Comparative Study of Microfluidic Chips in Cosmetic Industry Testing
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Technology Evolution in Cosmetics Testing
Microfluidic technology in cosmetics testing has undergone significant evolution over the past two decades, transforming from academic research tools to essential industrial testing platforms. The journey began in the early 2000s with rudimentary single-channel chips primarily used for basic rheological measurements of cosmetic formulations. These early systems offered limited functionality but demonstrated the potential for miniaturized testing environments.
By 2005-2010, the second generation of microfluidic platforms emerged, featuring multi-channel designs that enabled parallel testing of multiple cosmetic ingredients simultaneously. This period marked the first commercial adoption of microfluidic technology by major cosmetic companies like L'Oréal and Estée Lauder, who recognized the potential for accelerated ingredient screening and reduced sample consumption.
The 2010-2015 period witnessed a technological leap with the integration of optical sensors and imaging capabilities into microfluidic chips. This advancement allowed for real-time monitoring of skin cell responses to cosmetic compounds, providing unprecedented insights into product efficacy and potential irritation factors. The development of organ-on-chip models specifically designed to mimic human skin (skin-on-chip) represented a paradigm shift in testing methodologies.
From 2015-2020, microfluidic technology in cosmetics testing evolved toward automated high-throughput systems capable of conducting thousands of tests per day. These platforms incorporated advanced materials such as biocompatible polymers and hydrogels that better simulated skin tissue environments. The integration of artificial intelligence for data analysis became commonplace, enabling pattern recognition across vast datasets generated from microfluidic experiments.
The most recent evolution (2020-present) has seen the emergence of fully integrated testing ecosystems where microfluidic chips serve as the central component in end-to-end testing workflows. These systems now incorporate 3D bioprinting capabilities to create more physiologically relevant skin models directly on chips. Additionally, the miniaturization has continued to advance, with nanofluidic elements being incorporated to study molecular interactions at unprecedented scales.
A notable technological milestone was the development of "closed-loop" microfluidic systems that can automatically adjust testing parameters based on real-time results, enabling adaptive experimentation protocols. This has been particularly valuable for stability testing of complex cosmetic formulations under varying environmental conditions.
The evolution trajectory clearly points toward increasingly sophisticated biomimetic systems that can replicate not just skin tissue, but complete skin-microbiome interactions. Future developments are likely to focus on personalized testing capabilities, where microfluidic chips can be customized to match individual consumer skin profiles, enabling truly personalized cosmetic product development.
By 2005-2010, the second generation of microfluidic platforms emerged, featuring multi-channel designs that enabled parallel testing of multiple cosmetic ingredients simultaneously. This period marked the first commercial adoption of microfluidic technology by major cosmetic companies like L'Oréal and Estée Lauder, who recognized the potential for accelerated ingredient screening and reduced sample consumption.
The 2010-2015 period witnessed a technological leap with the integration of optical sensors and imaging capabilities into microfluidic chips. This advancement allowed for real-time monitoring of skin cell responses to cosmetic compounds, providing unprecedented insights into product efficacy and potential irritation factors. The development of organ-on-chip models specifically designed to mimic human skin (skin-on-chip) represented a paradigm shift in testing methodologies.
From 2015-2020, microfluidic technology in cosmetics testing evolved toward automated high-throughput systems capable of conducting thousands of tests per day. These platforms incorporated advanced materials such as biocompatible polymers and hydrogels that better simulated skin tissue environments. The integration of artificial intelligence for data analysis became commonplace, enabling pattern recognition across vast datasets generated from microfluidic experiments.
The most recent evolution (2020-present) has seen the emergence of fully integrated testing ecosystems where microfluidic chips serve as the central component in end-to-end testing workflows. These systems now incorporate 3D bioprinting capabilities to create more physiologically relevant skin models directly on chips. Additionally, the miniaturization has continued to advance, with nanofluidic elements being incorporated to study molecular interactions at unprecedented scales.
A notable technological milestone was the development of "closed-loop" microfluidic systems that can automatically adjust testing parameters based on real-time results, enabling adaptive experimentation protocols. This has been particularly valuable for stability testing of complex cosmetic formulations under varying environmental conditions.
