How Do Electrode Configurations in Microfluidic Chips Affect Results?
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Electrode Technology Background and Objectives
Microfluidic technology has evolved significantly over the past three decades, transforming from simple channel designs to sophisticated lab-on-a-chip systems capable of performing complex analytical tasks. The integration of electrodes within these microfluidic platforms represents a critical advancement that has expanded functionality and precision in various applications including biosensing, cell manipulation, and electrochemical detection.
The evolution of electrode configurations in microfluidic systems can be traced back to the early 2000s when researchers began exploring ways to incorporate electrical components into miniaturized fluidic channels. Initial designs featured simple planar electrodes fabricated using conventional photolithography techniques. As the field progressed, more complex three-dimensional electrode arrangements emerged, enabling enhanced control over electric fields and fluid dynamics at the microscale.
Current technological trends indicate a movement toward multi-functional electrode arrays that can simultaneously perform multiple operations such as dielectrophoresis, impedance sensing, and electrochemical detection. Additionally, there is growing interest in developing flexible and reconfigurable electrode systems that can adapt to different experimental requirements without requiring complete device redesign.
The primary objective of electrode configuration research in microfluidics is to optimize the spatial arrangement, geometry, material composition, and operational parameters of electrodes to achieve specific functional outcomes. These outcomes include improved sensitivity in biosensing applications, enhanced selectivity in particle manipulation, reduced power consumption, and minimized electrode fouling during extended operation.
Another critical goal is understanding the fundamental relationships between electrode design parameters and resulting electric field distributions within microfluidic channels. This understanding is essential for predicting how different configurations will affect experimental outcomes across various applications, from DNA analysis to point-of-care diagnostics.
Recent technological breakthroughs have enabled the integration of nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles into electrode structures, significantly enhancing performance characteristics. These advanced materials offer improved conductivity, larger surface areas, and unique electrochemical properties that conventional metal electrodes cannot provide.
The convergence of microfluidics with other emerging technologies, including artificial intelligence for real-time signal processing and advanced manufacturing techniques for electrode fabrication, is opening new possibilities for creating more sophisticated and efficient microfluidic systems. These interdisciplinary approaches are expected to drive the next generation of innovations in electrode-based microfluidic technologies.
The evolution of electrode configurations in microfluidic systems can be traced back to the early 2000s when researchers began exploring ways to incorporate electrical components into miniaturized fluidic channels. Initial designs featured simple planar electrodes fabricated using conventional photolithography techniques. As the field progressed, more complex three-dimensional electrode arrangements emerged, enabling enhanced control over electric fields and fluid dynamics at the microscale.
Current technological trends indicate a movement toward multi-functional electrode arrays that can simultaneously perform multiple operations such as dielectrophoresis, impedance sensing, and electrochemical detection. Additionally, there is growing interest in developing flexible and reconfigurable electrode systems that can adapt to different experimental requirements without requiring complete device redesign.
The primary objective of electrode configuration research in microfluidics is to optimize the spatial arrangement, geometry, material composition, and operational parameters of electrodes to achieve specific functional outcomes. These outcomes include improved sensitivity in biosensing applications, enhanced selectivity in particle manipulation, reduced power consumption, and minimized electrode fouling during extended operation.
Another critical goal is understanding the fundamental relationships between electrode design parameters and resulting electric field distributions within microfluidic channels. This understanding is essential for predicting how different configurations will affect experimental outcomes across various applications, from DNA analysis to point-of-care diagnostics.
Recent technological breakthroughs have enabled the integration of nanomaterials such as carbon nanotubes, graphene, and metal nanoparticles into electrode structures, significantly enhancing performance characteristics. These advanced materials offer improved conductivity, larger surface areas, and unique electrochemical properties that conventional metal electrodes cannot provide.
The convergence of microfluidics with other emerging technologies, including artificial intelligence for real-time signal processing and advanced manufacturing techniques for electrode fabrication, is opening new possibilities for creating more sophisticated and efficient microfluidic systems. These interdisciplinary approaches are expected to drive the next generation of innovations in electrode-based microfluidic technologies.
