Technical Advancements in Microfluidic Chips for Biochemical Analyses
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Chip Evolution and Research Objectives
Microfluidic technology has evolved significantly since its inception in the early 1990s, transforming from simple channel structures to sophisticated integrated systems capable of performing complex biochemical analyses. The initial development phase focused primarily on proof-of-concept demonstrations using polydimethylsiloxane (PDMS) and glass substrates. By the early 2000s, researchers had established fundamental fabrication techniques including soft lithography, which dramatically reduced production costs and accelerated innovation in the field.
The mid-2000s marked a pivotal transition toward application-specific designs, with microfluidic platforms being adapted for DNA analysis, protein detection, and cell culture applications. This period saw the emergence of droplet-based microfluidics, enabling high-throughput screening and digital PCR capabilities that revolutionized genomic research. Concurrently, paper-based microfluidics gained traction as a low-cost alternative for point-of-care diagnostics in resource-limited settings.
Recent technological advancements have focused on integration and automation, with the development of organ-on-a-chip platforms that simulate physiological environments for drug testing and disease modeling. These systems incorporate multiple tissue types and sensing elements to provide comprehensive analytical capabilities. Additionally, 3D printing technologies have emerged as promising fabrication methods, offering rapid prototyping capabilities and complex geometries previously unattainable through conventional techniques.
The current research landscape is increasingly oriented toward clinical translation, with emphasis on standardization, reproducibility, and regulatory compliance. Efforts to develop plug-and-play microfluidic modules compatible with existing laboratory equipment have gained momentum, addressing the historical challenges of technology adoption in clinical settings. Furthermore, the integration of artificial intelligence and machine learning algorithms with microfluidic systems has opened new possibilities for automated data analysis and interpretation.
Looking forward, the primary research objectives in microfluidic technology for biochemical analyses include enhancing sensitivity and specificity for early disease detection, improving throughput capabilities for population-scale screening, and developing fully integrated sample-to-answer systems that minimize user intervention. There is also growing interest in sustainable manufacturing approaches using biodegradable materials and energy-efficient designs to reduce environmental impact.
Another critical objective is bridging the gap between academic research and commercial implementation by addressing scalability challenges and establishing robust quality control measures. The convergence of microfluidics with complementary technologies such as nanomaterials, biosensors, and wireless communication systems represents a promising direction for creating next-generation analytical platforms with unprecedented capabilities for healthcare, environmental monitoring, and basic scientific research.
The mid-2000s marked a pivotal transition toward application-specific designs, with microfluidic platforms being adapted for DNA analysis, protein detection, and cell culture applications. This period saw the emergence of droplet-based microfluidics, enabling high-throughput screening and digital PCR capabilities that revolutionized genomic research. Concurrently, paper-based microfluidics gained traction as a low-cost alternative for point-of-care diagnostics in resource-limited settings.
Recent technological advancements have focused on integration and automation, with the development of organ-on-a-chip platforms that simulate physiological environments for drug testing and disease modeling. These systems incorporate multiple tissue types and sensing elements to provide comprehensive analytical capabilities. Additionally, 3D printing technologies have emerged as promising fabrication methods, offering rapid prototyping capabilities and complex geometries previously unattainable through conventional techniques.
The current research landscape is increasingly oriented toward clinical translation, with emphasis on standardization, reproducibility, and regulatory compliance. Efforts to develop plug-and-play microfluidic modules compatible with existing laboratory equipment have gained momentum, addressing the historical challenges of technology adoption in clinical settings. Furthermore, the integration of artificial intelligence and machine learning algorithms with microfluidic systems has opened new possibilities for automated data analysis and interpretation.
Looking forward, the primary research objectives in microfluidic technology for biochemical analyses include enhancing sensitivity and specificity for early disease detection, improving throughput capabilities for population-scale screening, and developing fully integrated sample-to-answer systems that minimize user intervention. There is also growing interest in sustainable manufacturing approaches using biodegradable materials and energy-efficient designs to reduce environmental impact.
