques. These approaches are particularly valuable for detecting low-abundance pathogens in complex biological matrices.
The evolution accelerated with the integration of various functional components such as micropumps, microvalves, and micromixers, allowing for precise fluid manipulation at the microscale. Recent advancements have seen the incorporation of biosensors, electrochemical detectors, and optical systems directly into microfluidic platforms, creating truly integrated analytical devices capable of sample-to-answer testing.
In the context of microbial testing, microfluidic technology has progressed from simple bacterial culture chambers to comprehensive systems capable of automated sample preparation, DNA amplification, and pathogen detection. The miniaturization of conventional laboratory processes has enabled faster analysis times, reduced reagent consumption, and enhanced sensitivity compared to traditional microbiology methods.
The primary objectives of implementing microfluidic chips in microbial testing include achieving rapid detection of pathogens in various matrices, from clinical samples to food and environmental specimens. Current targets aim for detection limits below 100 CFU/mL with analysis times under 60 minutes, representing significant improvements over conventional culture-based methods that typically require 24-72 hours.
Another critical objective is the development of point-of-care testing capabilities, allowing for decentralized microbial analysis in resource-limited settings. This includes creating robust, user-friendly devices that maintain analytical performance without requiring specialized laboratory infrastructure or trained personnel.
Automation represents a key technological goal, with efforts focused on integrating all testing steps—from sample introduction to result reporting—into a single microfluidic platform. This "sample-in, answer-out" paradigm aims to minimize human intervention and reduce the risk of contamination or operator error.
Multiplexing capabilities constitute another important objective, with current research targeting simultaneous detection of multiple pathogens or antimicrobial resistance markers from a single sample. Advanced microfluidic designs incorporating multiple detection chambers, reagent reservoirs, and detection modalities are being developed to address this need.
The trend toward digital microfluidics and droplet-based systems represents the cutting edge of the field, offering unprecedented control over sample partitioning and enabling absolute quantification of microbial loads through digital PCR and similar techniques. These approaches are particularly valuable for detecting low-abundance pathogens in complex biological matrices.
Market Analysis for Microfluidic-Based Microbial Detection
The global market for microfluidic-based microbial detection is experiencing robust growth, driven by increasing demand for rapid, accurate, and portable testing solutions across multiple industries. Current market valuations place this sector at approximately 3.2 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 11.7% through 2030, potentially reaching 7.1 billion USD by the end of the forecast period.
Healthcare applications represent the largest market segment, accounting for nearly 45% of the total market share. Within this segment, clinical diagnostics and point-of-care testing demonstrate particularly strong growth trajectories as healthcare systems worldwide seek to decentralize testing capabilities and reduce time-to-result for critical microbial analyses.
Food safety testing constitutes the second-largest application segment, representing approximately 28% of the market. Increasing regulatory requirements for pathogen detection in food production and processing facilities have accelerated adoption of microfluidic technologies that can detect contaminants like E. coli, Salmonella, and Listeria with greater sensitivity and speed than traditional culture-based methods.
Environmental monitoring applications, including water quality testing and industrial contamination assessment, comprise about 15% of the current market but show the highest growth potential at 13.2% CAGR through 2030. This growth is primarily driven by stricter environmental regulations and increasing public concern regarding water safety and industrial pollution.
Regionally, North America dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate at 14.3% CAGR, primarily due to expanding healthcare infrastructure, increasing food safety concerns, and growing industrial base in countries like China, India, and South Korea.
Key market drivers include increasing incidence of foodborne and waterborne diseases, growing demand for point-of-care diagnostics, technological advancements in microfluidic chip design, and rising adoption of automated testing systems in various industries. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic capabilities and decentralized testing infrastructure.
Market challenges include relatively high initial investment costs, technical complexity in chip design and manufacturing, and the need for specialized training for end-users. Additionally, standardization issues and regulatory hurdles present significant barriers to market entry, particularly for smaller companies and startups developing novel microfluidic solutions for microbial detection.
Healthcare applications represent the largest market segment, accounting for nearly 45% of the total market share. Within this segment, clinical diagnostics and point-of-care testing demonstrate particularly strong growth trajectories as healthcare systems worldwide seek to decentralize testing capabilities and reduce time-to-result for critical microbial analyses.
Food safety testing constitutes the second-largest application segment, representing approximately 28% of the market. Increasing regulatory requirements for pathogen detection in food production and processing facilities have accelerated adoption of microfluidic technologies that can detect contaminants like E. coli, Salmonella, and Listeria with greater sensitivity and speed than traditional culture-based methods.
Environmental monitoring applications, including water quality testing and industrial contamination assessment, comprise about 15% of the current market but show the highest growth potential at 13.2% CAGR through 2030. This growth is primarily driven by stricter environmental regulations and increasing public concern regarding water safety and industrial pollution.