Market Applications and Demand Analysis for Microfluidic Devices
The global microfluidic devices market has experienced substantial growth, valued at approximately $13.5 billion in 2020 and projected to reach $27.8 billion by 2025, with a compound annual growth rate (CAGR) of 15.6%. This remarkable expansion is driven by increasing applications across multiple sectors, particularly in healthcare, pharmaceuticals, and life sciences research.
In the healthcare sector, microfluidic devices have revolutionized point-of-care diagnostics, enabling rapid testing with minimal sample volumes. The COVID-19 pandemic significantly accelerated market demand, with microfluidic-based PCR and immunoassay tests becoming essential tools for mass testing strategies worldwide. This trend continues to evolve beyond the pandemic as healthcare systems increasingly value decentralized testing capabilities.
Pharmaceutical companies represent another major market segment, with growing investments in drug discovery platforms that utilize microfluidic technology. These platforms enable high-throughput screening and precise manipulation of small fluid volumes, reducing reagent costs and accelerating development timelines. The organ-on-a-chip subsegment is particularly noteworthy, with market analysts predicting 25% annual growth as these devices increasingly replace traditional animal testing methods.
Electrode configuration within microfluidic chips directly impacts market applications and adoption rates. Industries demand configurations that deliver consistent, reproducible results while maintaining manufacturing scalability. The market shows particular interest in electrode designs that enable multiplexed detection capabilities, allowing simultaneous analysis of multiple biomarkers from a single sample.
Regional market analysis reveals North America leading with approximately 40% market share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region demonstrates the fastest growth rate at 18.2% annually, driven by increasing research funding and manufacturing capabilities in China, South Korea, and Singapore.
Consumer preferences increasingly favor integrated systems that combine sample preparation, analysis, and data interpretation. This has created market opportunities for companies developing comprehensive microfluidic platforms rather than standalone components. Electrode configurations that support this integration while maintaining reliability are commanding premium pricing in the marketplace.
Environmental monitoring applications represent an emerging market segment with significant growth potential. Water quality testing, air pollution monitoring, and food safety applications increasingly utilize microfluidic technology, with particular demand for electrode configurations that remain stable in varied environmental conditions and can detect multiple contaminants simultaneously.
The industrial adoption of microfluidic technology faces challenges related to standardization and scalability. Market research indicates that electrode configurations demonstrating consistent performance across manufacturing batches gain significant competitive advantage, particularly in regulated industries like medical diagnostics and pharmaceutical manufacturing.
In the healthcare sector, microfluidic devices have revolutionized point-of-care diagnostics, enabling rapid testing with minimal sample volumes. The COVID-19 pandemic significantly accelerated market demand, with microfluidic-based PCR and immunoassay tests becoming essential tools for mass testing strategies worldwide. This trend continues to evolve beyond the pandemic as healthcare systems increasingly value decentralized testing capabilities.
Pharmaceutical companies represent another major market segment, with growing investments in drug discovery platforms that utilize microfluidic technology. These platforms enable high-throughput screening and precise manipulation of small fluid volumes, reducing reagent costs and accelerating development timelines. The organ-on-a-chip subsegment is particularly noteworthy, with market analysts predicting 25% annual growth as these devices increasingly replace traditional animal testing methods.
Electrode configuration within microfluidic chips directly impacts market applications and adoption rates. Industries demand configurations that deliver consistent, reproducible results while maintaining manufacturing scalability. The market shows particular interest in electrode designs that enable multiplexed detection capabilities, allowing simultaneous analysis of multiple biomarkers from a single sample.
Regional market analysis reveals North America leading with approximately 40% market share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region demonstrates the fastest growth rate at 18.2% annually, driven by increasing research funding and manufacturing capabilities in China, South Korea, and Singapore.
Consumer preferences increasingly favor integrated systems that combine sample preparation, analysis, and data interpretation. This has created market opportunities for companies developing comprehensive microfluidic platforms rather than standalone components. Electrode configurations that support this integration while maintaining reliability are commanding premium pricing in the marketplace.
