How Are Microfluidic Chips Revolutionizing Biopharmaceutical Research?
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Technology Evolution and Research Objectives
Microfluidic technology has evolved significantly since its inception in the early 1990s, transforming from simple channel systems to sophisticated lab-on-a-chip platforms. The journey began with fundamental research on fluid behavior at microscale, pioneered by scientists like George Whitesides at Harvard University, who developed soft lithography techniques for creating microfluidic devices using polydimethylsiloxane (PDMS). This breakthrough dramatically reduced fabrication costs and accelerated innovation in the field.
By the early 2000s, microfluidic technology had expanded beyond academic curiosity to practical applications in various industries, particularly biopharmaceuticals. The integration of microelectromechanical systems (MEMS) with biological applications gave rise to bio-MEMS, enabling precise manipulation of biological samples at microscale. This convergence created unprecedented opportunities for drug discovery, diagnostics, and personalized medicine.
The evolution of microfluidic chips has been characterized by increasing complexity and functionality. Early designs focused on simple channel networks for fluid transport, while contemporary chips incorporate multiple functional elements such as mixers, separators, detectors, and reaction chambers. Advanced fabrication techniques now allow for three-dimensional architectures and the integration of various materials beyond PDMS, including glass, thermoplastics, and paper-based substrates.
Recent technological advancements have further expanded the capabilities of microfluidic systems. Organ-on-a-chip platforms now simulate the physiological environment of human organs, providing more accurate models for drug testing than traditional cell cultures. Droplet microfluidics enables high-throughput screening by generating thousands of discrete reaction vessels, each serving as an independent experimental unit. These innovations have dramatically accelerated drug discovery processes while reducing costs and minimizing animal testing.
The primary research objectives in microfluidic technology for biopharmaceutical applications focus on several key areas. First, enhancing throughput and parallelization to enable massive screening capabilities for drug candidates. Second, improving the physiological relevance of microfluidic models to better predict drug efficacy and toxicity in humans. Third, developing integrated systems that combine multiple analytical functions on a single chip, streamlining complex workflows in drug development.
Additional research goals include increasing automation and standardization to improve reproducibility and facilitate regulatory approval. There is also significant interest in developing point-of-care diagnostic applications that leverage microfluidic technology for rapid, sensitive detection of biomarkers. The ultimate vision is to create fully integrated microfluidic platforms that can guide drug development from initial discovery through preclinical testing, potentially revolutionizing the biopharmaceutical research paradigm.
By the early 2000s, microfluidic technology had expanded beyond academic curiosity to practical applications in various industries, particularly biopharmaceuticals. The integration of microelectromechanical systems (MEMS) with biological applications gave rise to bio-MEMS, enabling precise manipulation of biological samples at microscale. This convergence created unprecedented opportunities for drug discovery, diagnostics, and personalized medicine.
The evolution of microfluidic chips has been characterized by increasing complexity and functionality. Early designs focused on simple channel networks for fluid transport, while contemporary chips incorporate multiple functional elements such as mixers, separators, detectors, and reaction chambers. Advanced fabrication techniques now allow for three-dimensional architectures and the integration of various materials beyond PDMS, including glass, thermoplastics, and paper-based substrates.
Recent technological advancements have further expanded the capabilities of microfluidic systems. Organ-on-a-chip platforms now simulate the physiological environment of human organs, providing more accurate models for drug testing than traditional cell cultures. Droplet microfluidics enables high-throughput screening by generating thousands of discrete reaction vessels, each serving as an independent experimental unit. These innovations have dramatically accelerated drug discovery processes while reducing costs and minimizing animal testing.
The primary research objectives in microfluidic technology for biopharmaceutical applications focus on several key areas. First, enhancing throughput and parallelization to enable massive screening capabilities for drug candidates. Second, improving the physiological relevance of microfluidic models to better predict drug efficacy and toxicity in humans. Third, developing integrated systems that combine multiple analytical functions on a single chip, streamlining complex workflows in drug development.