Regionally, North America dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate at 14.3% CAGR, primarily due to expanding healthcare infrastructure, increasing food safety concerns, and growing industrial base in countries like China, India, and South Korea.
Key market drivers include increasing incidence of foodborne and waterborne diseases, growing demand for point-of-care diagnostics, technological advancements in microfluidic chip design, and rising adoption of automated testing systems in various industries. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic capabilities and decentralized testing infrastructure.
Market challenges include relatively high initial investment costs, technical complexity in chip design and manufacturing, and the need for specialized training for end-users. Additionally, standardization issues and regulatory hurdles present significant barriers to market entry, particularly for smaller companies and startups developing novel microfluidic solutions for microbial detection.
Current Challenges in Microfluidic Chip Microbial Testing
Despite the significant advancements in microfluidic chip technology for microbial testing, several critical challenges continue to impede widespread adoption and optimal performance. One of the primary obstacles remains the standardization of fabrication processes. The variability in manufacturing techniques leads to inconsistent chip performance across different production batches, making quality control and result reproducibility problematic for commercial applications.
Sample preparation represents another substantial hurdle in microfluidic microbial testing. Complex biological samples often contain particulates, proteins, and other components that can cause channel clogging or interfere with detection mechanisms. Current filtration and sample preparation techniques integrated into microfluidic platforms still struggle with efficiently processing heterogeneous samples without compromising microbial viability or detection sensitivity.
Detection sensitivity limitations persist as a significant challenge, particularly when dealing with low microbial concentrations in clinical or environmental samples. While PCR-based methods have improved detection thresholds, they often require complex chip designs and precise temperature control systems that increase device complexity and cost. Alternative detection methods such as impedance measurement or optical detection face sensitivity constraints when dealing with samples containing fewer than 100 CFU/mL.
Integration challenges between microfluidic components and external detection systems continue to hinder the development of truly portable, point-of-care microbial testing devices. The interface between microchannels and sensors, pumps, or other external equipment often creates dead volumes, bubble formation, or connection failures that compromise system reliability and user-friendliness.
Biomolecule adsorption onto chip surfaces presents another persistent issue. Microbial cells and proteins tend to adhere to channel walls, particularly in polymer-based chips, leading to cross-contamination between samples and reduced detection accuracy. Current surface modification techniques provide only partial solutions and often add complexity to the manufacturing process.
Scaling production while maintaining cost-effectiveness remains problematic for commercial implementation. Many microfluidic chip designs that perform well in laboratory settings utilize fabrication techniques that are difficult to scale for mass production without significant cost increases. This creates a barrier to widespread adoption in resource-limited settings where microbial testing is most needed.
Regulatory challenges further complicate the path to market for microfluidic microbial testing platforms. The novel nature of these technologies often means they don't fit neatly into existing regulatory frameworks, creating uncertainty in validation requirements and approval pathways. This regulatory ambiguity discourages investment and slows commercialization efforts despite promising technical performance.
Sample preparation represents another substantial hurdle in microfluidic microbial testing. Complex biological samples often contain particulates, proteins, and other components that can cause channel clogging or interfere with detection mechanisms. Current filtration and sample preparation techniques integrated into microfluidic platforms still struggle with efficiently processing heterogeneous samples without compromising microbial viability or detection sensitivity.
Detection sensitivity limitations persist as a significant challenge, particularly when dealing with low microbial concentrations in clinical or environmental samples. While PCR-based methods have improved detection thresholds, they often require complex chip designs and precise temperature control systems that increase device complexity and cost. Alternative detection methods such as impedance measurement or optical detection face sensitivity constraints when dealing with samples containing fewer than 100 CFU/mL.
Integration challenges between microfluidic components and external detection systems continue to hinder the development of truly portable, point-of-care microbial testing devices. The interface between microchannels and sensors, pumps, or other external equipment often creates dead volumes, bubble formation, or connection failures that compromise system reliability and user-friendliness.
Biomolecule adsorption onto chip surfaces presents another persistent issue. Microbial cells and proteins tend to adhere to channel walls, particularly in polymer-based chips, leading to cross-contamination between samples and reduced detection accuracy. Current surface modification techniques provide only partial solutions and often add complexity to the manufacturing process.
Scaling production while maintaining cost-effectiveness remains problematic for commercial implementation. Many microfluidic chip designs that perform well in laboratory settings utilize fabrication techniques that are difficult to scale for mass production without significant cost increases. This creates a barrier to widespread adoption in resource-limited settings where microbial testing is most needed.
Regulatory challenges further complicate the path to market for microfluidic microbial testing platforms. The novel nature of these technologies often means they don't fit neatly into existing regulatory frameworks, creating uncertainty in validation requirements and approval pathways. This regulatory ambiguity discourages investment and slows commercialization efforts despite promising technical performance.