Another critical objective is bridging the gap between academic research and commercial implementation by addressing scalability challenges and establishing robust quality control measures. The convergence of microfluidics with complementary technologies such as nanomaterials, biosensors, and wireless communication systems represents a promising direction for creating next-generation analytical platforms with unprecedented capabilities for healthcare, environmental monitoring, and basic scientific research.
Market Analysis for Microfluidic-Based Biochemical Testing
The global microfluidic-based biochemical testing market has experienced substantial growth over the past decade, driven by increasing demand for point-of-care diagnostics, personalized medicine, and advanced research tools. Currently valued at approximately 23 billion USD, this market is projected to reach 50 billion USD by 2028, representing a compound annual growth rate of 16.7% during the forecast period.
Healthcare applications dominate the market landscape, accounting for nearly 60% of the total market share. Within this segment, point-of-care diagnostics represents the fastest-growing application area due to the rising prevalence of chronic diseases and the need for rapid, accurate testing in both clinical and home settings. The pharmaceutical and biotechnology sectors collectively contribute about 25% of market demand, primarily for drug discovery and development applications.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is witnessing the highest growth rate, fueled by increasing healthcare expenditure, growing research activities, and expanding biotechnology sectors in countries like China, Japan, and India.
The market is segmented by technology into electrokinetic, pressure-driven, and capillary systems, with pressure-driven systems currently holding the largest market share due to their reliability and precision in fluid handling. However, electrokinetic systems are gaining traction due to their minimal sample requirements and integration capabilities with electronic components.
Key market drivers include technological advancements in chip fabrication, increasing adoption of lab-on-a-chip technologies, growing demand for point-of-care diagnostics, and rising investment in healthcare infrastructure. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid, accurate diagnostic tools.
Challenges facing market expansion include high initial investment costs, technical complexities in device integration, and regulatory hurdles. The average cost of developing a commercial microfluidic device ranges from 2 million to 15 million USD, creating significant barriers to entry for smaller companies.
Consumer trends indicate growing preference for portable, user-friendly devices with smartphone connectivity and cloud-based data management. This shift is driving manufacturers to develop more integrated solutions that combine microfluidic technology with digital platforms, creating opportunities for cross-industry collaborations between traditional medical device manufacturers and technology companies.
Healthcare applications dominate the market landscape, accounting for nearly 60% of the total market share. Within this segment, point-of-care diagnostics represents the fastest-growing application area due to the rising prevalence of chronic diseases and the need for rapid, accurate testing in both clinical and home settings. The pharmaceutical and biotechnology sectors collectively contribute about 25% of market demand, primarily for drug discovery and development applications.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is witnessing the highest growth rate, fueled by increasing healthcare expenditure, growing research activities, and expanding biotechnology sectors in countries like China, Japan, and India.
The market is segmented by technology into electrokinetic, pressure-driven, and capillary systems, with pressure-driven systems currently holding the largest market share due to their reliability and precision in fluid handling. However, electrokinetic systems are gaining traction due to their minimal sample requirements and integration capabilities with electronic components.
Key market drivers include technological advancements in chip fabrication, increasing adoption of lab-on-a-chip technologies, growing demand for point-of-care diagnostics, and rising investment in healthcare infrastructure. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid, accurate diagnostic tools.
Challenges facing market expansion include high initial investment costs, technical complexities in device integration, and regulatory hurdles. The average cost of developing a commercial microfluidic device ranges from 2 million to 15 million USD, creating significant barriers to entry for smaller companies.
Consumer trends indicate growing preference for portable, user-friendly devices with smartphone connectivity and cloud-based data management. This shift is driving manufacturers to develop more integrated solutions that combine microfluidic technology with digital platforms, creating opportunities for cross-industry collaborations between traditional medical device manufacturers and technology companies.