Additional research goals include increasing automation and standardization to improve reproducibility and facilitate regulatory approval. There is also significant interest in developing point-of-care diagnostic applications that leverage microfluidic technology for rapid, sensitive detection of biomarkers. The ultimate vision is to create fully integrated microfluidic platforms that can guide drug development from initial discovery through preclinical testing, potentially revolutionizing the biopharmaceutical research paradigm.
Biopharmaceutical Market Demand for Microfluidic Solutions
The biopharmaceutical industry is experiencing unprecedented growth, with the global market projected to reach $856 billion by 2030, growing at a CAGR of 8.5% from 2023. This expansion is driving significant demand for innovative research and development tools, particularly microfluidic solutions that can accelerate drug discovery and development processes while reducing costs.
Pharmaceutical companies face mounting pressure to shorten development timelines, which traditionally span 10-15 years and cost upwards of $2.6 billion per successful drug. Microfluidic technologies directly address this challenge by enabling high-throughput screening, reducing sample volumes, and providing more physiologically relevant testing environments compared to conventional methods.
The demand for organ-on-a-chip and human-on-a-chip platforms has surged dramatically, with market analysts reporting a 25% annual growth rate in this segment. These platforms allow researchers to model human physiology and disease states with unprecedented accuracy, significantly improving the predictive value of preclinical testing and potentially reducing the 90% failure rate of drug candidates in clinical trials.
Personalized medicine initiatives are further driving demand for microfluidic solutions. As treatment paradigms shift toward targeted therapies based on individual genetic profiles, pharmaceutical companies require technologies capable of efficiently analyzing patient samples and testing drug responses at the individual level. Microfluidic devices that can perform rapid genetic analysis and drug sensitivity testing are experiencing particularly strong demand growth.
Contract research organizations (CROs) and academic institutions represent another significant market segment, collectively accounting for approximately 40% of the current demand for microfluidic technologies in biopharmaceutical applications. These organizations value the cost-efficiency and space-saving benefits of microfluidic platforms, which allow them to conduct sophisticated research with limited resources.
Regulatory considerations are also influencing market demand. As regulatory bodies increasingly recognize data from organ-on-chip models as complementary or alternative to animal testing, pharmaceutical companies are investing in these technologies to potentially streamline the approval process and address ethical concerns related to animal testing.
Geographically, North America currently dominates the market for microfluidic solutions in biopharmaceutical research, accounting for 45% of global demand. However, the Asia-Pacific region is experiencing the fastest growth rate at 12% annually, driven by expanding research infrastructure in China, Japan, and Singapore, along with increasing government investments in biotechnology innovation.
The COVID-19 pandemic has further accelerated demand for microfluidic technologies, as companies seek more efficient ways to develop vaccines and therapeutics under compressed timelines. This has resulted in a 35% increase in adoption of microfluidic platforms for infectious disease research since 2020.
Pharmaceutical companies face mounting pressure to shorten development timelines, which traditionally span 10-15 years and cost upwards of $2.6 billion per successful drug. Microfluidic technologies directly address this challenge by enabling high-throughput screening, reducing sample volumes, and providing more physiologically relevant testing environments compared to conventional methods.
The demand for organ-on-a-chip and human-on-a-chip platforms has surged dramatically, with market analysts reporting a 25% annual growth rate in this segment. These platforms allow researchers to model human physiology and disease states with unprecedented accuracy, significantly improving the predictive value of preclinical testing and potentially reducing the 90% failure rate of drug candidates in clinical trials.
Personalized medicine initiatives are further driving demand for microfluidic solutions. As treatment paradigms shift toward targeted therapies based on individual genetic profiles, pharmaceutical companies require technologies capable of efficiently analyzing patient samples and testing drug responses at the individual level. Microfluidic devices that can perform rapid genetic analysis and drug sensitivity testing are experiencing particularly strong demand growth.