Current Microfluidic Chip Designs for Microbial Detection
01 Fabrication techniques for microfluidic chips
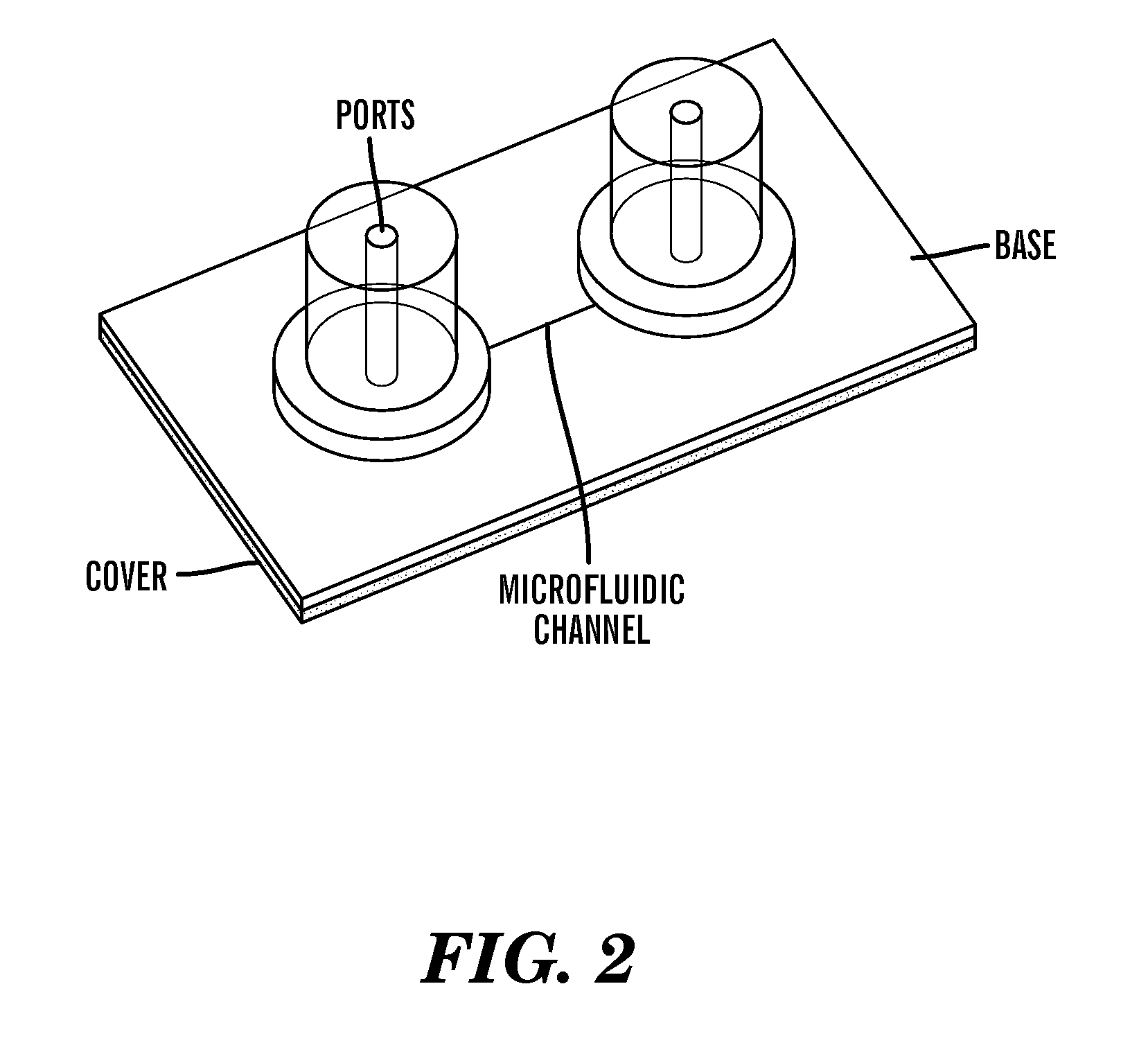
Various fabrication methods are employed to create microfluidic chips with precise channel geometries and surface properties. These techniques include soft lithography, injection molding, hot embossing, and 3D printing. The choice of fabrication method depends on the desired application, material properties, and production scale. Advanced fabrication techniques allow for the creation of complex microstructures with high precision, enabling efficient fluid handling and analysis.- Fabrication and manufacturing of microfluidic chips: Various techniques and methods are employed in the fabrication of microfluidic chips, including lithography, etching, and bonding processes. These manufacturing approaches enable the creation of precise microchannels, chambers, and other structures necessary for fluid manipulation at the microscale. Advanced fabrication methods allow for the integration of multiple functional elements on a single chip, enhancing their versatility and application range.
