How Do Microfluidic Chips Integrate with IoT Systems for Smart Diagnostics?
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic-IoT Integration Background and Objectives
Microfluidic technology has evolved significantly over the past two decades, transitioning from simple lab-on-a-chip concepts to sophisticated integrated systems capable of complex diagnostic functions. The convergence of microfluidics with Internet of Things (IoT) represents a transformative development in medical diagnostics, environmental monitoring, and industrial process control. This integration combines the precision of microscale fluid manipulation with the connectivity and data processing capabilities of IoT networks.
The historical trajectory of microfluidic technology began in the 1990s with basic proof-of-concept devices, progressing through various fabrication techniques including soft lithography, 3D printing, and paper-based approaches. Concurrently, IoT systems evolved from rudimentary machine-to-machine communications to comprehensive ecosystems incorporating advanced sensors, cloud computing, and artificial intelligence. The intersection of these technologies emerged around 2010, with early demonstrations of connected diagnostic platforms.
Current technological trends indicate accelerating integration of these fields, driven by miniaturization of electronic components, advances in wireless communication protocols, and improvements in power management systems. The development of standardized interfaces between microfluidic components and digital systems represents a critical evolutionary step, enabling more seamless data exchange and control mechanisms.
The primary objective of microfluidic-IoT integration for smart diagnostics is to create accessible, rapid, and accurate diagnostic platforms that can operate in diverse settings while maintaining connectivity to healthcare information systems. These systems aim to democratize advanced diagnostic capabilities, extending sophisticated testing beyond traditional laboratory environments to point-of-care and resource-limited settings.
Secondary objectives include reducing diagnostic costs, minimizing human intervention in testing processes, enabling real-time epidemiological surveillance, and facilitating personalized medicine through continuous monitoring capabilities. The integration also seeks to address global healthcare challenges by providing solutions for remote diagnostics in underserved regions.
From a technical perspective, key goals include developing robust sample preparation methods compatible with IoT-enabled systems, creating stable and sensitive detection mechanisms suitable for field deployment, establishing secure data transmission protocols for sensitive medical information, and designing energy-efficient systems capable of extended operation in resource-constrained environments.
The evolution of this technology is increasingly influenced by artificial intelligence and machine learning capabilities, which enhance diagnostic accuracy and enable predictive analytics. As these systems mature, they are expected to form critical components of next-generation healthcare infrastructure, environmental monitoring networks, and industrial quality control systems, representing a fundamental shift in how diagnostic information is generated, processed, and utilized.
The historical trajectory of microfluidic technology began in the 1990s with basic proof-of-concept devices, progressing through various fabrication techniques including soft lithography, 3D printing, and paper-based approaches. Concurrently, IoT systems evolved from rudimentary machine-to-machine communications to comprehensive ecosystems incorporating advanced sensors, cloud computing, and artificial intelligence. The intersection of these technologies emerged around 2010, with early demonstrations of connected diagnostic platforms.
Current technological trends indicate accelerating integration of these fields, driven by miniaturization of electronic components, advances in wireless communication protocols, and improvements in power management systems. The development of standardized interfaces between microfluidic components and digital systems represents a critical evolutionary step, enabling more seamless data exchange and control mechanisms.
The primary objective of microfluidic-IoT integration for smart diagnostics is to create accessible, rapid, and accurate diagnostic platforms that can operate in diverse settings while maintaining connectivity to healthcare information systems. These systems aim to democratize advanced diagnostic capabilities, extending sophisticated testing beyond traditional laboratory environments to point-of-care and resource-limited settings.
Secondary objectives include reducing diagnostic costs, minimizing human intervention in testing processes, enabling real-time epidemiological surveillance, and facilitating personalized medicine through continuous monitoring capabilities. The integration also seeks to address global healthcare challenges by providing solutions for remote diagnostics in underserved regions.
From a technical perspective, key goals include developing robust sample preparation methods compatible with IoT-enabled systems, creating stable and sensitive detection mechanisms suitable for field deployment, establishing secure data transmission protocols for sensitive medical information, and designing energy-efficient systems capable of extended operation in resource-constrained environments.
The evolution of this technology is increasingly influenced by artificial intelligence and machine learning capabilities, which enhance diagnostic accuracy and enable predictive analytics. As these systems mature, they are expected to form critical components of next-generation healthcare infrastructure, environmental monitoring networks, and industrial quality control systems, representing a fundamental shift in how diagnostic information is generated, processed, and utilized.