Contract research organizations (CROs) and academic institutions represent another significant market segment, collectively accounting for approximately 40% of the current demand for microfluidic technologies in biopharmaceutical applications. These organizations value the cost-efficiency and space-saving benefits of microfluidic platforms, which allow them to conduct sophisticated research with limited resources.
Regulatory considerations are also influencing market demand. As regulatory bodies increasingly recognize data from organ-on-chip models as complementary or alternative to animal testing, pharmaceutical companies are investing in these technologies to potentially streamline the approval process and address ethical concerns related to animal testing.
Geographically, North America currently dominates the market for microfluidic solutions in biopharmaceutical research, accounting for 45% of global demand. However, the Asia-Pacific region is experiencing the fastest growth rate at 12% annually, driven by expanding research infrastructure in China, Japan, and Singapore, along with increasing government investments in biotechnology innovation.
The COVID-19 pandemic has further accelerated demand for microfluidic technologies, as companies seek more efficient ways to develop vaccines and therapeutics under compressed timelines. This has resulted in a 35% increase in adoption of microfluidic platforms for infectious disease research since 2020.
Current Microfluidic Chip Capabilities and Technical Barriers
Microfluidic chip technology has evolved significantly over the past decade, enabling unprecedented capabilities in biopharmaceutical research. Current microfluidic platforms can manipulate fluids at the nanoliter to picoliter scale, allowing researchers to perform complex biological experiments with minimal sample consumption. These chips can integrate multiple laboratory functions including cell culture, sorting, lysis, and analysis on a single device, dramatically reducing experimental footprints and accelerating research timelines.
Advanced microfluidic systems now offer precise control over cellular microenvironments, enabling the creation of physiologically relevant conditions that better mimic in vivo settings. This capability has proven particularly valuable for drug screening applications, where researchers can observe cellular responses to pharmaceutical compounds in near-physiological conditions, potentially reducing the gap between laboratory findings and clinical outcomes.
Organ-on-a-chip technology represents one of the most promising applications, with current systems capable of replicating the functional units of human organs including liver, kidney, lung, and even blood-brain barrier models. These sophisticated platforms incorporate multiple cell types and can maintain their viability and functionality for extended periods, allowing for longitudinal studies of drug effects and toxicity profiles that were previously impossible with traditional cell culture methods.
Despite these advancements, significant technical barriers persist. Fabrication challenges remain a major limitation, particularly for mass production of complex microfluidic architectures. While PDMS (polydimethylsiloxane) remains the dominant material due to its optical transparency and biocompatibility, its tendency to absorb small hydrophobic molecules can interfere with drug testing applications. Alternative materials like thermoplastics offer better scalability but often lack the surface properties needed for certain biological applications.
Integration of sensing technologies presents another substantial challenge. While microfluidic chips excel at creating controlled environments, real-time monitoring of cellular responses often requires external equipment, limiting the portability and accessibility of these systems. Current on-chip sensing capabilities remain limited in sensitivity and specificity compared to conventional laboratory instruments.
Standardization issues further complicate widespread adoption. The field lacks unified design principles and manufacturing standards, resulting in significant variability between devices produced by different laboratories or companies. This hampers reproducibility of experimental results and slows technology transfer from academic research to industrial applications.
Biological complexity presents perhaps the most formidable barrier. While current systems can maintain viable cells, they still fall short of replicating the full complexity of human tissues. Vascularization, immune system components, and complex tissue-tissue interfaces remain difficult to incorporate, limiting the physiological relevance of current models for certain applications like immune-oncology research or studies of complex disease mechanisms.
Advanced microfluidic systems now offer precise control over cellular microenvironments, enabling the creation of physiologically relevant conditions that better mimic in vivo settings. This capability has proven particularly valuable for drug screening applications, where researchers can observe cellular responses to pharmaceutical compounds in near-physiological conditions, potentially reducing the gap between laboratory findings and clinical outcomes.