Current Microfluidic Technology Landscape and Barriers
Microfluidic technology has experienced significant growth over the past two decades, evolving from simple channel designs to sophisticated integrated systems capable of performing complex biochemical analyses. Currently, the global landscape of microfluidic chip technology is characterized by a diverse ecosystem of approaches, with droplet-based microfluidics, digital microfluidics, and continuous flow systems representing the dominant paradigms. Each approach offers distinct advantages for specific biochemical applications, with droplet-based systems excelling in high-throughput single-cell analysis, while continuous flow platforms provide superior control for reaction kinetics studies.
Despite remarkable progress, several critical barriers impede wider adoption of microfluidic technologies in biochemical analyses. Fabrication challenges remain significant, particularly the high costs associated with cleanroom facilities and specialized equipment required for producing high-precision microfluidic structures. The industry continues to struggle with standardization issues, as the lack of universal design protocols and connection interfaces hampers interoperability between different microfluidic platforms and peripheral equipment.
Material limitations present another substantial hurdle. While polydimethylsiloxane (PDMS) remains the most widely used substrate due to its optical transparency and ease of prototyping, it suffers from drawbacks including solvent absorption, protein adsorption, and limited long-term stability. Alternative materials such as thermoplastics offer improved chemical resistance but present their own fabrication challenges and often lack the favorable optical properties of PDMS.
Integration difficulties between microfluidic chips and detection systems constitute a major technical barrier. The miniaturization of sensing technologies that can be incorporated directly into microfluidic platforms without compromising sensitivity remains challenging. Current solutions often require external bulky instrumentation, undermining the portability advantages inherent to microfluidic systems.
Scaling production from laboratory prototypes to commercial manufacturing represents a significant obstacle. Many innovative microfluidic designs demonstrate excellent performance in research settings but encounter reproducibility issues when transitioning to mass production. The industry lacks robust quality control methodologies specifically tailored to microfluidic manufacturing processes.
Biological compatibility issues persist, particularly for long-term cell culture applications. Surface modifications to prevent non-specific binding and maintain cell viability over extended periods remain technically demanding. Additionally, achieving consistent cell seeding and maintaining uniform microenvironments across chip platforms continues to challenge researchers.
Regulatory uncertainties further complicate commercial development, especially for diagnostic applications. The novel nature of many microfluidic technologies creates ambiguity in classification and validation requirements, extending development timelines and increasing costs for bringing products to market. These combined factors have created a fragmented landscape where technological innovation outpaces practical implementation in many biochemical analysis applications.
Despite remarkable progress, several critical barriers impede wider adoption of microfluidic technologies in biochemical analyses. Fabrication challenges remain significant, particularly the high costs associated with cleanroom facilities and specialized equipment required for producing high-precision microfluidic structures. The industry continues to struggle with standardization issues, as the lack of universal design protocols and connection interfaces hampers interoperability between different microfluidic platforms and peripheral equipment.
Material limitations present another substantial hurdle. While polydimethylsiloxane (PDMS) remains the most widely used substrate due to its optical transparency and ease of prototyping, it suffers from drawbacks including solvent absorption, protein adsorption, and limited long-term stability. Alternative materials such as thermoplastics offer improved chemical resistance but present their own fabrication challenges and often lack the favorable optical properties of PDMS.
Integration difficulties between microfluidic chips and detection systems constitute a major technical barrier. The miniaturization of sensing technologies that can be incorporated directly into microfluidic platforms without compromising sensitivity remains challenging. Current solutions often require external bulky instrumentation, undermining the portability advantages inherent to microfluidic systems.
Scaling production from laboratory prototypes to commercial manufacturing represents a significant obstacle. Many innovative microfluidic designs demonstrate excellent performance in research settings but encounter reproducibility issues when transitioning to mass production. The industry lacks robust quality control methodologies specifically tailored to microfluidic manufacturing processes.
Biological compatibility issues persist, particularly for long-term cell culture applications. Surface modifications to prevent non-specific binding and maintain cell viability over extended periods remain technically demanding. Additionally, achieving consistent cell seeding and maintaining uniform microenvironments across chip platforms continues to challenge researchers.