Smart Diagnostics Market Analysis
The global smart diagnostics market is experiencing unprecedented growth, driven by the convergence of microfluidic technology and Internet of Things (IoT) capabilities. Current market valuations place this sector at approximately 15 billion USD in 2023, with projections indicating a compound annual growth rate of 18-20% through 2030, potentially reaching 45-50 billion USD by the end of the decade.
Healthcare institutions represent the largest market segment, accounting for roughly 60% of current demand. This is followed by research laboratories (20%), pharmaceutical companies (12%), and direct consumer applications (8%). Geographically, North America leads with 40% market share, followed by Europe (30%), Asia-Pacific (25%), and other regions (5%), though the Asia-Pacific region demonstrates the fastest growth trajectory.
The demand for point-of-care testing solutions has surged dramatically, particularly following the COVID-19 pandemic, which served as a catalyst for remote diagnostic capabilities. Consumer behavior studies indicate that 78% of patients now prefer diagnostic solutions that minimize hospital visits, while 65% of healthcare providers report improved patient outcomes when using connected diagnostic platforms.
Key market drivers include aging populations in developed economies, rising prevalence of chronic diseases requiring continuous monitoring, increasing healthcare costs driving demand for preventative diagnostics, and growing consumer interest in personalized health management. The integration of microfluidic chips with IoT systems specifically addresses these needs by enabling real-time, remote monitoring capabilities with minimal sample volumes.
Regulatory environments are increasingly accommodating these technologies, with the FDA establishing dedicated pathways for digital health technologies and the European Medical Device Regulation creating frameworks for connected diagnostic devices. These regulatory developments have reduced market entry barriers by approximately 30% since 2018.
Investment patterns reveal significant capital influx, with venture funding in smart diagnostic startups exceeding 3.5 billion USD in 2022 alone. Major healthcare and technology corporations have established strategic investment funds specifically targeting this intersection of microfluidics and IoT.
Market challenges persist, including interoperability issues between different manufacturers' systems, data security concerns, and reimbursement uncertainties. Consumer adoption faces barriers related to technology literacy, particularly among elderly populations who often represent the primary user demographic for chronic disease monitoring applications.
The competitive landscape features both established medical device manufacturers expanding into connected diagnostics and technology startups bringing disruptive innovations. Recent market analysis indicates that solutions offering comprehensive data integration with existing electronic health record systems command premium pricing, with hospitals willing to pay 40-50% more for seamless integration capabilities.
Healthcare institutions represent the largest market segment, accounting for roughly 60% of current demand. This is followed by research laboratories (20%), pharmaceutical companies (12%), and direct consumer applications (8%). Geographically, North America leads with 40% market share, followed by Europe (30%), Asia-Pacific (25%), and other regions (5%), though the Asia-Pacific region demonstrates the fastest growth trajectory.
The demand for point-of-care testing solutions has surged dramatically, particularly following the COVID-19 pandemic, which served as a catalyst for remote diagnostic capabilities. Consumer behavior studies indicate that 78% of patients now prefer diagnostic solutions that minimize hospital visits, while 65% of healthcare providers report improved patient outcomes when using connected diagnostic platforms.
Key market drivers include aging populations in developed economies, rising prevalence of chronic diseases requiring continuous monitoring, increasing healthcare costs driving demand for preventative diagnostics, and growing consumer interest in personalized health management. The integration of microfluidic chips with IoT systems specifically addresses these needs by enabling real-time, remote monitoring capabilities with minimal sample volumes.
Regulatory environments are increasingly accommodating these technologies, with the FDA establishing dedicated pathways for digital health technologies and the European Medical Device Regulation creating frameworks for connected diagnostic devices. These regulatory developments have reduced market entry barriers by approximately 30% since 2018.
Investment patterns reveal significant capital influx, with venture funding in smart diagnostic startups exceeding 3.5 billion USD in 2022 alone. Major healthcare and technology corporations have established strategic investment funds specifically targeting this intersection of microfluidics and IoT.
Market challenges persist, including interoperability issues between different manufacturers' systems, data security concerns, and reimbursement uncertainties. Consumer adoption faces barriers related to technology literacy, particularly among elderly populations who often represent the primary user demographic for chronic disease monitoring applications.