The evolution trajectory clearly points toward increasingly sophisticated biomimetic systems that can replicate not just skin tissue, but complete skin-microbiome interactions. Future developments are likely to focus on personalized testing capabilities, where microfluidic chips can be customized to match individual consumer skin profiles, enabling truly personalized cosmetic product development.
Market Demand Analysis for Advanced Cosmetic Testing Solutions
The global cosmetic testing market is experiencing significant growth, driven by increasing consumer demand for safe, effective, and innovative beauty products. Current market analysis indicates that the cosmetic testing sector is valued at approximately $8.2 billion in 2023, with projections suggesting a compound annual growth rate of 5.7% through 2028. This growth is particularly pronounced in regions with stringent regulatory frameworks such as Europe, North America, and increasingly in Asia-Pacific markets.
Advanced testing solutions, particularly microfluidic chip technologies, are emerging as a critical need within the cosmetics industry. This demand is fueled by several converging factors. First, regulatory bodies worldwide are implementing stricter safety assessment requirements for cosmetic products, necessitating more sophisticated testing methodologies. The European Union's complete ban on animal testing for cosmetics has accelerated the need for alternative testing platforms, with microfluidic organ-on-chip technologies positioned as promising solutions.
Consumer preferences are also reshaping market demands. Modern consumers increasingly seek transparency regarding product ingredients, efficacy claims, and testing methods. This has created a market pull for testing technologies that can provide comprehensive safety and efficacy data while aligning with ethical considerations. Industry surveys indicate that 73% of global consumers consider a brand's testing practices when making purchasing decisions, highlighting the commercial importance of advanced testing solutions.
The personalized cosmetics trend represents another significant market driver. As brands move toward customized formulations tailored to individual skin types and concerns, there is growing demand for testing platforms capable of evaluating product performance across diverse skin conditions and genetic backgrounds. Microfluidic technologies, with their ability to simulate various skin environments and conditions, offer compelling advantages in this context.
From an industry perspective, cosmetic manufacturers are seeking testing solutions that reduce time-to-market while maintaining rigorous safety standards. Traditional testing methods often require weeks or months to complete, creating bottlenecks in product development pipelines. Advanced microfluidic testing platforms can potentially reduce testing timelines by 40-60%, representing significant competitive advantages for early adopters.
Cost considerations also influence market demand patterns. While initial investment in advanced testing technologies may be substantial, the long-term economic benefits—including reduced product failure rates, faster development cycles, and decreased regulatory compliance costs—present compelling value propositions for cosmetic manufacturers. Market analysis suggests that companies implementing advanced testing solutions can achieve return on investment within 18-24 months through operational efficiencies and accelerated product launches.
Advanced testing solutions, particularly microfluidic chip technologies, are emerging as a critical need within the cosmetics industry. This demand is fueled by several converging factors. First, regulatory bodies worldwide are implementing stricter safety assessment requirements for cosmetic products, necessitating more sophisticated testing methodologies. The European Union's complete ban on animal testing for cosmetics has accelerated the need for alternative testing platforms, with microfluidic organ-on-chip technologies positioned as promising solutions.
Consumer preferences are also reshaping market demands. Modern consumers increasingly seek transparency regarding product ingredients, efficacy claims, and testing methods. This has created a market pull for testing technologies that can provide comprehensive safety and efficacy data while aligning with ethical considerations. Industry surveys indicate that 73% of global consumers consider a brand's testing practices when making purchasing decisions, highlighting the commercial importance of advanced testing solutions.
The personalized cosmetics trend represents another significant market driver. As brands move toward customized formulations tailored to individual skin types and concerns, there is growing demand for testing platforms capable of evaluating product performance across diverse skin conditions and genetic backgrounds. Microfluidic technologies, with their ability to simulate various skin environments and conditions, offer compelling advantages in this context.
From an industry perspective, cosmetic manufacturers are seeking testing solutions that reduce time-to-market while maintaining rigorous safety standards. Traditional testing methods often require weeks or months to complete, creating bottlenecks in product development pipelines. Advanced microfluidic testing platforms can potentially reduce testing timelines by 40-60%, representing significant competitive advantages for early adopters.