- Microfluidic chips for biological and medical applications: Microfluidic chips are extensively used in biological and medical fields for applications such as cell culture, DNA analysis, and diagnostic testing. These chips enable precise control over small sample volumes, reducing reagent consumption and enhancing sensitivity. They facilitate rapid analysis of biological samples, making them valuable tools for point-of-care diagnostics, drug discovery, and personalized medicine approaches.
- Integration of sensors and detection systems in microfluidic chips: Modern microfluidic chips incorporate various sensing and detection technologies to monitor and analyze samples in real-time. These integrated systems may include optical, electrochemical, or mechanical sensors that enable quantitative measurements of biological or chemical parameters. The integration of detection systems directly onto microfluidic platforms enhances analytical capabilities while maintaining the compact nature of these devices.
- Droplet-based microfluidic systems: Droplet-based microfluidics involves the generation and manipulation of discrete droplets within microfluidic channels. This approach enables compartmentalization of reactions, high-throughput screening, and digital analysis of samples. Droplet generation, merging, splitting, and sorting are key operations in these systems, allowing for precise control over reaction conditions and sample processing at extremely small volumes.
- Novel materials and design innovations for microfluidic chips: Research in microfluidic technology has led to the development of novel materials and innovative designs that enhance chip performance and functionality. These advancements include the use of flexible polymers, paper-based substrates, and hybrid material systems that offer advantages such as reduced cost, improved biocompatibility, or enhanced durability. New design approaches also focus on creating reconfigurable systems, 3D microfluidic networks, and chips with integrated valves and pumps for more sophisticated fluid handling capabilities.
02 Integration of sensing and detection systems
Microfluidic chips can be integrated with various sensing and detection systems to enable real-time monitoring and analysis of samples. These systems include optical sensors, electrochemical detectors, and spectroscopic instruments that can detect and quantify analytes within the microfluidic channels. The integration of these detection systems enhances the functionality of microfluidic chips, allowing for automated and high-throughput analysis in applications such as diagnostics, environmental monitoring, and drug discovery.Expand Specific Solutions03 Microfluidic chip designs for biological applications
Specialized microfluidic chip designs have been developed for various biological applications, including cell culture, DNA analysis, protein separation, and organ-on-a-chip systems. These designs incorporate features such as cell trapping structures, gradient generators, and mixing chambers to mimic physiological conditions and enable complex biological studies. The miniaturized format of these chips allows for reduced sample volumes, faster reaction times, and more controlled experimental conditions compared to traditional methods.Expand Specific Solutions04 Flow control and manipulation in microfluidic systems
Various mechanisms are employed to control and manipulate fluid flow within microfluidic chips, including valves, pumps, and mixers. These components enable precise control over sample movement, reagent mixing, and reaction timing. Advanced flow control systems utilize pneumatic, hydraulic, or electrokinetic principles to achieve automated and programmable fluid handling. Effective flow control is essential for applications requiring precise timing, sequential operations, or gradient formation within the microfluidic environment.Expand Specific Solutions05 Materials and surface modifications for microfluidic chips
The choice of materials and surface modifications significantly impacts the performance of microfluidic chips. Common materials include glass, silicon, and various polymers such as PDMS, PMMA, and COC, each offering different optical, mechanical, and chemical properties. Surface modifications can alter wettability, prevent non-specific adsorption, or introduce functional groups for biomolecule immobilization. These material considerations are crucial for optimizing chip performance in specific applications and ensuring compatibility with biological samples and detection methods.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
Microfluidic chip implementation in microbial testing is currently in a growth phase, with the market expanding rapidly due to increasing demand for rapid, point-of-care diagnostic solutions. The technology is approaching maturity with several key players advancing commercial applications. Companies like Pattern Bioscience and Lansion Biotechnology are pioneering innovative platforms that significantly reduce testing time compared to traditional culture methods. Academic institutions including National Cheng Kung University and Yale University are driving fundamental research, while established corporations such as Samsung Electronics and BOE Technology are leveraging their manufacturing expertise to scale production. The competitive landscape features both specialized biotech firms like Holosensor Medical and Loop Medical focusing on specific applications, alongside larger diagnostic companies like Siemens Healthcare Diagnostics integrating microfluidic capabilities into comprehensive testing systems.
Pattern Bioscience, Inc.