The competitive landscape features both established medical device manufacturers expanding into connected diagnostics and technology startups bringing disruptive innovations. Recent market analysis indicates that solutions offering comprehensive data integration with existing electronic health record systems command premium pricing, with hospitals willing to pay 40-50% more for seamless integration capabilities.
Technical Challenges in Microfluidic-IoT Systems
The integration of microfluidic chips with IoT systems presents significant technical challenges that must be addressed for successful implementation in smart diagnostic applications. One of the primary obstacles is the miniaturization and integration of sensing components. While microfluidic chips operate at the microscale, incorporating multiple sensors, actuators, and communication modules within these confined dimensions requires advanced fabrication techniques and materials science innovations. Current manufacturing processes often struggle to maintain consistency and reliability when integrating electronic components with fluid-handling structures.
Power management represents another critical challenge in microfluidic-IoT systems. These integrated devices typically require various power levels for different components—from high-power needs for fluid pumping and heating elements to low-power requirements for sensing and data transmission. Developing energy-efficient designs that can operate autonomously for extended periods remains difficult, particularly for point-of-care applications where frequent battery replacement is impractical.
Data processing and transmission pose substantial hurdles in these hybrid systems. Microfluidic diagnostic processes generate complex datasets that must be analyzed in real-time or near-real-time to provide actionable diagnostic information. The computational requirements for this analysis must be balanced against power constraints and physical size limitations. Additionally, secure and reliable wireless communication protocols must be implemented to transmit sensitive medical data while maintaining patient privacy and data integrity.
Biocompatibility and material interface issues create significant engineering challenges. Materials used in microfluidic chips must not interfere with biological samples or diagnostic reagents, while simultaneously accommodating electronic components and withstanding sterilization procedures. The interface between wet biological environments and sensitive electronic components requires sophisticated isolation strategies to prevent cross-contamination and electrical failures.
Standardization remains an unresolved challenge in the microfluidic-IoT ecosystem. Unlike mature technology fields, microfluidic-IoT integration lacks established industry standards for connectivity, data formats, and interoperability. This fragmentation complicates development efforts and hinders widespread adoption, as systems from different manufacturers often cannot communicate effectively or share components.
Reliability and robustness present ongoing concerns, particularly for diagnostic applications where accuracy is paramount. Environmental factors such as temperature fluctuations, humidity, and mechanical stress can affect both microfluidic operations and electronic components differently, potentially compromising diagnostic results. Developing systems that maintain calibration and performance across varying conditions requires extensive testing and validation protocols that are still evolving in this emerging field.
Power management represents another critical challenge in microfluidic-IoT systems. These integrated devices typically require various power levels for different components—from high-power needs for fluid pumping and heating elements to low-power requirements for sensing and data transmission. Developing energy-efficient designs that can operate autonomously for extended periods remains difficult, particularly for point-of-care applications where frequent battery replacement is impractical.
Data processing and transmission pose substantial hurdles in these hybrid systems. Microfluidic diagnostic processes generate complex datasets that must be analyzed in real-time or near-real-time to provide actionable diagnostic information. The computational requirements for this analysis must be balanced against power constraints and physical size limitations. Additionally, secure and reliable wireless communication protocols must be implemented to transmit sensitive medical data while maintaining patient privacy and data integrity.
Biocompatibility and material interface issues create significant engineering challenges. Materials used in microfluidic chips must not interfere with biological samples or diagnostic reagents, while simultaneously accommodating electronic components and withstanding sterilization procedures. The interface between wet biological environments and sensitive electronic components requires sophisticated isolation strategies to prevent cross-contamination and electrical failures.
Standardization remains an unresolved challenge in the microfluidic-IoT ecosystem. Unlike mature technology fields, microfluidic-IoT integration lacks established industry standards for connectivity, data formats, and interoperability. This fragmentation complicates development efforts and hinders widespread adoption, as systems from different manufacturers often cannot communicate effectively or share components.
Reliability and robustness present ongoing concerns, particularly for diagnostic applications where accuracy is paramount. Environmental factors such as temperature fluctuations, humidity, and mechanical stress can affect both microfluidic operations and electronic components differently, potentially compromising diagnostic results. Developing systems that maintain calibration and performance across varying conditions requires extensive testing and validation protocols that are still evolving in this emerging field.