Cost considerations also influence market demand patterns. While initial investment in advanced testing technologies may be substantial, the long-term economic benefits—including reduced product failure rates, faster development cycles, and decreased regulatory compliance costs—present compelling value propositions for cosmetic manufacturers. Market analysis suggests that companies implementing advanced testing solutions can achieve return on investment within 18-24 months through operational efficiencies and accelerated product launches.
Current Microfluidic Chip Technologies and Limitations
Microfluidic chip technology has evolved significantly over the past decade, with various platforms now available for cosmetic industry testing applications. Currently, the most prevalent technologies include polydimethylsiloxane (PDMS)-based chips, glass/silicon-based platforms, paper-based microfluidics, and thermoplastic-based systems. Each offers distinct advantages for specific cosmetic testing scenarios, though all share the fundamental capability to manipulate fluids at the microscale.
PDMS-based microfluidic chips dominate the market due to their optical transparency, gas permeability, and relatively straightforward fabrication process. These properties make them particularly suitable for cell-based assays and skin-on-chip models used in cosmetic toxicity and efficacy testing. However, PDMS exhibits significant limitations including absorption of hydrophobic compounds—a critical concern when testing lipophilic cosmetic ingredients—and challenges in mass production scalability.
Glass and silicon-based microfluidic platforms offer superior chemical resistance and thermal stability compared to polymer alternatives. These characteristics make them ideal for applications requiring precise temperature control or involving aggressive solvents often used in cosmetic formulation analysis. The primary limitations include higher manufacturing costs, brittleness, and more complex fabrication processes requiring specialized equipment and clean room facilities.
Paper-based microfluidic devices represent a low-cost alternative gaining traction for point-of-use cosmetic testing. Their capillary-driven flow eliminates the need for external pumps, and their disposable nature addresses contamination concerns. However, these systems suffer from limited sensitivity, poor reproducibility, and restricted channel geometries, making them unsuitable for sophisticated cosmetic formulation testing requiring precise fluid control.
Thermoplastic-based microfluidic chips (including PMMA, COC, and PC) offer a middle ground between PDMS and glass platforms. Their industrial-scale manufacturing compatibility through injection molding makes them attractive for commercial applications. Nevertheless, they typically exhibit autofluorescence that can interfere with optical detection methods commonly employed in cosmetic ingredient analysis.
A significant limitation across all current microfluidic technologies is the challenge of standardization. The cosmetic industry lacks universally accepted protocols for microfluidic testing, creating barriers to regulatory acceptance and cross-laboratory result comparison. Additionally, most existing platforms struggle with effective integration of sensing elements, requiring external instrumentation that limits portability and increases overall system complexity.
Another critical limitation is the difficulty in achieving physiologically relevant models that accurately mimic human skin structure and function. While organ-on-chip technologies have advanced considerably, current microfluidic skin models still fail to fully recapitulate the complexity of human skin, particularly regarding barrier function and metabolism of cosmetic compounds.
PDMS-based microfluidic chips dominate the market due to their optical transparency, gas permeability, and relatively straightforward fabrication process. These properties make them particularly suitable for cell-based assays and skin-on-chip models used in cosmetic toxicity and efficacy testing. However, PDMS exhibits significant limitations including absorption of hydrophobic compounds—a critical concern when testing lipophilic cosmetic ingredients—and challenges in mass production scalability.
Glass and silicon-based microfluidic platforms offer superior chemical resistance and thermal stability compared to polymer alternatives. These characteristics make them ideal for applications requiring precise temperature control or involving aggressive solvents often used in cosmetic formulation analysis. The primary limitations include higher manufacturing costs, brittleness, and more complex fabrication processes requiring specialized equipment and clean room facilities.
Paper-based microfluidic devices represent a low-cost alternative gaining traction for point-of-use cosmetic testing. Their capillary-driven flow eliminates the need for external pumps, and their disposable nature addresses contamination concerns. However, these systems suffer from limited sensitivity, poor reproducibility, and restricted channel geometries, making them unsuitable for sophisticated cosmetic formulation testing requiring precise fluid control.