Technical Solution: Pattern Bioscience has pioneered a microfluidic-based rapid diagnostic platform specifically designed for antimicrobial susceptibility testing and pathogen identification. Their technology employs droplet microfluidics to encapsulate individual bacterial cells in nanoliter-sized droplets, creating thousands of isolated micro-reactors. Within these droplets, bacteria are exposed to various antibiotics while their growth and metabolic responses are continuously monitored using advanced imaging and phenotypic analysis techniques. The system utilizes machine learning algorithms to analyze bacterial growth patterns and determine susceptibility profiles within 4-8 hours, compared to 2-3 days with conventional methods[2]. Pattern's microfluidic chips incorporate multiple parallel testing channels with integrated sensors that detect subtle changes in bacterial metabolism, enabling rapid determination of minimum inhibitory concentrations (MICs) for various antibiotics simultaneously.
Strengths: Extremely rapid results (hours vs. days), ability to test multiple antibiotics simultaneously, and generation of phenotypic rather than genetic resistance data which correlates better with clinical outcomes. Weaknesses: Limited to culturable organisms and potential challenges in polymicrobial infections where multiple species may interact with antibiotics differently.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed an integrated microfluidic lab-on-a-chip platform for rapid microbial detection and analysis. Their system utilizes semiconductor manufacturing techniques to create high-precision microfluidic channels with integrated