Organ-on-a-chip technology represents one of the most promising applications, with current systems capable of replicating the functional units of human organs including liver, kidney, lung, and even blood-brain barrier models. These sophisticated platforms incorporate multiple cell types and can maintain their viability and functionality for extended periods, allowing for longitudinal studies of drug effects and toxicity profiles that were previously impossible with traditional cell culture methods.
Despite these advancements, significant technical barriers persist. Fabrication challenges remain a major limitation, particularly for mass production of complex microfluidic architectures. While PDMS (polydimethylsiloxane) remains the dominant material due to its optical transparency and biocompatibility, its tendency to absorb small hydrophobic molecules can interfere with drug testing applications. Alternative materials like thermoplastics offer better scalability but often lack the surface properties needed for certain biological applications.
Integration of sensing technologies presents another substantial challenge. While microfluidic chips excel at creating controlled environments, real-time monitoring of cellular responses often requires external equipment, limiting the portability and accessibility of these systems. Current on-chip sensing capabilities remain limited in sensitivity and specificity compared to conventional laboratory instruments.
Standardization issues further complicate widespread adoption. The field lacks unified design principles and manufacturing standards, resulting in significant variability between devices produced by different laboratories or companies. This hampers reproducibility of experimental results and slows technology transfer from academic research to industrial applications.
Biological complexity presents perhaps the most formidable barrier. While current systems can maintain viable cells, they still fall short of replicating the full complexity of human tissues. Vascularization, immune system components, and complex tissue-tissue interfaces remain difficult to incorporate, limiting the physiological relevance of current models for certain applications like immune-oncology research or studies of complex disease mechanisms.
State-of-the-Art Microfluidic Chip Architectures
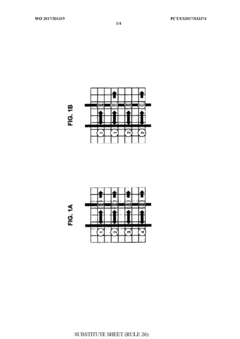
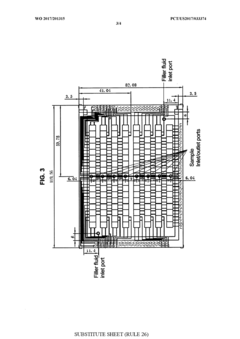
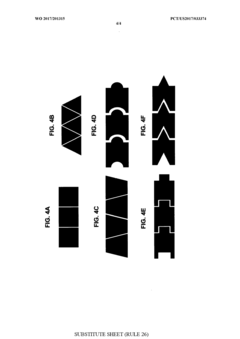
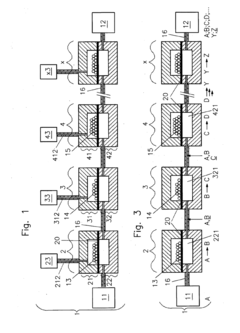
01 Microfluidic chip design and fabrication techniques
Advanced design and fabrication methods for microfluidic chips have revolutionized their capabilities. These techniques include multi-layer fabrication, 3D printing, and integration of various materials to create complex channel networks and structures. The innovations in fabrication allow for precise control over fluid flow, mixing, and separation at the microscale, enabling more sophisticated applications across multiple fields.- Microfluidic chip design and fabrication technologies: Advanced design and fabrication techniques for microfluidic chips have revolutionized their capabilities. These include novel materials, 3D printing methods, and integration of multiple functional layers. These fabrication technologies enable precise control of fluid flow at microscale, allowing for complex chip architectures that can perform sophisticated analytical functions while maintaining small form factors.
- Lab-on-a-chip diagnostic applications: Microfluidic chips are transforming diagnostic capabilities by integrating laboratory functions onto small devices. These lab-on-a-chip systems enable rapid, portable, and cost-effective testing for various diseases and conditions. They require minimal sample volumes and can perform multiple analytical steps including sample preparation, reaction, separation, and detection, making sophisticated diagnostics accessible in resource-limited settings.