Thermoplastic-based microfluidic chips (including PMMA, COC, and PC) offer a middle ground between PDMS and glass platforms. Their industrial-scale manufacturing compatibility through injection molding makes them attractive for commercial applications. Nevertheless, they typically exhibit autofluorescence that can interfere with optical detection methods commonly employed in cosmetic ingredient analysis.
A significant limitation across all current microfluidic technologies is the challenge of standardization. The cosmetic industry lacks universally accepted protocols for microfluidic testing, creating barriers to regulatory acceptance and cross-laboratory result comparison. Additionally, most existing platforms struggle with effective integration of sensing elements, requiring external instrumentation that limits portability and increases overall system complexity.
Another critical limitation is the difficulty in achieving physiologically relevant models that accurately mimic human skin structure and function. While organ-on-chip technologies have advanced considerably, current microfluidic skin models still fail to fully recapitulate the complexity of human skin, particularly regarding barrier function and metabolism of cosmetic compounds.
Comparative Analysis of Current Microfluidic Testing Platforms
01 Fabrication techniques for microfluidic chips
Various fabrication methods are employed to create microfluidic chips with precise channel geometries and surface properties. These techniques include soft lithography, hot embossing, injection molding, and 3D printing. The choice of fabrication method depends on the desired application, material properties, and production scale. Advanced fabrication techniques allow for the creation of complex microstructures with feature sizes down to the micrometer scale, enabling precise fluid control and manipulation within the chip.- Fabrication techniques for microfluidic chips: Various fabrication methods are employed to create microfluidic chips with precise channel geometries and surface properties. These techniques include soft lithography, injection molding, hot embossing, and 3D printing. The choice of fabrication method depends on the desired material properties, feature resolution, and production volume. Advanced manufacturing approaches enable the creation of complex microfluidic structures with integrated components such as valves, pumps, and sensors.
- Integration of sensing and detection systems: Microfluidic chips can be integrated with various sensing and detection systems to enable real-time monitoring and analysis. These systems include optical sensors, electrochemical detectors, impedance-based sensors, and fluorescence detection modules. The integration of these sensing technologies allows for the detection of specific analytes, monitoring of chemical reactions, and characterization of biological samples within the microfluidic environment, enhancing the analytical capabilities of these devices.
- Applications in biological and medical analysis: Microfluidic chips have extensive applications in biological and medical analysis, including DNA sequencing, protein analysis, cell sorting, and disease diagnostics. These chips enable the manipulation of small sample volumes, reducing reagent consumption and analysis time. The controlled microenvironment provided by these devices allows for precise cell culture, drug screening, and personalized medicine applications. The integration of multiple analytical steps on a single chip facilitates point-of-care diagnostics and rapid testing capabilities.
- Flow control and manipulation techniques: Various techniques are employed to control and manipulate fluid flow within microfluidic chips. These include passive methods such as capillary forces and geometric designs, as well as active methods involving external pressure sources, electrokinetic forces, and acoustic waves. Advanced flow control mechanisms enable precise sample handling, mixing, separation, and concentration within the microchannels. The development of integrated valves and pumps further enhances the functionality of these devices by allowing for automated and programmable fluid manipulation.
- Materials and surface modifications: The selection of materials and surface modifications plays a crucial role in the performance of microfluidic chips. Common materials include glass, silicon, polymers (PDMS, PMMA), and paper. Surface modifications such as hydrophilic/hydrophobic patterning, chemical functionalization, and biomolecule immobilization enhance the chip's functionality for specific applications. These modifications can prevent non-specific adsorption, control wetting properties, and enable selective binding of target molecules, improving the overall efficiency and reliability of microfluidic devices.