Environmental monitoring applications represent an emerging market segment with significant growth potential. Water quality testing, air pollution monitoring, and food safety applications increasingly utilize microfluidic technology, with particular demand for electrode configurations that remain stable in varied environmental conditions and can detect multiple contaminants simultaneously.
The industrial adoption of microfluidic technology faces challenges related to standardization and scalability. Market research indicates that electrode configurations demonstrating consistent performance across manufacturing batches gain significant competitive advantage, particularly in regulated industries like medical diagnostics and pharmaceutical manufacturing.
Current Electrode Configuration Challenges in Microfluidics
Microfluidic chips employ various electrode configurations to manipulate fluids and particles at the microscale, yet several significant challenges persist in current designs. The most prevalent issue involves electrode fouling and degradation, where prolonged operation leads to surface contamination from biological samples or chemical reactions. This contamination layer alters the electrode's electrical properties, resulting in diminished performance and inconsistent experimental outcomes over time.
Bubble formation presents another critical challenge, occurring when electrolysis generates gas bubbles at electrode surfaces during operation. These bubbles disrupt fluid flow patterns, create electrical discontinuities, and can completely obstruct microchannels, compromising experimental reliability and reproducibility. Researchers have attempted various surface treatments and pulsed voltage techniques to mitigate this issue, but a comprehensive solution remains elusive.
Spatial resolution limitations significantly constrain the precision of manipulation in microfluidic systems. Current fabrication techniques struggle to produce electrode features below certain dimensions (typically 5-10 μm), restricting the ability to manipulate individual cells or particles with high precision. This limitation becomes particularly problematic when working with heterogeneous biological samples requiring single-cell analysis.
Non-uniform electric field distribution represents a persistent challenge in electrode design. Field strength variations across microchannels lead to inconsistent forces on particles or cells, resulting in unpredictable movement patterns and reduced experimental control. Complex geometries and channel configurations further exacerbate this issue, creating "dead zones" where field effects are minimal or distorted.
Biocompatibility concerns arise when electrode materials or their degradation products prove toxic to biological samples. Materials like gold and platinum offer good biocompatibility but at high cost, while more affordable alternatives often compromise either performance or sample viability. Additionally, electrochemical reactions at electrode surfaces can alter local pH or generate reactive species that damage sensitive biological specimens.
Integration challenges emerge when incorporating electrodes with other functional components. Current fabrication approaches often require separate processing steps for electrodes and microfluidic channels, leading to alignment issues, increased fabrication complexity, and higher production costs. These integration difficulties limit the commercial viability and widespread adoption of electrode-equipped microfluidic devices.
Power management presents significant hurdles, particularly for portable or point-of-care applications. High voltage requirements for certain electrode configurations conflict with miniaturization goals, while Joule heating from current flow can create detrimental temperature gradients affecting both sample integrity and experimental outcomes. These thermal effects become particularly problematic in temperature-sensitive applications like PCR or enzyme-based assays.
Bubble formation presents another critical challenge, occurring when electrolysis generates gas bubbles at electrode surfaces during operation. These bubbles disrupt fluid flow patterns, create electrical discontinuities, and can completely obstruct microchannels, compromising experimental reliability and reproducibility. Researchers have attempted various surface treatments and pulsed voltage techniques to mitigate this issue, but a comprehensive solution remains elusive.
Spatial resolution limitations significantly constrain the precision of manipulation in microfluidic systems. Current fabrication techniques struggle to produce electrode features below certain dimensions (typically 5-10 μm), restricting the ability to manipulate individual cells or particles with high precision. This limitation becomes particularly problematic when working with heterogeneous biological samples requiring single-cell analysis.
Non-uniform electric field distribution represents a persistent challenge in electrode design. Field strength variations across microchannels lead to inconsistent forces on particles or cells, resulting in unpredictable movement patterns and reduced experimental control. Complex geometries and channel configurations further exacerbate this issue, creating "dead zones" where field effects are minimal or distorted.
Biocompatibility concerns arise when electrode materials or their degradation products prove toxic to biological samples. Materials like gold and platinum offer good biocompatibility but at high cost, while more affordable alternatives often compromise either performance or sample viability. Additionally, electrochemical reactions at electrode surfaces can alter local pH or generate reactive species that damage sensitive biological specimens.