- Integration with sensing and detection systems: Microfluidic chips integrated with advanced sensing and detection systems enable real-time monitoring and analysis. These systems incorporate optical, electrochemical, or mechanical sensors directly into the chip architecture. The integration allows for highly sensitive detection of biomarkers, pathogens, or chemical compounds at extremely low concentrations, enhancing analytical capabilities while maintaining the compact nature of the devices.
- Organ-on-a-chip and biological applications: Microfluidic technology has enabled the development of organ-on-a-chip platforms that mimic the physiological functions of human organs. These systems recreate tissue-tissue interfaces, mechanical forces, and biochemical environments found in living organs. They provide more accurate models for drug testing, disease modeling, and personalized medicine applications than traditional cell culture methods, potentially reducing animal testing and accelerating drug development processes.
- Automated and high-throughput microfluidic systems: Automated microfluidic platforms enable high-throughput analysis and processing capabilities. These systems incorporate programmable fluid handling, parallel processing channels, and integration with robotic systems. The automation reduces human error, increases reproducibility, and allows for continuous operation, making microfluidic technology suitable for industrial applications, large-scale screening, and complex multi-step protocols that would be labor-intensive if performed manually.
02 Integration of sensing and detection systems
Microfluidic chips with integrated sensing and detection capabilities have transformed analytical processes. These systems incorporate various detection methods such as optical, electrochemical, and impedance-based sensors directly into the chip architecture. This integration enables real-time monitoring and analysis of samples, significantly improving sensitivity, specificity, and throughput for applications in diagnostics, environmental monitoring, and chemical analysis.Expand Specific Solutions03 Lab-on-a-chip diagnostic applications
Microfluidic chips have revolutionized diagnostic testing by miniaturizing laboratory processes onto a single chip. These lab-on-a-chip devices enable rapid, point-of-care diagnostics with minimal sample volumes and reagent consumption. The technology allows for multiplexed testing, automation of complex protocols, and integration with data analysis systems, making sophisticated diagnostic capabilities accessible in resource-limited settings and enabling faster medical decision-making.Expand Specific Solutions04 Organ-on-a-chip and biological applications
Microfluidic technology has enabled the development of organ-on-a-chip platforms that mimic the physiological functions of human organs. These systems recreate tissue microenvironments with controlled fluid flow, mechanical forces, and cell-cell interactions. The technology provides more physiologically relevant models for drug testing, disease modeling, and personalized medicine applications, potentially reducing animal testing and accelerating drug development processes.Expand Specific Solutions05 Automated fluid handling and control systems
Advanced fluid handling and control systems have transformed microfluidic chip capabilities. These innovations include precise pumping mechanisms, valve systems, droplet generation, and flow control technologies that enable complex fluid manipulations at the microscale. Automated systems allow for programmable operations, reducing human intervention and error while increasing reproducibility and throughput for applications in research, diagnostics, and industrial processes.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
The microfluidic chip market in biopharmaceutical research is experiencing rapid growth, currently transitioning from early adoption to mainstream implementation. The market is projected to expand significantly, driven by increasing demand for point-of-care diagnostics and personalized medicine. Technologically, the field shows varying maturity levels across applications, with companies demonstrating different specializations. Established players like Roche Diagnostics and HP Development are investing heavily in platform technologies, while innovative startups like Lansion Biotechnology and Pattern Bioscience focus on novel applications. Academic institutions including Tsinghua University, Zhejiang University, and Yale University are advancing fundamental research, while research organizations like Agency for Science, Technology & Research and Dalian Institute of Chemical Physics are bridging the gap between academic innovation and commercial implementation.
Roche Sequencing Solutions, Inc.