02 Integration of sensing and detection systems
Microfluidic chips can be integrated with various sensing and detection systems to enable real-time monitoring and analysis of samples. These systems include optical sensors, electrochemical detectors, and spectroscopic instruments that can be miniaturized and incorporated directly into the chip design. The integration of sensing technologies enhances the functionality of microfluidic devices, allowing for automated detection of analytes, monitoring of reaction kinetics, and quantification of target molecules with high sensitivity and specificity.Expand Specific Solutions03 Applications in biological and medical analysis
Microfluidic chips have extensive applications in biological and medical analysis, including DNA sequencing, protein analysis, cell sorting, and diagnostic testing. These chips enable the manipulation of small sample volumes, reducing reagent consumption and analysis time. The controlled microenvironment within the chips allows for precise cell culture conditions, single-cell analysis, and high-throughput screening of drugs or biomarkers. Microfluidic technology has revolutionized point-of-care diagnostics by providing rapid, portable, and sensitive analytical platforms.Expand Specific Solutions04 Flow control and manipulation mechanisms
Microfluidic chips incorporate various mechanisms for controlling and manipulating fluid flow within the microchannels. These mechanisms include valves, pumps, mixers, and gradient generators that enable precise control over fluid dynamics at the microscale. Advanced flow control systems can create laminar flow patterns, generate droplets, or establish concentration gradients for specific applications. The ability to precisely control fluid behavior is essential for applications requiring accurate dosing, mixing, separation, or reaction timing within the microfluidic environment.Expand Specific Solutions05 Materials and surface modifications for microfluidic chips
The selection of materials and surface modifications plays a crucial role in the performance of microfluidic chips. Common materials include glass, silicon, polymers (PDMS, PMMA), and paper, each offering different properties in terms of optical transparency, chemical resistance, and fabrication ease. Surface modifications can alter the hydrophobicity, charge, or biocompatibility of channel walls, influencing fluid flow characteristics and preventing non-specific adsorption of biomolecules. Advanced materials and coatings can enhance the functionality and durability of microfluidic devices for specific applications.Expand Specific Solutions
Leading Companies and Research Institutions in Cosmetic Microfluidics
The microfluidic chip market in cosmetic industry testing is currently in a growth phase, with increasing adoption driven by demands for more efficient and precise testing methods. The global market size is expanding rapidly, estimated to reach significant value as cosmetic companies invest in advanced testing technologies. Technologically, the field shows varying maturity levels, with established players like Amorepacific, L'Oréal, and BOE Technology Group demonstrating advanced capabilities through dedicated research centers. Academic institutions including Zhejiang University and KAIST are contributing fundamental research, while specialized companies such as Pattern Bioscience and Lansion Biotechnology are developing innovative microfluidic solutions. The competitive landscape features diverse players from cosmetic giants to technology companies and research institutions, creating a dynamic ecosystem where collaboration between industry and academia is accelerating technological advancement.
Amorepacific Corp.
Technical Solution: Amorepacific has developed innovative microfluidic chip technology specifically for cosmetic efficacy and safety testing. Their "AmoreSkin-Chip" platform incorporates multi-layered microchannels that simulate the stratified structure of human skin, allowing for precise evaluation of ingredient penetration and distribution. The company's microfluidic systems feature specialized chambers that maintain viable skin cells for extended periods, enabling long-term exposure studies that better reflect real-world product usage. Amorepacific's chips integrate advanced imaging capabilities that provide real-time visualization of cellular responses to cosmetic ingredients, including inflammation markers, melanin production, and collagen synthesis. Their technology employs gradient generators to simultaneously test multiple concentrations of active ingredients, optimizing formulation efficiency. The company has also pioneered microfluidic models that incorporate Korean skin-specific characteristics, allowing for targeted product development for Asian markets. These systems have significantly reduced Amorepacific's reliance on animal testing while providing more physiologically relevant data for cosmetic development.
Strengths: Specialized expertise in Asian skin biology integration; excellent correlation with clinical outcomes; rapid testing capabilities for ingredient screening. Weaknesses: Regional focus may limit global applicability; relatively new technology with limited long-term validation data; higher initial investment costs compared to traditional testing methods.
Canon U.S. Life Sciences, Inc.