Integration challenges emerge when incorporating electrodes with other functional components. Current fabrication approaches often require separate processing steps for electrodes and microfluidic channels, leading to alignment issues, increased fabrication complexity, and higher production costs. These integration difficulties limit the commercial viability and widespread adoption of electrode-equipped microfluidic devices.
Power management presents significant hurdles, particularly for portable or point-of-care applications. High voltage requirements for certain electrode configurations conflict with miniaturization goals, while Joule heating from current flow can create detrimental temperature gradients affecting both sample integrity and experimental outcomes. These thermal effects become particularly problematic in temperature-sensitive applications like PCR or enzyme-based assays.
Prevalent Electrode Configuration Solutions and Implementations
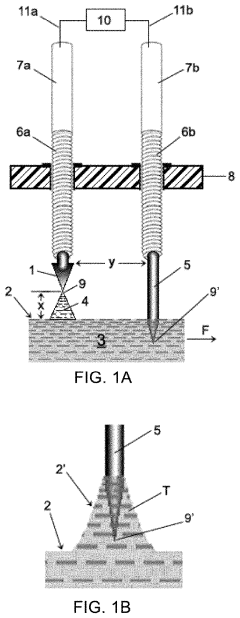
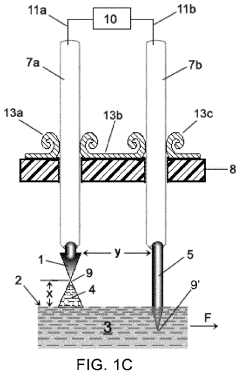
01 Interdigitated electrode configurations
Interdigitated electrode configurations are commonly used in microfluidic chips for various applications including particle manipulation, sensing, and detection. These configurations consist of alternating finger-like electrodes that create non-uniform electric fields, enabling precise control over fluid flow and particle movement within microchannels. The design allows for enhanced sensitivity in biosensing applications and efficient dielectrophoretic manipulation of cells and biomolecules.- Interdigitated electrode configurations: Interdigitated electrode configurations are commonly used in microfluidic chips for various applications including particle manipulation, sensing, and detection. These configurations consist of alternating finger-like electrodes that maximize the effective surface area and create uniform electric fields. This design enhances sensitivity in electrochemical detection and enables efficient dielectrophoretic manipulation of particles or cells within microfluidic channels.
- 3D electrode structures in microfluidic devices: Three-dimensional electrode configurations provide enhanced functionality in microfluidic chips by enabling control of particles and fluids in multiple spatial dimensions. These structures can be fabricated using techniques such as metal deposition on 3D surfaces, through-substrate vias, or stacked electrode layers. 3D electrode configurations allow for more complex field patterns and improved manipulation capabilities compared to planar electrodes, particularly useful for cell sorting, focusing, and trapping applications.
- Electrode configurations for dielectrophoresis applications: Specialized electrode configurations designed specifically for dielectrophoresis (DEP) applications in microfluidic chips enable precise manipulation of particles and cells based on their electrical properties. These configurations include quadrupole electrodes, castellated electrodes, and curved electrode arrays that generate non-uniform electric fields. The electrode geometries can be optimized to create specific field gradients for trapping, sorting, or focusing particles in microfluidic channels without direct contact with the electrodes.
- Integrated sensing electrode configurations: Microfluidic chips incorporate integrated sensing electrode configurations for real-time detection and analysis of samples. These configurations include impedance measurement electrodes, amperometric sensors, and potentiometric electrodes positioned strategically within microchannels. The integration of sensing electrodes with microfluidic structures enables label-free detection of analytes, monitoring of electrochemical reactions, and characterization of cells or particles as they flow through the device, enhancing analytical capabilities.
- Electrode materials and surface modifications: Advanced electrode materials and surface modifications enhance the performance and functionality of microfluidic chip electrodes. Materials such as gold, platinum, carbon, and conductive polymers offer different electrical properties and biocompatibility profiles. Surface modifications including nanopatterning, chemical functionalization, and protective coatings can improve electrode stability, reduce fouling, and enable specific interactions with target analytes. These material choices and modifications are critical for optimizing electrode performance in various microfluidic applications.