Regulatory uncertainties further complicate commercial development, especially for diagnostic applications. The novel nature of many microfluidic technologies creates ambiguity in classification and validation requirements, extending development timelines and increasing costs for bringing products to market. These combined factors have created a fragmented landscape where technological innovation outpaces practical implementation in many biochemical analysis applications.
State-of-the-Art Microfluidic Solutions for Biochemical Analysis
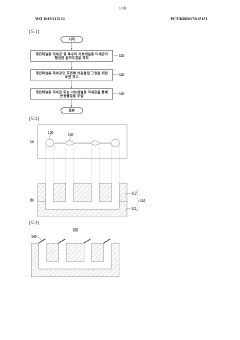
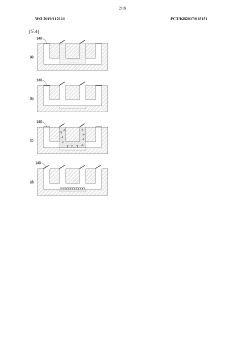
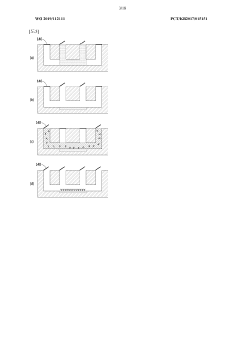
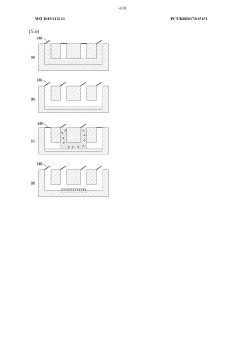
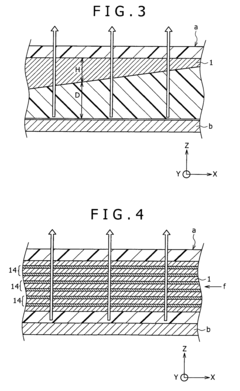
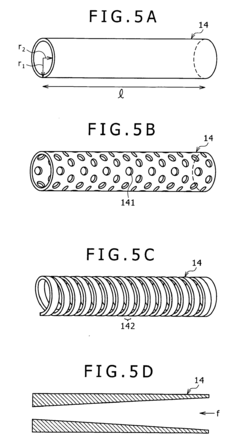
01 Fabrication techniques for microfluidic chips
Various fabrication methods are employed to create microfluidic chips with precise channel geometries and surface properties. These techniques include soft lithography, hot embossing, injection molding, and 3D printing. The choice of fabrication method depends on the desired application, material properties, and production scale. Advanced fabrication techniques allow for the creation of complex microstructures with high resolution and reproducibility, enabling sophisticated fluid handling capabilities.- Fabrication techniques for microfluidic chips: Various fabrication methods are employed to create microfluidic chips with precise channel geometries and surface properties. These techniques include soft lithography, injection molding, hot embossing, and 3D printing. The choice of fabrication method depends on the desired application, material properties, and production scale. Advanced manufacturing approaches enable the creation of complex microstructures with feature sizes down to the micrometer scale, allowing for precise fluid control and manipulation.
- Integration of sensing and detection systems: Microfluidic chips can be integrated with various sensing and detection systems to enable real-time monitoring and analysis of samples. These systems include optical sensors, electrochemical detectors, and impedance-based sensors that can detect biological or chemical analytes. The integration of these sensing technologies with microfluidic platforms enhances the functionality and applicability of the chips for diagnostic, analytical, and research purposes, allowing for rapid and sensitive detection of target molecules.
- Applications in biological and medical analysis: Microfluidic chips have extensive applications in biological and medical analysis, including DNA sequencing, protein analysis, cell sorting, and disease diagnostics. These chips enable the manipulation of small sample volumes, reducing reagent consumption and analysis time. The controlled microenvironment provided by these chips allows for precise cell culture, drug screening, and personalized medicine approaches. Their ability to perform multiple analytical steps on a single platform makes them valuable tools in biomedical research and clinical diagnostics.