Technical Solution: Canon U.S. Life Sciences has leveraged its expertise in precision engineering and microfluidics to develop advanced chip platforms applicable to cosmetic testing. Their "BioAnalyzer" microfluidic system utilizes high-precision inkjet technology to create exceptionally uniform droplet-based testing environments, enabling quantitative analysis of cosmetic ingredient interactions with biological samples. The company's chips feature integrated optical detection systems that provide real-time monitoring of cellular responses to cosmetic compounds, including changes in morphology, viability, and functional markers. Canon's microfluidic technology incorporates proprietary surface treatments that prevent non-specific binding of test substances, enhancing measurement accuracy and reproducibility. Their systems employ parallel processing capabilities that allow simultaneous testing of multiple formulations under identical conditions, significantly increasing throughput compared to traditional methods. The company has also developed specialized microfluidic chips that simulate specific skin conditions, such as sensitive skin or photoaged skin, providing targeted testing platforms for specialized cosmetic products. Canon's microfluidic solutions integrate seamlessly with their advanced imaging systems, creating comprehensive analytical platforms for cosmetic research and development.
Strengths: Exceptional precision engineering and manufacturing capabilities; outstanding optical integration for high-resolution analysis; robust quality control and reproducibility. Weaknesses: Less specialized focus on skin biology compared to cosmetic industry leaders; higher cost systems compared to some competitors; relatively newer entrant to cosmetic testing applications.
Key Patents and Innovations in Cosmetic Microfluidic Testing
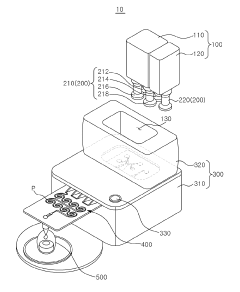
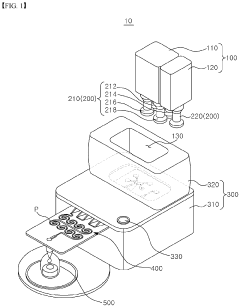
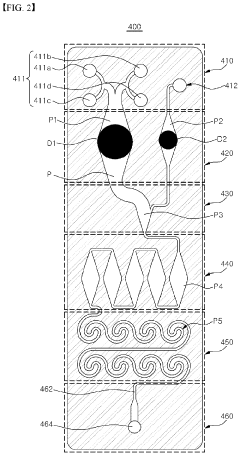
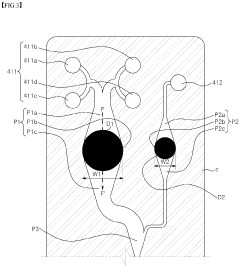
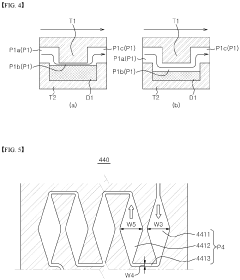
Microfluidic chip and cosmetic manufacturing apparatus including same
PatentPendingKR1020240063526A
Innovation
- A microfluidic chip with integrated microchannels for fluid mixing and emulsification, capable of producing various cosmetic contents by combining and emulsifying multiple fluids, including a vortexing unit to form emulsified particles, and designed for miniaturization and user customization.
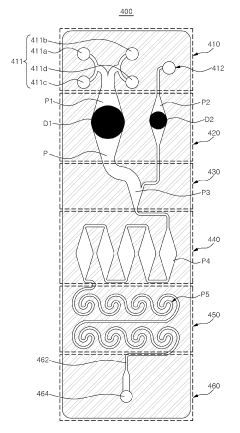
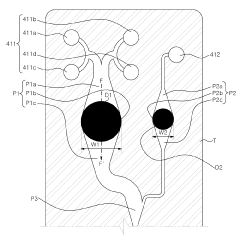
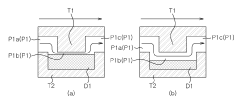
Microfluidic chip and cosmetic manufacturing apparatus including same
PatentPendingEP4364832A1
Innovation
- A microfluidic chip with modular raw materials and a miniaturized cosmetic manufacturing apparatus that allows for the instant production of various cosmetic contents by mixing and emulsifying fluids, featuring a micro flow path design that includes inlet, dissolution, confluence, stir, and vortexing portions to create emulsion particles, enabling customizable cosmetic production with replaceable microfluidic chips.