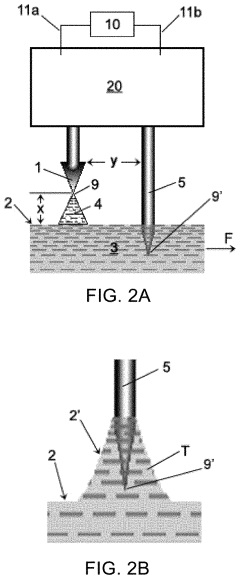
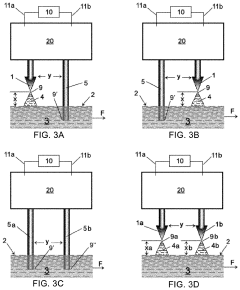
02 3D electrode structures in microfluidic devices
Three-dimensional electrode structures provide enhanced functionality in microfluidic chips by enabling control of electric fields in multiple spatial dimensions. These configurations can include vertical electrodes, stacked electrode layers, or electrodes embedded at different depths within the microfluidic channels. 3D electrode arrangements allow for more complex manipulation of particles and fluids, improving separation efficiency, detection sensitivity, and enabling advanced applications such as 3D cell culture and tissue engineering within microfluidic environments.Expand Specific Solutions03 Electrode configurations for dielectrophoresis applications
Specialized electrode configurations designed specifically for dielectrophoresis (DEP) applications in microfluidic chips enable the manipulation, sorting, and characterization of cells and particles. These configurations create non-uniform electric fields that exert forces on polarizable particles, allowing for label-free separation based on their electrical properties. Various geometries including castellated electrodes, curved electrodes, and gradient electrode arrays are employed to optimize DEP force distribution for specific applications such as cell sorting, particle concentration, and rare cell isolation.Expand Specific Solutions04 Integrated sensing electrode configurations
Microfluidic chips with integrated sensing electrode configurations combine fluid handling with electrical detection capabilities. These designs incorporate electrodes for impedance measurements, amperometric detection, or capacitive sensing directly within the microfluidic channels. The integration enables real-time monitoring of biochemical reactions, detection of analytes, and characterization of cells or particles as they flow through the device. Various electrode materials and surface modifications are employed to enhance sensitivity, selectivity, and biocompatibility for specific sensing applications.Expand Specific Solutions05 Reconfigurable and addressable electrode arrays
Reconfigurable and addressable electrode arrays in microfluidic chips provide dynamic control over electric field distributions, enabling adaptive manipulation of fluids and particles. These systems typically consist of individually addressable electrode elements that can be selectively activated to create customizable electric field patterns. The reconfigurability allows for multiple operations to be performed sequentially on the same device, such as mixing, separation, and detection, without requiring physical reconfiguration of the chip. Advanced control systems and multiplexing techniques are employed to manage complex electrode arrays for applications in digital microfluidics and adaptive lab-on-chip systems.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
The microfluidic electrode configuration market is currently in a growth phase, with increasing adoption across biomedical, analytical, and industrial applications. The market size is projected to expand significantly due to rising demand for point-of-care diagnostics and lab-on-chip technologies. Technologically, the field shows varying maturity levels, with companies like BOE Technology Group and Huawei Technologies leading in advanced manufacturing capabilities, while specialized players such as SBT Instruments and Clear Gene focus on application-specific innovations. Academic institutions including California Institute of Technology and Hong Kong University of Science & Technology are driving fundamental research, while established corporations like NXP Semiconductors and Murata Manufacturing contribute expertise in miniaturization and integration. The competitive landscape reflects a blend of large electronics manufacturers, specialized biotech firms, and research institutions collaborating to overcome challenges in electrode design, material selection, and signal processing.