sensors. Samsung's technology employs dielectrophoresis (DEP) to manipulate and concentrate microbial cells within designated detection zones on the chip. The platform incorporates CMOS-based biosensors that can detect electrical impedance changes or optical signals when microorganisms interact with specific recognition elements immobilized within the microchannels[3]. For sample preparation, Samsung has implemented acoustic microfluidics that use ultrasonic standing waves to separate microbial cells from complex matrices. Their system achieves detection limits as low as 10-100 CFU/mL within 30-60 minutes, significantly faster than traditional culture methods which require 24-48 hours[4]. The chips include multiple testing chambers allowing simultaneous analysis of different pathogens from a single sample.
Strengths: Leverages Samsung's semiconductor manufacturing expertise for high-precision, mass-producible chips; achieves very low detection limits; and offers rapid results suitable for point-of-care applications. Weaknesses: May require specialized equipment for operation and analysis, potentially limiting deployment in resource-limited settings.
Key Patents and Innovations in Microfluidic Microbial Testing
Detection chip
PatentActiveUS11986821B2
Innovation
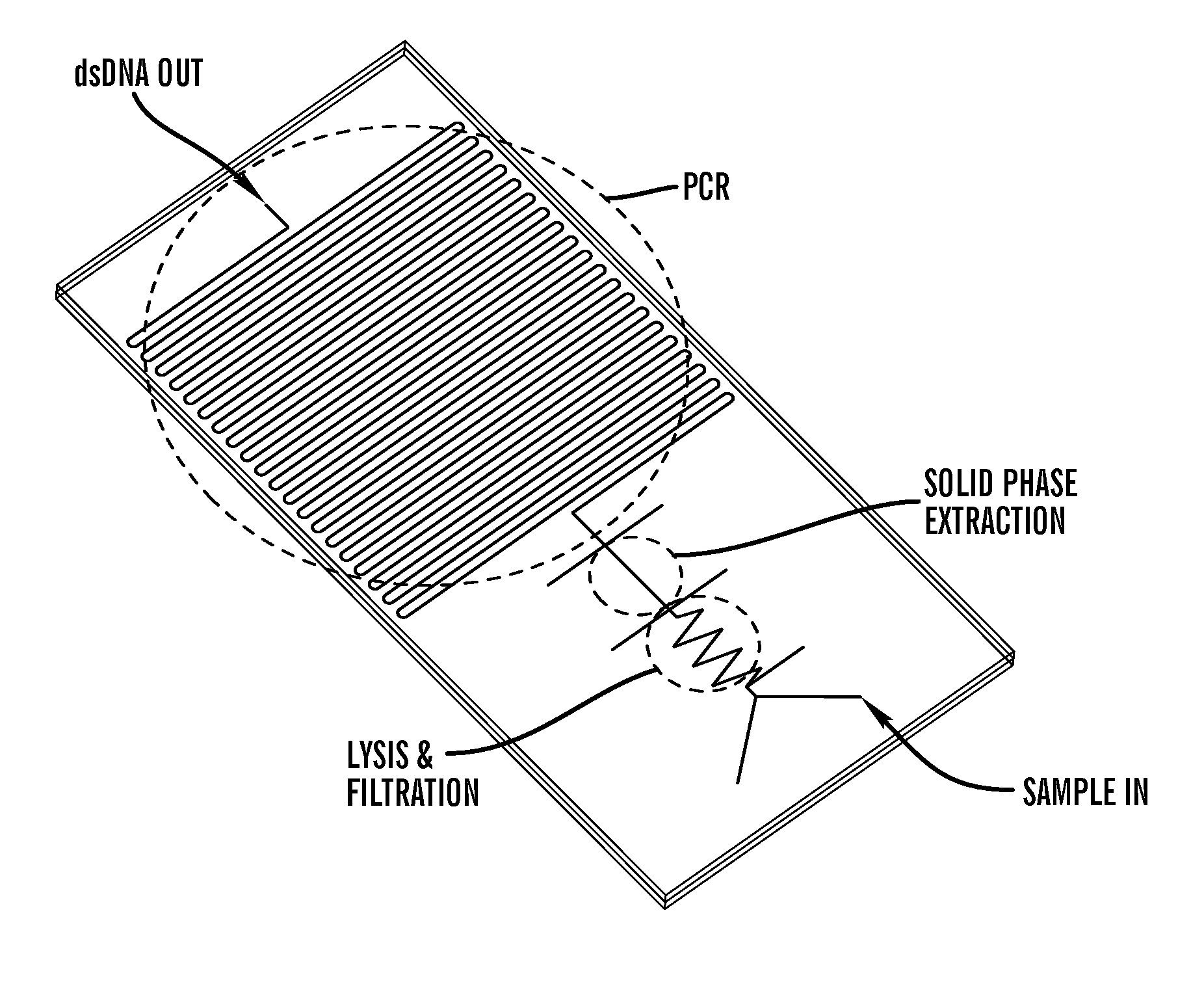
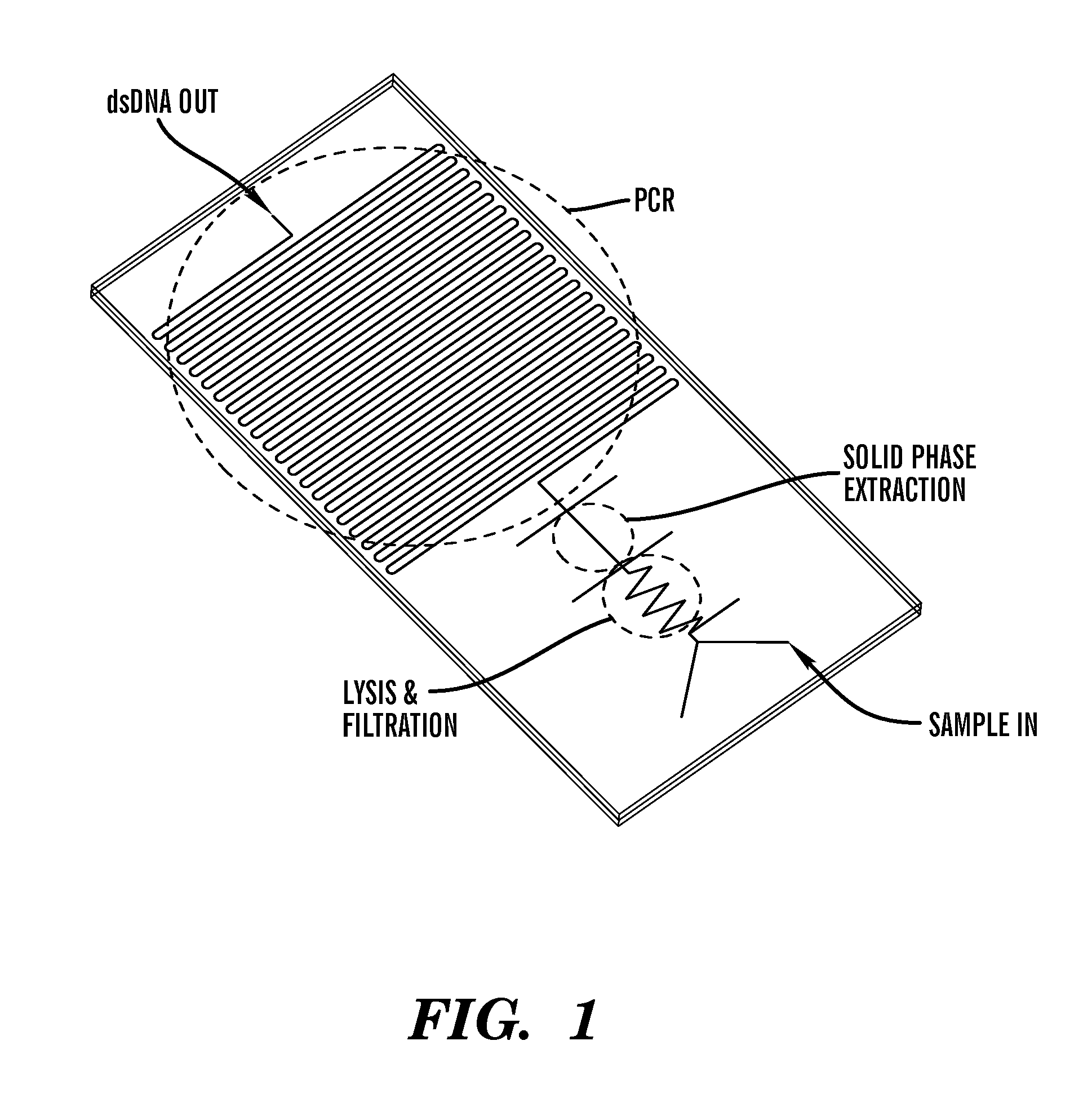
- A detection chip design featuring a sample injection structure, a filter structure with a unique inlet and outlet geometry, and a mixing structure, all connected through flow channels, which facilitates lateral flow filtration and prevents leakage by using a compressed filter film and optimized channel dimensions.