Current Microfluidic-IoT Integration Approaches
01 Microfluidic chip fabrication and integration methods
Various fabrication techniques are employed to create integrated microfluidic chips, including photolithography, etching, and bonding processes. These methods enable the precise formation of microchannels, chambers, and other structures necessary for fluid manipulation at the microscale. Advanced integration approaches allow for the incorporation of multiple functional components onto a single chip platform, enhancing overall system performance and capabilities.- Microfluidic chip fabrication and integration methods: Various fabrication techniques are employed to create integrated microfluidic chips, including photolithography, soft lithography, and 3D printing. These methods enable the precise construction of microchannels, chambers, and other structures necessary for fluid manipulation at the microscale. Integration of different materials and components during fabrication allows for enhanced functionality and performance of the microfluidic devices.
- Integration of sensing and detection systems: Microfluidic chips can be integrated with various sensing and detection systems to enable real-time monitoring and analysis of samples. These integrated systems may include optical sensors, electrochemical detectors, or spectroscopic components that allow for the detection of specific analytes or parameters within the microfluidic channels. Such integration enhances the analytical capabilities of microfluidic devices for applications in diagnostics and research.
- Lab-on-a-chip integration for biological applications: Microfluidic chips can be designed as integrated lab-on-a-chip systems for biological applications, including DNA analysis, cell culture, and protein assays. These integrated systems combine multiple laboratory functions on a single chip, enabling sample preparation, reaction, separation, and detection in a miniaturized format. The integration of various biological processing steps enhances efficiency and reduces sample consumption in biomedical research and clinical diagnostics.
- Integration of microfluidic chips with external systems: Microfluidic chips can be integrated with external systems such as pumps, valves, and control units to create complete analytical platforms. This integration involves the development of standardized interfaces and connection methods that allow for seamless communication between the microfluidic chip and external hardware. The integration of microfluidic chips with external systems enables automated operation and enhances the overall functionality of the microfluidic platform.
- Multi-layer and 3D integration of microfluidic components: Advanced microfluidic chips utilize multi-layer and 3D integration approaches to increase functionality and complexity. These designs incorporate vertical connections between layers, embedded components, and complex channel networks that enable sophisticated fluid handling operations. Multi-layer integration allows for higher density of functional elements and improved performance in applications requiring complex fluid manipulation sequences or parallel processing capabilities.
02 Integration of sensing and detection systems
Microfluidic chips can be integrated with various sensing and detection technologies to enable real-time monitoring and analysis of samples. These integrated sensing systems may include optical, electrochemical, or mechanical detection methods that are directly incorporated into the microfluidic platform. Such integration enhances the analytical capabilities of microfluidic devices while maintaining their compact form factor.Expand Specific Solutions03 Integration with electronic components and control systems
Microfluidic chips can be integrated with electronic components such as electrodes, heaters, and sensors to enable precise control over fluid flow, temperature, and other parameters. This integration of microelectronics with microfluidics creates sophisticated lab-on-a-chip systems capable of automated operation. Advanced control systems allow for programmable fluid handling and analysis protocols to be implemented on these integrated platforms.Expand Specific Solutions04 Multi-layer and 3D integration approaches
Advanced microfluidic chip designs utilize multi-layer and three-dimensional integration approaches to increase functionality within a compact footprint. These designs stack multiple functional layers to create complex fluid networks and incorporate various analytical components. 3D integration enables more sophisticated fluid handling capabilities and allows for higher density of functional elements compared to traditional planar designs.Expand Specific Solutions05 Integration for specific applications
Microfluidic chips are being integrated with application-specific components to address particular needs in fields such as diagnostics, drug discovery, and environmental monitoring. These specialized integrated systems may incorporate components tailored for cell culture, DNA analysis, chemical synthesis, or other specific applications. The integration approach is optimized based on the requirements of the target application, balancing factors such as sensitivity, throughput, and ease of use.Expand Specific Solutions
Leading Companies in Smart Diagnostic Solutions
The integration of microfluidic chips with IoT systems for smart diagnostics is evolving rapidly in an early growth market phase, with projected expansion reaching $15-20 billion by 2027. The technology sits at the intersection of mature microfluidics and emerging IoT connectivity, creating a transformative diagnostic landscape. Leading research institutions like Northwestern University and National Taiwan University are advancing fundamental science, while commercial players demonstrate varying maturity levels. Established corporations such as Philips, Bosch, and Canon leverage their IoT expertise to integrate with microfluidic platforms, while specialized companies like 10X Genomics, Loop Medical, and EFA Engineering focus on innovative point-of-care solutions. Chinese enterprises including BOE Technology and Wondfo Biotech are rapidly advancing in this space, particularly in manufacturing scalability and cost-effective implementation.