Sbt Instruments A/S
Technical Solution: Sbt Instruments has developed specialized microfluidic platforms with unique electrode configurations focused on rapid bacterial detection and antimicrobial susceptibility testing. Their proprietary BacWatcher technology employs interdigitated microelectrode arrays with precisely controlled spacing to measure impedance changes caused by bacterial metabolism. The electrode configuration features gold electrodes with nanoscale surface modifications that enhance sensitivity while reducing biofouling. Their microfluidic chips incorporate a dual-chamber design with reference and sample electrodes that enable differential measurements, effectively eliminating environmental noise and drift. The company has optimized electrode geometries through extensive computational fluid dynamics modeling to ensure uniform electric field distribution across measurement zones while minimizing electrolysis effects that could interfere with bacterial growth[6]. Recent innovations include temperature-compensated electrode systems with integrated heating elements that maintain optimal conditions for bacterial metabolism while simultaneously performing electrical measurements. Their electrode configurations are specifically designed to detect subtle changes in media conductivity resulting from bacterial metabolic activity, achieving detection limits in the range of 10³ CFU/mL within hours rather than days required by traditional methods.
Strengths: Extremely sensitive detection of bacterial metabolism through optimized electrode spacing and surface treatments; excellent signal-to-noise ratio through differential measurement design; minimal sample preparation requirements. Weaknesses: Electrode configurations optimized specifically for bacterial applications may have limited versatility for other uses; potential for electrode fouling during extended measurements with complex biological samples; relatively higher cost per test compared to traditional methods.
Covaris, Inc.
Technical Solution: Covaris has pioneered Adaptive Focused Acoustics (AFA) technology that works synergistically with specialized electrode configurations in their microfluidic platforms. Their approach combines acoustic energy with strategically positioned electrodes to enhance sample processing efficiency. The company's microfluidic chips feature platinum-iridium alloy electrodes arranged in a proprietary pattern that creates highly uniform electric fields across the sample chamber. This configuration enables precise control of acoustic-electric interactions for applications such as nucleic acid extraction and chromatin shearing. Covaris employs a unique three-electrode system where the central electrode generates the primary field while two auxiliary electrodes modulate field strength and direction in response to real-time feedback from integrated sensors. This adaptive electrode configuration automatically adjusts to sample conductivity variations, maintaining consistent performance across different buffer conditions[3]. Their latest designs incorporate nanopatterned electrode surfaces that increase effective surface area while minimizing bubble formation at electrode interfaces.
Strengths: Exceptional field uniformity across sample chambers; adaptive electrode systems that respond to changing sample conditions; reduced electrode fouling through proprietary surface treatments. Weaknesses: Complex manufacturing process increases production costs; requires specialized power delivery systems; electrode configurations optimized primarily for nucleic acid applications may be less versatile for other applications.
Critical Patents and Research on Electrode-Result Relationships
Continuous methods for treating liquids and manufacturing certain constituents (e.g., nanoparticles) in liquds, apparatuses and nanoparticles and nanoparticle/liquid solution(s) resulting therefrom
PatentInactiveUS20210052638A1
Innovation
- A continuous process using adjustable plasmas and electrochemical techniques, where adjustable plasmas are created between electrodes and a liquid surface, allowing for the formation of nanoparticles of various compositions, sizes, and shapes, with simultaneous electrochemical processing, and the use of metal-based or non-metallic electrodes to control the plasma and electrochemical reactions.
Localized droplet heating with surface electrodes in microfluidic chips
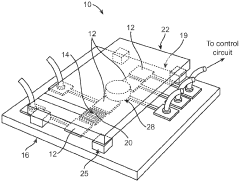
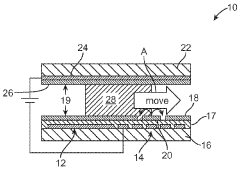
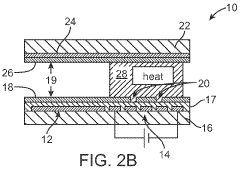
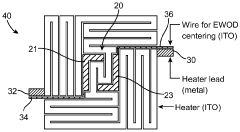
PatentWO2010141104A2
Innovation
- Integration of patterned thin-film electrodes on microfluidic chips for localized heating and temperature sensing, allowing for electrical control of droplet manipulation and thermal cycling without external components, using electrowetting-based actuation and resistance temperature detectors.