Technical Solution: Roche Sequencing Solutions has developed advanced microfluidic chip platforms for next-generation sequencing applications in biopharmaceutical research. Their technology integrates sample preparation, DNA amplification, and sequencing chemistry on a single microfluidic device. The company's Nanopore sequencing platform utilizes microfluidic channels to control the movement of DNA molecules through nanopores, enabling real-time, long-read sequencing with minimal sample preparation. This approach allows researchers to analyze complex genomic structures and identify novel biomarkers for drug development. Their microfluidic chips incorporate precision flow control systems that maintain consistent reagent delivery and reaction conditions, critical for reproducible sequencing results in pharmaceutical R&D applications[1][3]. The technology enables multiplexed analysis of multiple samples simultaneously, significantly increasing throughput while reducing reagent consumption by up to 90% compared to conventional methods.
Strengths: Superior integration of multiple analytical processes on a single chip platform, reducing contamination risks and improving workflow efficiency. Their technology excels at handling limited sample volumes, making it ideal for precious clinical samples. Weaknesses: Higher initial investment costs compared to traditional sequencing methods and requires specialized expertise for operation and data interpretation.
F. Hoffmann-La Roche Ltd.
Technical Solution: F. Hoffmann-La Roche has developed sophisticated microfluidic chip platforms that revolutionize drug discovery and development processes. Their technology integrates multiple analytical functions on single microfluidic devices, enabling high-throughput screening of drug candidates against biological targets. Roche's microfluidic chips feature precision-engineered microchannels that control fluid dynamics at the nanoliter scale, allowing for precise manipulation of biological samples and reagents. The company has implemented digital microfluidics technology that uses electrowetting principles to move discrete droplets containing cells, proteins, or nucleic acids across arrays of electrodes without requiring continuous flow systems[5]. This approach enables complex assay protocols to be performed with minimal sample consumption. Their advanced chips incorporate integrated biosensors that provide real-time monitoring of biochemical reactions, protein-protein interactions, and cellular responses to drug compounds. Particularly noteworthy is Roche's application of microfluidic technology to antibody discovery, where their chips can screen thousands of single B cells to identify those producing antibodies with desired therapeutic properties, dramatically accelerating the identification of potential biologic drug candidates[6].
Strengths: Exceptional precision in fluid handling that enables consistent results even with minimal sample volumes, and comprehensive integration with downstream analytical systems for seamless data acquisition. Their platforms demonstrate remarkable versatility across different drug modalities including small molecules, antibodies, and nucleic acid therapeutics. Weaknesses: High manufacturing costs for their more sophisticated chip designs limit widespread adoption, and some applications require specialized expertise to optimize assay conditions for specific biological targets.
Breakthrough Patents and Publications in Microfluidic Technology
Quantitative real time PCR amplification using an electrowetting-based device
PatentWO2017201315A1
Innovation
- An electrowetting-based device is used to automate nucleic acid amplification by creating and manipulating droplets containing primers and target nucleic acids, allowing for parallel amplification, real-time quantitation, and recovery of the amplification product for further analysis.
Microfluidic bioreactor with modular design for synthesizing cell metabolites, method for using same, and use thereof
PatentInactiveUS20160326476A1
Innovation
- A modular microfluidic bioreactor with modules mimicking tissue structures, where different cell types communicate and exchange materials through porous membranes, allowing for the production of valuable metabolites by connecting cell and material chambers in series or parallel, enabling the upregulation of metabolic steps and the production of complex compounds not feasible in natural systems.
Regulatory Considerations for Microfluidic-Based Diagnostics
The regulatory landscape for microfluidic-based diagnostics presents a complex framework that developers must navigate to bring innovative solutions to market. In the United States, the Food and Drug Administration (FDA) classifies most microfluidic diagnostic devices as in vitro diagnostic (IVD) medical devices, requiring either premarket approval (PMA) or 510(k) clearance depending on risk classification. High-risk devices typically undergo more rigorous clinical validation studies, while moderate-risk devices may follow the 510(k) pathway by demonstrating substantial equivalence to predicate devices.