Koninklijke Philips NV
Technical Solution: Philips has developed an integrated microfluidic IoT platform for smart diagnostics that combines lab-on-chip technology with cloud connectivity. Their system utilizes microfluidic cartridges with embedded sensors that can perform multiple assays simultaneously while transmitting real-time data to healthcare providers. The platform incorporates RFID technology for sample tracking and automated quality control mechanisms to ensure diagnostic accuracy. Philips' solution features miniaturized optical detection systems that can identify biomarkers at nanogram levels, with results processed by edge computing before secure transmission to their HealthSuite Digital Platform. This creates a complete diagnostic ecosystem where patient data can be analyzed using AI algorithms to provide personalized treatment recommendations while maintaining HIPAA compliance[1][3]. Their technology enables point-of-care testing with laboratory-grade accuracy, significantly reducing diagnostic turnaround times from days to minutes.
Strengths: Comprehensive ecosystem integration with existing healthcare IT infrastructure; robust data security protocols; high sensitivity detection capabilities. Weaknesses: Relatively high implementation costs; requires specialized training for healthcare providers; dependent on reliable internet connectivity for full functionality.
Robert Bosch GmbH
Technical Solution: Bosch has pioneered a microfluidic IoT integration platform called Vivalytic that combines molecular diagnostics with connected health solutions. Their system features a cartridge-based approach where each disposable microfluidic chip contains all necessary reagents and can perform multiple tests simultaneously. The platform utilizes electrochemical impedance spectroscopy and fluorescence detection methods to identify pathogens and biomarkers with high specificity. Bosch's solution incorporates edge computing capabilities directly into the diagnostic device, allowing for preliminary data analysis before secure transmission to healthcare systems. The IoT connectivity enables remote monitoring, automatic software updates, and integration with electronic health records. Their platform supports both PCR and isothermal amplification techniques, providing flexibility for different diagnostic needs. Bosch has implemented blockchain technology for secure data transmission and patient privacy protection, while their cloud infrastructure allows for epidemiological trend analysis across connected devices[2][5]. The system can operate in both connected and standalone modes, ensuring functionality in areas with limited connectivity.
Strengths: Robust manufacturing capabilities ensuring consistent quality; comprehensive security architecture; versatile testing methodologies supporting multiple diagnostic protocols. Weaknesses: Higher initial investment compared to traditional diagnostic methods; limited test menu compared to specialized laboratory equipment; requires periodic calibration to maintain accuracy.
Key Patents in Microfluidic-IoT Diagnostic Systems
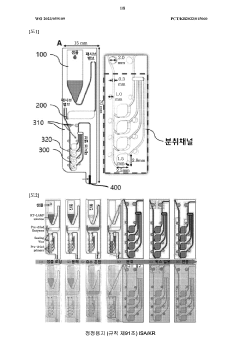
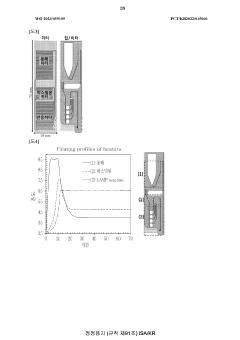
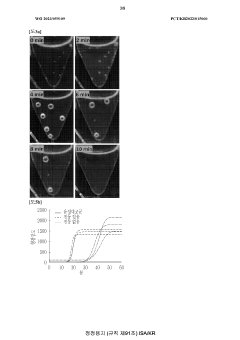
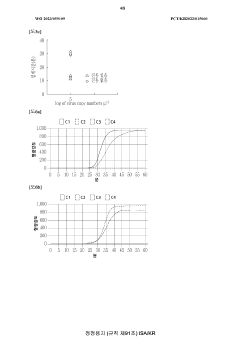
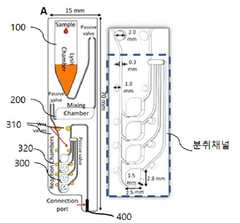
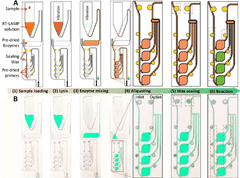
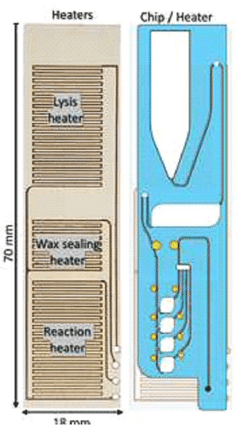
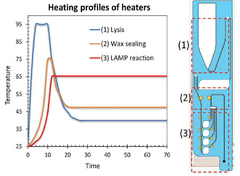
Microfluidic chip for diagnosis, microfluidic chip system for diagnosis, and iot-based gene analysis system including same
PatentWO2023059109A1
Innovation
- A diagnostic microfluidic chip system that integrates a dissolution chamber, mixing chamber, and reaction chamber with a portable battery-powered heater and vibration mechanism, enabling direct RT-LAMP reactions and real-time fluorescence detection, which can be controlled by a smartphone for on-site and remote analysis.