Materials Science Impact on Electrode Performance
The selection of electrode materials in microfluidic chip design represents a critical factor that significantly influences experimental outcomes and device performance. Traditional electrode materials such as gold, platinum, and silver have dominated the field due to their excellent conductivity and chemical stability. However, recent advances in materials science have expanded the available options to include carbon-based materials, conductive polymers, and various metal alloys, each offering unique advantages for specific applications.
Material properties directly impact electrode performance through several key mechanisms. Conductivity variations between materials affect signal transmission efficiency and sensitivity, with noble metals generally providing superior conductivity but at higher cost. Surface chemistry of electrode materials determines interaction with analytes and biological samples, influencing adsorption characteristics and potential for non-specific binding that can compromise measurement accuracy.
Biocompatibility considerations become paramount when microfluidic devices are employed for biological applications. Materials such as titanium and certain polymers demonstrate superior biocompatibility compared to conventional metals, reducing cellular toxicity and inflammatory responses. This property is particularly crucial for long-term cell culture applications and implantable microfluidic systems.
Durability and resistance to electrochemical degradation vary significantly across material classes. While platinum electrodes exhibit exceptional resistance to corrosion in various electrolyte solutions, silver electrodes may undergo rapid degradation in chloride-containing environments. This degradation not only compromises electrode functionality but can introduce contaminants into the microfluidic system, potentially affecting experimental results.
Nanomaterial integration represents a frontier in electrode development, with carbon nanotubes, graphene, and metal nanoparticles offering enhanced surface area and unique electrochemical properties. These materials enable increased sensitivity and can be functionalized to target specific analytes, though challenges in uniform integration and long-term stability remain.
Manufacturing considerations also influence material selection, as some advanced materials require specialized fabrication techniques that may limit scalability. The integration of electrode materials with the substrate materials of microfluidic chips presents additional challenges, particularly regarding adhesion strength and thermal expansion compatibility during fabrication processes.
Cost-effectiveness ultimately determines practical implementation, with precious metals offering superior performance but at prohibitive costs for many applications. The development of composite materials and novel fabrication techniques aims to balance performance requirements with economic constraints, enabling broader adoption of advanced microfluidic technologies across various fields.
Material properties directly impact electrode performance through several key mechanisms. Conductivity variations between materials affect signal transmission efficiency and sensitivity, with noble metals generally providing superior conductivity but at higher cost. Surface chemistry of electrode materials determines interaction with analytes and biological samples, influencing adsorption characteristics and potential for non-specific binding that can compromise measurement accuracy.
Biocompatibility considerations become paramount when microfluidic devices are employed for biological applications. Materials such as titanium and certain polymers demonstrate superior biocompatibility compared to conventional metals, reducing cellular toxicity and inflammatory responses. This property is particularly crucial for long-term cell culture applications and implantable microfluidic systems.
Durability and resistance to electrochemical degradation vary significantly across material classes. While platinum electrodes exhibit exceptional resistance to corrosion in various electrolyte solutions, silver electrodes may undergo rapid degradation in chloride-containing environments. This degradation not only compromises electrode functionality but can introduce contaminants into the microfluidic system, potentially affecting experimental results.
Nanomaterial integration represents a frontier in electrode development, with carbon nanotubes, graphene, and metal nanoparticles offering enhanced surface area and unique electrochemical properties. These materials enable increased sensitivity and can be functionalized to target specific analytes, though challenges in uniform integration and long-term stability remain.
Manufacturing considerations also influence material selection, as some advanced materials require specialized fabrication techniques that may limit scalability. The integration of electrode materials with the substrate materials of microfluidic chips presents additional challenges, particularly regarding adhesion strength and thermal expansion compatibility during fabrication processes.
Cost-effectiveness ultimately determines practical implementation, with precious metals offering superior performance but at prohibitive costs for many applications. The development of composite materials and novel fabrication techniques aims to balance performance requirements with economic constraints, enabling broader adoption of advanced microfluidic technologies across various fields.
Standardization and Quality Control in Microfluidic Manufacturing
The standardization and quality control in microfluidic manufacturing represent critical factors that directly influence electrode configuration performance and result reliability. Manufacturing variability in electrode placement, geometry, and surface properties can significantly impact experimental outcomes, creating challenges for result reproducibility across different microfluidic platforms.