European regulatory frameworks have undergone significant transformation with the implementation of the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous In Vitro Diagnostic Directive (IVDD). This transition has introduced more stringent requirements for clinical evidence, post-market surveillance, and risk classification, particularly affecting novel microfluidic technologies that may not have clear precedents.
The regulatory considerations extend beyond initial approval to include manufacturing standards. Good Manufacturing Practices (GMP) compliance is essential, with particular attention to consistency in microfluidic chip production, where minor variations in channel dimensions or surface properties can significantly impact diagnostic performance. Quality management systems must address these unique challenges specific to microfluidics manufacturing.
Validation protocols for microfluidic diagnostics present unique challenges due to the integration of multiple analytical steps on a single platform. Regulatory bodies increasingly require demonstration of analytical validity (accuracy, precision, sensitivity, specificity) and clinical validity (clinical sensitivity, specificity, positive and negative predictive values) in intended use populations. The miniaturized nature of these systems often necessitates novel validation approaches that may not be fully addressed in existing regulatory guidelines.
Data management considerations have gained prominence with the integration of microfluidic diagnostics into digital health ecosystems. Regulations concerning data privacy (such as GDPR in Europe and HIPAA in the US) intersect with medical device regulations when diagnostic results are transmitted, stored, or analyzed using connected systems. Manufacturers must implement appropriate cybersecurity measures and data protection protocols as part of their regulatory compliance strategy.
Global harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) initiatives, are gradually creating more consistent pathways for microfluidic diagnostic approval across major markets. However, significant regional variations persist, creating challenges for companies seeking multinational deployment of their technologies. Strategic regulatory planning must account for these differences early in the development process.
Emerging regulatory frameworks for companion diagnostics are particularly relevant for microfluidic platforms in biopharmaceutical research, as these technologies increasingly enable personalized medicine approaches. Co-development and co-approval pathways between diagnostic devices and therapeutic products require careful coordination with multiple regulatory divisions and may influence clinical trial design for both components.
European regulatory frameworks have undergone significant transformation with the implementation of the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous In Vitro Diagnostic Directive (IVDD). This transition has introduced more stringent requirements for clinical evidence, post-market surveillance, and risk classification, particularly affecting novel microfluidic technologies that may not have clear precedents.
The regulatory considerations extend beyond initial approval to include manufacturing standards. Good Manufacturing Practices (GMP) compliance is essential, with particular attention to consistency in microfluidic chip production, where minor variations in channel dimensions or surface properties can significantly impact diagnostic performance. Quality management systems must address these unique challenges specific to microfluidics manufacturing.
Validation protocols for microfluidic diagnostics present unique challenges due to the integration of multiple analytical steps on a single platform. Regulatory bodies increasingly require demonstration of analytical validity (accuracy, precision, sensitivity, specificity) and clinical validity (clinical sensitivity, specificity, positive and negative predictive values) in intended use populations. The miniaturized nature of these systems often necessitates novel validation approaches that may not be fully addressed in existing regulatory guidelines.
Data management considerations have gained prominence with the integration of microfluidic diagnostics into digital health ecosystems. Regulations concerning data privacy (such as GDPR in Europe and HIPAA in the US) intersect with medical device regulations when diagnostic results are transmitted, stored, or analyzed using connected systems. Manufacturers must implement appropriate cybersecurity measures and data protection protocols as part of their regulatory compliance strategy.
Global harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and International Medical Device Regulators Forum (IMDRF) initiatives, are gradually creating more consistent pathways for microfluidic diagnostic approval across major markets. However, significant regional variations persist, creating challenges for companies seeking multinational deployment of their technologies. Strategic regulatory planning must account for these differences early in the development process.
Emerging regulatory frameworks for companion diagnostics are particularly relevant for microfluidic platforms in biopharmaceutical research, as these technologies increasingly enable personalized medicine approaches. Co-development and co-approval pathways between diagnostic devices and therapeutic products require careful coordination with multiple regulatory divisions and may influence clinical trial design for both components.