Method for bacterial lysis
PatentInactiveUS20100203521A1
Innovation
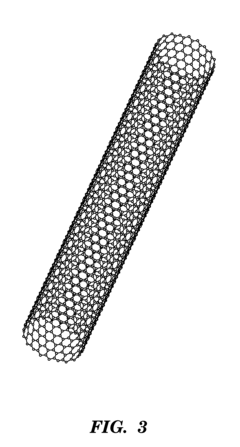
- A microfluidic chip with channels embedded with a porous polymer monolith containing carbon particles, such as carbon nanotubes, for mechanical-based cell lysis, allowing for rapid and efficient lysis of both gram-positive and gram-negative bacteria without the need for external chemicals or power sources, integrated with modules for subsequent biomolecule isolation and detection.
Standardization and Validation Protocols for Microfluidic Testing
The standardization and validation of microfluidic testing protocols represents a critical challenge in the widespread adoption of microfluidic technology for microbial testing applications. Currently, the field lacks comprehensive, universally accepted standards, which hampers reproducibility across different laboratories and impedes regulatory approval processes.
International organizations such as ISO (International Organization for Standardization) and ASTM International have begun developing preliminary guidelines for microfluidic device validation, though these remain in early stages compared to established laboratory methods. The FDA has also published guidance documents addressing aspects of microfluidic-based diagnostic validation, particularly focusing on analytical sensitivity, specificity, and reproducibility metrics.
Validation protocols for microfluidic microbial testing typically encompass several key parameters. Limit of detection (LOD) assessment requires establishing the minimum concentration of target microorganisms that can be reliably detected. Analytical specificity validation ensures the system can distinguish between target pathogens and similar non-target microorganisms. Reproducibility testing across different operators, instruments, and manufacturing lots is essential for establishing robust performance characteristics.
Reference standards present a particular challenge in microfluidic microbial testing. While conventional microbiology relies on established culture-based reference methods, microfluidic approaches often detect microbial presence through different mechanisms, creating comparison difficulties. Several approaches have emerged to address this challenge, including parallel testing with gold-standard methods and the development of certified reference materials specifically designed for microfluidic platforms.
Inter-laboratory validation studies have proven valuable in establishing protocol reliability. These collaborative efforts involve multiple facilities following identical protocols to verify consistent results across different settings. Such studies have been instrumental in identifying variables that affect test outcomes, including sample preparation techniques, environmental conditions, and operator training requirements.
Quality control measures represent another critical aspect of standardization. Regular system performance verification using control samples, calibration protocols, and system suitability tests ensures ongoing reliability. Documentation standards for these procedures are increasingly being formalized to support regulatory compliance and facilitate technology transfer between laboratories.
The emergence of digital microfluidics has introduced additional validation considerations related to droplet generation consistency, manipulation precision, and cross-contamination prevention. Specialized protocols addressing these unique aspects are being developed by industry consortia and academic research groups to establish standardized approaches for this rapidly evolving technology subset.
International organizations such as ISO (International Organization for Standardization) and ASTM International have begun developing preliminary guidelines for microfluidic device validation, though these remain in early stages compared to established laboratory methods. The FDA has also published guidance documents addressing aspects of microfluidic-based diagnostic validation, particularly focusing on analytical sensitivity, specificity, and reproducibility metrics.
Validation protocols for microfluidic microbial testing typically encompass several key parameters. Limit of detection (LOD) assessment requires establishing the minimum concentration of target microorganisms that can be reliably detected. Analytical specificity validation ensures the system can distinguish between target pathogens and similar non-target microorganisms. Reproducibility testing across different operators, instruments, and manufacturing lots is essential for establishing robust performance characteristics.
Reference standards present a particular challenge in microfluidic microbial testing. While conventional microbiology relies on established culture-based reference methods, microfluidic approaches often detect microbial presence through different mechanisms, creating comparison difficulties. Several approaches have emerged to address this challenge, including parallel testing with gold-standard methods and the development of certified reference materials specifically designed for microfluidic platforms.
Inter-laboratory validation studies have proven valuable in establishing protocol reliability. These collaborative efforts involve multiple facilities following identical protocols to verify consistent results across different settings. Such studies have been instrumental in identifying variables that affect test outcomes, including sample preparation techniques, environmental conditions, and operator training requirements.