Current industry standards for electrode manufacturing in microfluidic chips remain fragmented, with various fabrication techniques yielding different levels of precision. Photolithography-based methods typically achieve positioning accuracy within 1-5 μm, while screen printing techniques may introduce variations of 10-20 μm. These discrepancies become particularly problematic when studying phenomena requiring precise electric field distributions, such as dielectrophoresis or electrochemical detection.
Quality control protocols have evolved to address these manufacturing challenges, incorporating multi-stage inspection processes. Advanced optical characterization techniques, including confocal microscopy and white light interferometry, enable dimensional verification of electrode geometries with sub-micron resolution. Electrical characterization methods such as impedance spectroscopy provide functional validation of electrode performance before chip deployment.
Statistical process control (SPC) methodologies have been adapted specifically for microfluidic manufacturing, establishing control limits for critical electrode parameters. These include surface roughness (typically maintained below 50 nm RMS), edge definition (with deviation tolerances under 2 μm), and electrical conductivity uniformity (with acceptable variation under 5% across the electrode surface). Manufacturers increasingly implement automated vision systems for real-time monitoring during production.
Material consistency represents another quality control challenge, as electrode performance depends heavily on material purity and structural integrity. Gold electrodes require verification of layer thickness uniformity and adhesion strength to substrate materials, while carbon-based electrodes demand careful monitoring of functionalization processes to ensure consistent surface chemistry.
The development of reference materials and calibration standards specifically designed for microfluidic electrode systems has accelerated in recent years. These standards enable benchmarking of electrode performance across different manufacturing batches and facilities. Organizations including NIST and SEMI have initiated working groups focused on establishing standardized testing protocols for microfluidic electrode characterization.
Implementation of comprehensive quality management systems, incorporating elements from ISO 9001 and industry-specific guidelines, has demonstrated significant improvements in manufacturing consistency. Companies adopting these approaches report reduction in batch-to-batch variability by 30-50%, directly enhancing the reliability of experimental results obtained from electrode-based microfluidic systems.
Current industry standards for electrode manufacturing in microfluidic chips remain fragmented, with various fabrication techniques yielding different levels of precision. Photolithography-based methods typically achieve positioning accuracy within 1-5 μm, while screen printing techniques may introduce variations of 10-20 μm. These discrepancies become particularly problematic when studying phenomena requiring precise electric field distributions, such as dielectrophoresis or electrochemical detection.
Quality control protocols have evolved to address these manufacturing challenges, incorporating multi-stage inspection processes. Advanced optical characterization techniques, including confocal microscopy and white light interferometry, enable dimensional verification of electrode geometries with sub-micron resolution. Electrical characterization methods such as impedance spectroscopy provide functional validation of electrode performance before chip deployment.
Statistical process control (SPC) methodologies have been adapted specifically for microfluidic manufacturing, establishing control limits for critical electrode parameters. These include surface roughness (typically maintained below 50 nm RMS), edge definition (with deviation tolerances under 2 μm), and electrical conductivity uniformity (with acceptable variation under 5% across the electrode surface). Manufacturers increasingly implement automated vision systems for real-time monitoring during production.
Material consistency represents another quality control challenge, as electrode performance depends heavily on material purity and structural integrity. Gold electrodes require verification of layer thickness uniformity and adhesion strength to substrate materials, while carbon-based electrodes demand careful monitoring of functionalization processes to ensure consistent surface chemistry.
The development of reference materials and calibration standards specifically designed for microfluidic electrode systems has accelerated in recent years. These standards enable benchmarking of electrode performance across different manufacturing batches and facilities. Organizations including NIST and SEMI have initiated working groups focused on establishing standardized testing protocols for microfluidic electrode characterization.
Implementation of comprehensive quality management systems, incorporating elements from ISO 9001 and industry-specific guidelines, has demonstrated significant improvements in manufacturing consistency. Companies adopting these approaches report reduction in batch-to-batch variability by 30-50%, directly enhancing the reliability of experimental results obtained from electrode-based microfluidic systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!