Regulatory Compliance and Safety Standards for Microfluidic Testing
The regulatory landscape for microfluidic testing in cosmetics is increasingly complex, with different regions implementing varying standards. In the European Union, the Cosmetic Products Regulation (EC) No 1223/2009 establishes comprehensive requirements for safety assessment and prohibits animal testing, making microfluidic technologies particularly valuable as alternative testing methods. These technologies must comply with the principles outlined in OECD Guidelines for the Testing of Chemicals, which provide internationally agreed testing methods for safety evaluation.
In the United States, the FDA regulates cosmetics under the Federal Food, Drug, and Cosmetic Act, though with less stringent pre-market approval requirements than in the EU. However, the FDA has shown increasing interest in organ-on-chip and microfluidic technologies as potential tools for safety assessment, particularly through its Predictive Toxicology Roadmap initiative. Manufacturers utilizing microfluidic testing must ensure their methods provide adequate safety data to meet the "reasonable certainty of no harm" standard.
ISO standards play a crucial role in standardizing microfluidic testing methodologies. ISO 10993 series, while primarily focused on medical devices, provides relevant guidance for biocompatibility testing that can be adapted to cosmetic applications. Additionally, ISO/TC 48/SC 8 specifically addresses microfluidic devices, establishing standards for terminology, performance, and calibration that testing laboratories must follow.
Good Laboratory Practice (GLP) principles are fundamental for ensuring the quality and integrity of microfluidic testing data. Laboratories conducting these tests must maintain appropriate facilities, equipment validation protocols, standard operating procedures, and comprehensive documentation systems. The OECD Principles of GLP provide an internationally recognized framework that testing facilities should adhere to when generating safety data.
Validation of alternative testing methods represents a significant regulatory hurdle. Organizations such as the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) and the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) in the US evaluate and validate new testing approaches. Microfluidic chip-based methods must demonstrate scientific validity, relevance, and reliability through these validation processes before gaining regulatory acceptance.
Data integrity and security requirements are increasingly important as microfluidic testing generates complex datasets. Compliance with data protection regulations such as GDPR in Europe or HIPAA in the US may be necessary when handling human cell samples or personal data. Furthermore, electronic records from automated microfluidic systems must meet requirements similar to those outlined in 21 CFR Part 11 for electronic records and signatures.
In the United States, the FDA regulates cosmetics under the Federal Food, Drug, and Cosmetic Act, though with less stringent pre-market approval requirements than in the EU. However, the FDA has shown increasing interest in organ-on-chip and microfluidic technologies as potential tools for safety assessment, particularly through its Predictive Toxicology Roadmap initiative. Manufacturers utilizing microfluidic testing must ensure their methods provide adequate safety data to meet the "reasonable certainty of no harm" standard.
ISO standards play a crucial role in standardizing microfluidic testing methodologies. ISO 10993 series, while primarily focused on medical devices, provides relevant guidance for biocompatibility testing that can be adapted to cosmetic applications. Additionally, ISO/TC 48/SC 8 specifically addresses microfluidic devices, establishing standards for terminology, performance, and calibration that testing laboratories must follow.
Good Laboratory Practice (GLP) principles are fundamental for ensuring the quality and integrity of microfluidic testing data. Laboratories conducting these tests must maintain appropriate facilities, equipment validation protocols, standard operating procedures, and comprehensive documentation systems. The OECD Principles of GLP provide an internationally recognized framework that testing facilities should adhere to when generating safety data.
Validation of alternative testing methods represents a significant regulatory hurdle. Organizations such as the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) and the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) in the US evaluate and validate new testing approaches. Microfluidic chip-based methods must demonstrate scientific validity, relevance, and reliability through these validation processes before gaining regulatory acceptance.
Data integrity and security requirements are increasingly important as microfluidic testing generates complex datasets. Compliance with data protection regulations such as GDPR in Europe or HIPAA in the US may be necessary when handling human cell samples or personal data. Furthermore, electronic records from automated microfluidic systems must meet requirements similar to those outlined in 21 CFR Part 11 for electronic records and signatures.