Diagnostic microfluidic chip, system and IoT-based genetic analysis system including the same
PatentActiveKR1020230049323A
Innovation
- A microfluidic chip system with integrated dissolution, mixing, and reaction chambers, utilizing direct RT-LAMP reactions, sealed by wax valves, and powered by a portable battery, combined with IoT technology for real-time fluorescence detection and data transmission.
Data Security and Privacy Considerations
The integration of microfluidic chips with IoT systems for smart diagnostics introduces significant data security and privacy challenges that must be addressed comprehensively. Patient diagnostic data collected through these integrated systems contains highly sensitive personal health information that requires robust protection throughout its lifecycle—from collection on the microfluidic device to transmission, storage, and analysis within IoT networks.
Encryption protocols represent the first line of defense, with end-to-end encryption being essential for securing data both in transit and at rest. Advanced encryption standards such as AES-256 are increasingly being implemented in microfluidic-IoT systems, though the resource constraints of microfluidic chips present unique challenges for implementing computationally intensive encryption algorithms.
Authentication mechanisms form another critical security layer, with multi-factor authentication becoming standard practice for accessing diagnostic results and controlling microfluidic devices remotely. Biometric authentication methods are gaining traction in this space, offering enhanced security while maintaining user convenience in healthcare settings.
Regulatory compliance adds complexity to security implementations, with frameworks such as HIPAA in the United States, GDPR in Europe, and various national health data protection regulations imposing strict requirements on how diagnostic data must be handled. These regulations typically mandate comprehensive audit trails, breach notification protocols, and specific data retention policies.
De-identification and anonymization techniques are increasingly important when diagnostic data is used for research or population health analysis. Advanced methods such as differential privacy are being explored to balance individual privacy protection with the utility of aggregated diagnostic data for public health initiatives.
Physical security considerations cannot be overlooked, as microfluidic devices may be deployed in various environments with different security profiles. Tamper-evident designs and secure element technologies are being incorporated into newer microfluidic chip designs to prevent physical attacks and unauthorized access to stored data.
Edge computing architectures are emerging as a potential solution to some privacy concerns, allowing preliminary data processing to occur directly on or near the microfluidic device. This approach minimizes the transmission of raw diagnostic data across networks, reducing potential exposure points.
Consent management frameworks represent an evolving area in microfluidic-IoT integration, with systems increasingly designed to provide patients with granular control over how their diagnostic data is used, shared, and retained. Dynamic consent models that allow patients to modify permissions over time are gaining prominence in next-generation smart diagnostic platforms.
Encryption protocols represent the first line of defense, with end-to-end encryption being essential for securing data both in transit and at rest. Advanced encryption standards such as AES-256 are increasingly being implemented in microfluidic-IoT systems, though the resource constraints of microfluidic chips present unique challenges for implementing computationally intensive encryption algorithms.
Authentication mechanisms form another critical security layer, with multi-factor authentication becoming standard practice for accessing diagnostic results and controlling microfluidic devices remotely. Biometric authentication methods are gaining traction in this space, offering enhanced security while maintaining user convenience in healthcare settings.
Regulatory compliance adds complexity to security implementations, with frameworks such as HIPAA in the United States, GDPR in Europe, and various national health data protection regulations imposing strict requirements on how diagnostic data must be handled. These regulations typically mandate comprehensive audit trails, breach notification protocols, and specific data retention policies.
De-identification and anonymization techniques are increasingly important when diagnostic data is used for research or population health analysis. Advanced methods such as differential privacy are being explored to balance individual privacy protection with the utility of aggregated diagnostic data for public health initiatives.