- Fluid control and manipulation mechanisms: Microfluidic chips incorporate various mechanisms for fluid control and manipulation, including valves, pumps, mixers, and droplet generators. These components enable precise control over fluid flow, mixing, separation, and reaction conditions within the microchannels. Advanced designs utilize electrokinetic, pneumatic, or capillary forces to drive and control fluid movement. The integration of these control mechanisms allows for complex fluid handling operations to be performed on a miniaturized scale with high precision and reproducibility.
- Materials and surface modifications: The performance of microfluidic chips is significantly influenced by the materials used in their construction and the surface modifications applied. Common materials include glass, silicon, polymers (PDMS, PMMA), and paper. Surface modifications such as hydrophilic or hydrophobic coatings, chemical functionalization, and biomolecule immobilization can enhance specific interactions, prevent non-specific binding, and improve biocompatibility. The selection of appropriate materials and surface treatments is crucial for optimizing chip performance for specific applications.
02 Microfluidic chip materials and design
The selection of materials for microfluidic chips is critical for their performance and application. Common materials include polydimethylsiloxane (PDMS), glass, polymethyl methacrylate (PMMA), and silicon. The design of microfluidic chips incorporates various elements such as channels, chambers, valves, and mixers to control fluid flow and facilitate specific reactions or analyses. Material properties affect surface chemistry, optical transparency, mechanical strength, and compatibility with biological samples.Expand Specific Solutions03 Biological applications of microfluidic chips
Microfluidic chips are extensively used in biological research and diagnostics. Applications include cell culture, DNA analysis, protein separation, and single-cell analysis. These chips provide controlled microenvironments for studying cellular behavior, enable high-throughput screening of drugs, and facilitate point-of-care diagnostic tests. The small sample volumes required and the ability to integrate multiple analytical steps make microfluidic platforms particularly valuable for biological applications where sample quantities are limited.Expand Specific Solutions04 Integration of sensors and detection systems
Modern microfluidic chips often incorporate various sensing and detection technologies to monitor and analyze samples in real-time. These include optical sensors, electrochemical detectors, impedance sensors, and fluorescence detection systems. The integration of these technologies enables automated analysis, improves sensitivity, and allows for multiplexed detection of multiple analytes simultaneously. Advanced chips may also include temperature control elements and pressure sensors to maintain optimal conditions for specific applications.Expand Specific Solutions05 Microfluidic chip automation and control systems
Automation systems for microfluidic chips enable precise control of fluid flow, mixing, and separation processes. These systems may include micropumps, microvalves, flow controllers, and electronic interfaces for programmable operation. Advanced control systems allow for complex protocols to be executed with minimal user intervention, improving reproducibility and throughput. Integration with software platforms enables real-time monitoring, data acquisition, and analysis, making microfluidic systems more accessible for a wider range of applications.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
The microfluidic chip market for biochemical analyses is experiencing rapid growth, transitioning from early adoption to mainstream implementation. The global market is projected to reach significant scale, driven by increasing demand for point-of-care diagnostics and personalized medicine. Companies like Agilent Technologies, HP Development, and Sony Group represent established players with robust R&D capabilities, while specialized firms such as Lansion Biotechnology, 10X Genomics, and Cytonome are driving innovation with novel microfluidic technologies. Academic institutions including Tsinghua University, Fudan University, and Johns Hopkins University contribute significantly to technological advancement through research collaborations. The integration of microfluidics with AI and automation, led by companies like Tianma Microelectronics and Philips, is accelerating market maturation, though standardization challenges remain.