Quality control measures represent another critical aspect of standardization. Regular system performance verification using control samples, calibration protocols, and system suitability tests ensures ongoing reliability. Documentation standards for these procedures are increasingly being formalized to support regulatory compliance and facilitate technology transfer between laboratories.
The emergence of digital microfluidics has introduced additional validation considerations related to droplet generation consistency, manipulation precision, and cross-contamination prevention. Specialized protocols addressing these unique aspects are being developed by industry consortia and academic research groups to establish standardized approaches for this rapidly evolving technology subset.
Integration with IoT and AI for Advanced Microbial Diagnostics
The convergence of microfluidic technology with Internet of Things (IoT) and Artificial Intelligence (AI) represents a transformative advancement in microbial diagnostics. This integration creates intelligent diagnostic systems capable of real-time monitoring, automated analysis, and predictive capabilities that far exceed traditional testing methods.
IoT connectivity enables microfluidic chips to transmit testing data instantaneously to cloud platforms, allowing for remote monitoring and analysis. This connectivity facilitates continuous surveillance of microbial populations in various environments, from hospital settings to food processing facilities, without requiring constant human intervention. The real-time data collection capabilities significantly reduce the time between sample collection and actionable results, enabling rapid response to potential pathogenic threats.
AI algorithms, particularly machine learning models, enhance the analytical capabilities of microfluidic systems by identifying patterns in complex microbial data that might be imperceptible to human analysts. These algorithms can be trained to recognize specific microbial signatures, predict antimicrobial resistance patterns, and even forecast potential outbreaks based on subtle changes in microbial populations. Deep learning approaches have demonstrated remarkable accuracy in classifying microorganisms from microscopic images captured within microfluidic channels.
The integration of edge computing with microfluidic devices allows for preliminary data processing directly at the testing site, reducing bandwidth requirements and enabling functionality in areas with limited connectivity. This distributed intelligence architecture supports deployment in resource-limited settings where traditional laboratory infrastructure is unavailable.
Advanced sensor fusion techniques combine data from multiple sensing modalities within microfluidic platforms, creating comprehensive microbial profiles that include genetic, metabolic, and morphological characteristics. This multi-parameter analysis significantly improves diagnostic accuracy and provides deeper insights into microbial behavior and pathogenicity.
Predictive analytics powered by AI can anticipate antimicrobial resistance development by analyzing subtle changes in microbial response patterns over time. This capability is particularly valuable for healthcare settings, where early identification of resistance emergence can guide appropriate therapeutic interventions and stewardship programs.
The development of digital twins for microfluidic testing systems enables virtual simulation and optimization of testing protocols before physical implementation. This approach accelerates the development cycle for new diagnostic applications and facilitates personalized testing strategies based on specific environmental or clinical contexts.
IoT connectivity enables microfluidic chips to transmit testing data instantaneously to cloud platforms, allowing for remote monitoring and analysis. This connectivity facilitates continuous surveillance of microbial populations in various environments, from hospital settings to food processing facilities, without requiring constant human intervention. The real-time data collection capabilities significantly reduce the time between sample collection and actionable results, enabling rapid response to potential pathogenic threats.
AI algorithms, particularly machine learning models, enhance the analytical capabilities of microfluidic systems by identifying patterns in complex microbial data that might be imperceptible to human analysts. These algorithms can be trained to recognize specific microbial signatures, predict antimicrobial resistance patterns, and even forecast potential outbreaks based on subtle changes in microbial populations. Deep learning approaches have demonstrated remarkable accuracy in classifying microorganisms from microscopic images captured within microfluidic channels.
The integration of edge computing with microfluidic devices allows for preliminary data processing directly at the testing site, reducing bandwidth requirements and enabling functionality in areas with limited connectivity. This distributed intelligence architecture supports deployment in resource-limited settings where traditional laboratory infrastructure is unavailable.
Advanced sensor fusion techniques combine data from multiple sensing modalities within microfluidic platforms, creating comprehensive microbial profiles that include genetic, metabolic, and morphological characteristics. This multi-parameter analysis significantly improves diagnostic accuracy and provides deeper insights into microbial behavior and pathogenicity.
Predictive analytics powered by AI can anticipate antimicrobial resistance development by analyzing subtle changes in microbial response patterns over time. This capability is particularly valuable for healthcare settings, where early identification of resistance emergence can guide appropriate therapeutic interventions and stewardship programs.
The development of digital twins for microfluidic testing systems enables virtual simulation and optimization of testing protocols before physical implementation. This approach accelerates the development cycle for new diagnostic applications and facilitates personalized testing strategies based on specific environmental or clinical contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!