Sustainability and Ethical Considerations in Microfluidic Cosmetic Testing
The integration of microfluidic technology in cosmetic testing presents significant sustainability and ethical implications that warrant careful consideration. Traditional cosmetic testing methods often consume substantial resources, generate considerable waste, and may involve animal testing—practices increasingly scrutinized by consumers, regulatory bodies, and industry stakeholders alike.
Microfluidic chips offer remarkable sustainability advantages through dramatic reduction in reagent consumption. These systems typically require only microliters or nanoliters of testing materials, representing a 90-95% reduction compared to conventional methods. This minimization directly translates to decreased chemical waste generation and reduced environmental impact throughout the testing lifecycle.
Energy efficiency constitutes another critical sustainability benefit. Microfluidic systems generally operate at room temperature with minimal power requirements, particularly when compared to traditional laboratory equipment. Studies indicate potential energy savings of 40-60% when implementing microfluidic testing platforms at scale, contributing significantly to reduced carbon footprints in cosmetic research facilities.
From an ethical perspective, microfluidic organ-on-chip and skin-on-chip technologies represent transformative alternatives to animal testing. These platforms recreate human tissue environments with remarkable fidelity, enabling more relevant safety and efficacy assessments while addressing ethical concerns surrounding animal welfare. The alignment with global regulatory shifts toward animal testing alternatives positions microfluidic technology as ethically advantageous.
Material selection for microfluidic chips presents both challenges and opportunities for sustainability. While many current chips utilize petroleum-based polymers like PDMS, research into biodegradable alternatives is advancing rapidly. Bio-based polymers derived from renewable resources and recyclable glass or silicon substrates are emerging as environmentally preferable options, though cost and performance barriers remain.
The lifecycle assessment of microfluidic testing platforms reveals complex sustainability considerations. While operational resource efficiency is clear, manufacturing processes for precision microfluidic components can be energy-intensive. Comprehensive sustainability requires addressing the entire value chain, from raw material sourcing through end-of-life disposal or recycling pathways.
Democratization of testing technology represents an often-overlooked ethical dimension. Microfluidic platforms, with their potential for cost reduction and accessibility, may enable smaller cosmetic companies and developing markets to conduct sophisticated safety testing previously available only to industry giants, potentially fostering more equitable industry practices and innovation.
Microfluidic chips offer remarkable sustainability advantages through dramatic reduction in reagent consumption. These systems typically require only microliters or nanoliters of testing materials, representing a 90-95% reduction compared to conventional methods. This minimization directly translates to decreased chemical waste generation and reduced environmental impact throughout the testing lifecycle.
Energy efficiency constitutes another critical sustainability benefit. Microfluidic systems generally operate at room temperature with minimal power requirements, particularly when compared to traditional laboratory equipment. Studies indicate potential energy savings of 40-60% when implementing microfluidic testing platforms at scale, contributing significantly to reduced carbon footprints in cosmetic research facilities.
From an ethical perspective, microfluidic organ-on-chip and skin-on-chip technologies represent transformative alternatives to animal testing. These platforms recreate human tissue environments with remarkable fidelity, enabling more relevant safety and efficacy assessments while addressing ethical concerns surrounding animal welfare. The alignment with global regulatory shifts toward animal testing alternatives positions microfluidic technology as ethically advantageous.
Material selection for microfluidic chips presents both challenges and opportunities for sustainability. While many current chips utilize petroleum-based polymers like PDMS, research into biodegradable alternatives is advancing rapidly. Bio-based polymers derived from renewable resources and recyclable glass or silicon substrates are emerging as environmentally preferable options, though cost and performance barriers remain.
The lifecycle assessment of microfluidic testing platforms reveals complex sustainability considerations. While operational resource efficiency is clear, manufacturing processes for precision microfluidic components can be energy-intensive. Comprehensive sustainability requires addressing the entire value chain, from raw material sourcing through end-of-life disposal or recycling pathways.
Democratization of testing technology represents an often-overlooked ethical dimension. Microfluidic platforms, with their potential for cost reduction and accessibility, may enable smaller cosmetic companies and developing markets to conduct sophisticated safety testing previously available only to industry giants, potentially fostering more equitable industry practices and innovation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!