HP Development Co. LP
Technical Solution: HP has leveraged its expertise in precision fluid handling from inkjet printing technology to develop innovative microfluidic platforms for biochemical analysis. Their approach utilizes digital microfluidics where discrete droplets are manipulated on an array of electrodes using electrowetting principles. This technology enables programmable fluid routing without physical channels, offering unprecedented flexibility in protocol design. HP's microfluidic chips incorporate high-density electrode arrays with hydrophobic surface coatings and dielectric layers that enable precise control of droplet movement, splitting, and merging operations. Recent advancements include the integration of thermal control elements for on-chip PCR reactions, development of specialized surface treatments to minimize protein adsorption, and implementation of impedance-based sensing for droplet composition analysis. Their manufacturing approach leverages semiconductor fabrication techniques to produce highly reproducible electrode structures at scale. HP has also pioneered software control systems that allow users to design and execute complex biochemical protocols through intuitive graphical interfaces, making advanced microfluidic capabilities accessible to non-specialists.
Strengths: Exceptional flexibility in protocol design and execution; minimal dead volume reducing reagent consumption; ability to perform complex multi-step protocols on a single chip. Weaknesses: Lower throughput compared to continuous-flow systems; challenges with certain biochemical applications requiring continuous flow; higher complexity in electronic control systems.
AGILENT TECHNOLOGIES INC
Technical Solution: Agilent has developed advanced microfluidic lab-on-a-chip platforms that integrate multiple biochemical analysis functions onto a single chip. Their technology utilizes precision-etched microchannels in glass or polymer substrates with integrated electrodes for electrokinetic sample manipulation. Agilent's Bioanalyzer system employs microfluidic chips for nucleic acid and protein analysis, featuring automated sample loading, separation, staining, detection, and data analysis. The chips contain interconnected microchannels and reservoirs that enable precise control of nanoliter-volume samples. Their recent advancements include enhanced surface treatments to reduce biomolecule adsorption, improved detection sensitivity through integrated optical components, and development of multiplexed assay capabilities allowing simultaneous analysis of multiple samples or analytes on a single chip. Agilent has also pioneered microvalve technology for precise fluid control and mixing chambers for on-chip reagent preparation.
Strengths: Industry-leading precision in fluid handling at nanoliter volumes; robust manufacturing processes ensuring chip-to-chip reproducibility; comprehensive integration with analytical software. Weaknesses: Higher cost compared to conventional methods; limited flexibility for customization by end-users; requires specialized equipment for operation.
Key Patents and Scientific Breakthroughs in Microfluidics
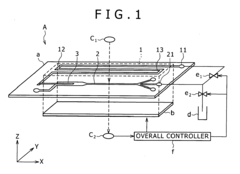
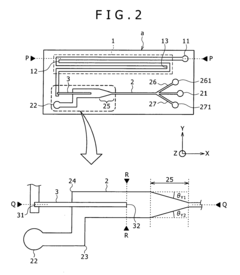
Microfluid analysis chip including micro injector and method for manufacturing same and method for using same
PatentWO2019112111A1
Innovation
- The microfluidic analysis chip incorporates a chip housing with a main channel microtubule and subchannel microtubules, equipped with an injector and a sealing film, along with a control unit that uses impedance, magnetic field, and optical measurements to determine sample or reagent presence, flow rate, and type, allowing for precise injection and mixing of samples and reagents.
Microparticle analysis device, microfluidic chip for microparticle analysis, and microparticle analysis method
PatentInactiveEP2191895A1
Innovation
- A microparticle analysis device with a micro-fluidic chip that uses a magnetic field to separate magnetic and non-magnetic microparticles, where the magnetic microparticles are held in the region and the non-magnetic microparticles are sent to a microfluidic channel for analysis, allowing for efficient sorting and detection using optical, electrical, or magnetic characteristics.
Materials Science Advancements for Microfluidic Applications
The evolution of microfluidic chip materials represents a critical advancement pathway for biochemical analysis applications. Traditional materials like glass and silicon, while offering excellent chemical resistance and optical transparency, present limitations in terms of manufacturing complexity and cost. Recent developments have shifted focus toward polymeric materials such as polydimethylsiloxane (PDMS), polymethyl methacrylate (PMMA), and cyclic olefin copolymer (COC), which offer improved fabrication flexibility and reduced production costs.