Scalability and Manufacturing Challenges for Microfluidic Devices
Despite the tremendous potential of microfluidic technology in biopharmaceutical research, significant scalability and manufacturing challenges persist that hinder widespread industrial adoption. The transition from laboratory prototypes to commercial-scale production represents a critical bottleneck in the microfluidics value chain.
Material selection presents a fundamental challenge, as materials must simultaneously satisfy biocompatibility requirements, manufacturing constraints, and cost considerations. While polydimethylsiloxane (PDMS) remains popular for prototyping due to its optical transparency and ease of fabrication, its limitations in mass production include absorption of small hydrophobic molecules, potential leaching of uncured oligomers, and challenges in standardization.
Fabrication techniques constitute another significant hurdle. Traditional methods like soft lithography excel in research settings but prove inefficient for high-volume manufacturing. Alternative approaches such as injection molding, hot embossing, and 3D printing each present distinct trade-offs between resolution, throughput, and cost-effectiveness. The industry continues to search for the optimal balance that can maintain nanoscale precision while enabling industrial-scale production.
Quality control and standardization represent persistent challenges in microfluidic manufacturing. The microscale nature of these devices demands extremely tight tolerances, with even minor variations potentially compromising functionality. Current inspection technologies struggle to provide rapid, non-destructive assessment of internal microfluidic structures at production speeds.
Integration with existing pharmaceutical manufacturing workflows presents additional complexity. Microfluidic platforms must interface seamlessly with established processes, requiring standardized connections, control systems, and validation protocols that satisfy stringent regulatory requirements for pharmaceutical production.
Cost considerations remain paramount, as current manufacturing approaches often involve specialized equipment and expertise. The economic viability of microfluidic solutions depends on achieving economies of scale that can compete with traditional batch processing methods in biopharmaceutical production.
Recent innovations addressing these challenges include advances in thermoplastic manufacturing, modular design approaches, and automated assembly techniques. Companies like Dolomite Microfluidics and Fluigent have developed standardized microfluidic components that facilitate scaling, while organizations such as the National Institute of Standards and Technology (NIST) are working to establish industry-wide standards for microfluidic manufacturing and testing.
Material selection presents a fundamental challenge, as materials must simultaneously satisfy biocompatibility requirements, manufacturing constraints, and cost considerations. While polydimethylsiloxane (PDMS) remains popular for prototyping due to its optical transparency and ease of fabrication, its limitations in mass production include absorption of small hydrophobic molecules, potential leaching of uncured oligomers, and challenges in standardization.
Fabrication techniques constitute another significant hurdle. Traditional methods like soft lithography excel in research settings but prove inefficient for high-volume manufacturing. Alternative approaches such as injection molding, hot embossing, and 3D printing each present distinct trade-offs between resolution, throughput, and cost-effectiveness. The industry continues to search for the optimal balance that can maintain nanoscale precision while enabling industrial-scale production.
Quality control and standardization represent persistent challenges in microfluidic manufacturing. The microscale nature of these devices demands extremely tight tolerances, with even minor variations potentially compromising functionality. Current inspection technologies struggle to provide rapid, non-destructive assessment of internal microfluidic structures at production speeds.
Integration with existing pharmaceutical manufacturing workflows presents additional complexity. Microfluidic platforms must interface seamlessly with established processes, requiring standardized connections, control systems, and validation protocols that satisfy stringent regulatory requirements for pharmaceutical production.
Cost considerations remain paramount, as current manufacturing approaches often involve specialized equipment and expertise. The economic viability of microfluidic solutions depends on achieving economies of scale that can compete with traditional batch processing methods in biopharmaceutical production.
Recent innovations addressing these challenges include advances in thermoplastic manufacturing, modular design approaches, and automated assembly techniques. Companies like Dolomite Microfluidics and Fluigent have developed standardized microfluidic components that facilitate scaling, while organizations such as the National Institute of Standards and Technology (NIST) are working to establish industry-wide standards for microfluidic manufacturing and testing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!