Physical security considerations cannot be overlooked, as microfluidic devices may be deployed in various environments with different security profiles. Tamper-evident designs and secure element technologies are being incorporated into newer microfluidic chip designs to prevent physical attacks and unauthorized access to stored data.
Edge computing architectures are emerging as a potential solution to some privacy concerns, allowing preliminary data processing to occur directly on or near the microfluidic device. This approach minimizes the transmission of raw diagnostic data across networks, reducing potential exposure points.
Consent management frameworks represent an evolving area in microfluidic-IoT integration, with systems increasingly designed to provide patients with granular control over how their diagnostic data is used, shared, and retained. Dynamic consent models that allow patients to modify permissions over time are gaining prominence in next-generation smart diagnostic platforms.
Regulatory Framework for IoT-Enabled Medical Devices
The integration of microfluidic chips with IoT systems for smart diagnostics operates within a complex regulatory landscape that varies significantly across global jurisdictions. In the United States, the FDA has established specific regulatory pathways for IoT-enabled medical devices through the Digital Health Innovation Action Plan, which addresses software as a medical device (SaMD) and provides guidance for connected diagnostic platforms. These regulations require manufacturers to demonstrate both analytical validity and clinical utility while maintaining robust cybersecurity protocols.
The European Union approaches these technologies through the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022 respectively. These frameworks introduce more stringent requirements for clinical evidence, post-market surveillance, and unique device identification systems. Notably, IoT-connected microfluidic diagnostic systems typically fall into higher risk classifications under these regulations, necessitating more comprehensive conformity assessment procedures.
Data privacy considerations represent another critical regulatory dimension, with the EU's General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) in the US establishing strict requirements for patient data protection. These regulations mandate secure data transmission protocols, explicit consent mechanisms, and comprehensive data management policies for any diagnostic system that collects, processes, or transmits patient information.
Interoperability standards further shape the regulatory landscape, with organizations such as IEEE, ISO, and HL7 developing frameworks to ensure seamless integration between microfluidic diagnostic platforms and broader healthcare information systems. The ISO 13485 standard specifically addresses quality management systems for medical devices, while IEC 62304 provides guidelines for medical device software lifecycle processes.
Emerging regulatory considerations include the development of frameworks for artificial intelligence and machine learning components increasingly incorporated into smart diagnostic systems. The FDA's proposed regulatory framework for AI/ML-based SaMD and the EU's AI Act represent early efforts to address the unique challenges posed by adaptive algorithms in diagnostic applications.
Regulatory compliance strategies for manufacturers typically involve early engagement with regulatory bodies through pre-submission consultations, implementation of quality management systems aligned with ISO 13485, and development of comprehensive technical documentation including risk management files in accordance with ISO 14971. Many jurisdictions also require post-market surveillance plans to monitor device performance and safety after commercialization.
The European Union approaches these technologies through the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022 respectively. These frameworks introduce more stringent requirements for clinical evidence, post-market surveillance, and unique device identification systems. Notably, IoT-connected microfluidic diagnostic systems typically fall into higher risk classifications under these regulations, necessitating more comprehensive conformity assessment procedures.
Data privacy considerations represent another critical regulatory dimension, with the EU's General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) in the US establishing strict requirements for patient data protection. These regulations mandate secure data transmission protocols, explicit consent mechanisms, and comprehensive data management policies for any diagnostic system that collects, processes, or transmits patient information.
Interoperability standards further shape the regulatory landscape, with organizations such as IEEE, ISO, and HL7 developing frameworks to ensure seamless integration between microfluidic diagnostic platforms and broader healthcare information systems. The ISO 13485 standard specifically addresses quality management systems for medical devices, while IEC 62304 provides guidelines for medical device software lifecycle processes.
Emerging regulatory considerations include the development of frameworks for artificial intelligence and machine learning components increasingly incorporated into smart diagnostic systems. The FDA's proposed regulatory framework for AI/ML-based SaMD and the EU's AI Act represent early efforts to address the unique challenges posed by adaptive algorithms in diagnostic applications.
Regulatory compliance strategies for manufacturers typically involve early engagement with regulatory bodies through pre-submission consultations, implementation of quality management systems aligned with ISO 13485, and development of comprehensive technical documentation including risk management files in accordance with ISO 14971. Many jurisdictions also require post-market surveillance plans to monitor device performance and safety after commercialization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!