PDMS has emerged as a particularly versatile material due to its gas permeability, optical transparency, and biocompatibility. However, its hydrophobic nature can lead to non-specific protein adsorption, potentially compromising analytical results. To address this limitation, surface modification techniques including plasma treatment, chemical functionalization, and polymer grafting have been developed to enhance hydrophilicity and reduce biomolecule adsorption.
Paper-based microfluidic devices represent another significant materials advancement, offering extremely low-cost alternatives for point-of-care diagnostics in resource-limited settings. These cellulose-based platforms leverage capillary action for fluid transport, eliminating the need for external pumping systems and significantly simplifying device operation.
Hybrid material systems combining the advantages of different substrates have gained traction in recent years. Glass-polymer and silicon-polymer combinations offer enhanced performance characteristics while maintaining reasonable manufacturing costs. These hybrid approaches enable integration of specific functional elements like electrodes or sensors within microfluidic channels.
Stimuli-responsive materials represent the cutting edge of microfluidic material science, enabling dynamic control over surface properties and fluid behavior. Materials responsive to temperature, pH, light, or electrical stimuli can facilitate on-demand changes in hydrophobicity, permeability, or mechanical properties, creating programmable microenvironments for complex biochemical analyses.
Nanomaterial incorporation, including graphene, carbon nanotubes, and metal nanoparticles, has enhanced detection sensitivity and expanded functionality in microfluidic systems. These materials provide exceptional electrical conductivity, mechanical strength, and unique optical properties that significantly improve analytical performance parameters.
Biodegradable and environmentally friendly materials are gaining importance as sustainability concerns grow. Materials like polylactic acid (PLA), polyglycolic acid (PGA), and various biopolymers offer reduced environmental impact while maintaining performance characteristics suitable for many biochemical applications.
PDMS has emerged as a particularly versatile material due to its gas permeability, optical transparency, and biocompatibility. However, its hydrophobic nature can lead to non-specific protein adsorption, potentially compromising analytical results. To address this limitation, surface modification techniques including plasma treatment, chemical functionalization, and polymer grafting have been developed to enhance hydrophilicity and reduce biomolecule adsorption.
Paper-based microfluidic devices represent another significant materials advancement, offering extremely low-cost alternatives for point-of-care diagnostics in resource-limited settings. These cellulose-based platforms leverage capillary action for fluid transport, eliminating the need for external pumping systems and significantly simplifying device operation.
Hybrid material systems combining the advantages of different substrates have gained traction in recent years. Glass-polymer and silicon-polymer combinations offer enhanced performance characteristics while maintaining reasonable manufacturing costs. These hybrid approaches enable integration of specific functional elements like electrodes or sensors within microfluidic channels.
Stimuli-responsive materials represent the cutting edge of microfluidic material science, enabling dynamic control over surface properties and fluid behavior. Materials responsive to temperature, pH, light, or electrical stimuli can facilitate on-demand changes in hydrophobicity, permeability, or mechanical properties, creating programmable microenvironments for complex biochemical analyses.
Nanomaterial incorporation, including graphene, carbon nanotubes, and metal nanoparticles, has enhanced detection sensitivity and expanded functionality in microfluidic systems. These materials provide exceptional electrical conductivity, mechanical strength, and unique optical properties that significantly improve analytical performance parameters.
Biodegradable and environmentally friendly materials are gaining importance as sustainability concerns grow. Materials like polylactic acid (PLA), polyglycolic acid (PGA), and various biopolymers offer reduced environmental impact while maintaining performance characteristics suitable for many biochemical applications.
Standardization and Integration Challenges in Microfluidic Systems
Despite significant advancements in microfluidic chip technology for biochemical analyses, the field faces substantial challenges in standardization and integration that hinder widespread adoption and commercialization. The lack of universal standards for microfluidic components, connections, and interfaces creates significant barriers to interoperability between different systems and manufacturers, limiting the potential for modular design approaches.
Manufacturing inconsistencies represent a critical challenge, as variations in fabrication processes can lead to performance discrepancies between supposedly identical devices. This undermines reproducibility of experimental results and complicates quality control in commercial production. The absence of standardized testing protocols further exacerbates this issue, making it difficult to compare performance metrics across different microfluidic platforms.
Integration with existing laboratory infrastructure presents another significant hurdle. Many microfluidic systems require specialized equipment for operation, creating compatibility issues with conventional laboratory instruments. This incompatibility often necessitates complete workflow redesigns, increasing implementation costs and reducing adoption incentives for established laboratories with substantial investments in traditional equipment.
Data standardization remains underdeveloped in the microfluidic field, with inconsistent formats for reporting experimental parameters and results. This impedes data sharing, meta-analyses, and the development of comprehensive databases that could accelerate innovation. The lack of standardized data protocols also complicates regulatory approval processes for clinical applications, as validation becomes more challenging without clear benchmarks.
Material compatibility issues further complicate integration efforts. Different biochemical analyses may require specific surface properties or material characteristics, yet standardizing these aspects while maintaining versatility remains difficult. Chemical interactions between samples and device materials can affect analysis results, necessitating careful consideration of material selection for each application.
Scaling production while maintaining performance consistency represents a significant challenge for commercialization. Many microfluidic designs that perform well in research settings encounter difficulties when translated to mass production, often requiring substantial redesign to accommodate manufacturing constraints. This "valley of death" between laboratory prototype and commercial product has slowed market penetration of many promising microfluidic technologies.
Addressing these standardization and integration challenges requires coordinated efforts across academia, industry, and regulatory bodies. Several international initiatives have emerged to develop consensus standards, including the Microfluidics Association's working groups on interface standardization and the ISO Technical Committee on microfluidic devices. These collaborative efforts aim to establish common frameworks that could accelerate innovation while ensuring reliability and interoperability in microfluidic systems for biochemical analyses.
Manufacturing inconsistencies represent a critical challenge, as variations in fabrication processes can lead to performance discrepancies between supposedly identical devices. This undermines reproducibility of experimental results and complicates quality control in commercial production. The absence of standardized testing protocols further exacerbates this issue, making it difficult to compare performance metrics across different microfluidic platforms.
Integration with existing laboratory infrastructure presents another significant hurdle. Many microfluidic systems require specialized equipment for operation, creating compatibility issues with conventional laboratory instruments. This incompatibility often necessitates complete workflow redesigns, increasing implementation costs and reducing adoption incentives for established laboratories with substantial investments in traditional equipment.
Data standardization remains underdeveloped in the microfluidic field, with inconsistent formats for reporting experimental parameters and results. This impedes data sharing, meta-analyses, and the development of comprehensive databases that could accelerate innovation. The lack of standardized data protocols also complicates regulatory approval processes for clinical applications, as validation becomes more challenging without clear benchmarks.
Material compatibility issues further complicate integration efforts. Different biochemical analyses may require specific surface properties or material characteristics, yet standardizing these aspects while maintaining versatility remains difficult. Chemical interactions between samples and device materials can affect analysis results, necessitating careful consideration of material selection for each application.
Scaling production while maintaining performance consistency represents a significant challenge for commercialization. Many microfluidic designs that perform well in research settings encounter difficulties when translated to mass production, often requiring substantial redesign to accommodate manufacturing constraints. This "valley of death" between laboratory prototype and commercial product has slowed market penetration of many promising microfluidic technologies.
Addressing these standardization and integration challenges requires coordinated efforts across academia, industry, and regulatory bodies. Several international initiatives have emerged to develop consensus standards, including the Microfluidics Association's working groups on interface standardization and the ISO Technical Committee on microfluidic devices. These collaborative efforts aim to establish common frameworks that could accelerate innovation while ensuring reliability and interoperability in microfluidic systems for biochemical analyses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!