What Influences the Safety and Compliance of Microfluidic Chips?
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Chip Safety Background and Objectives
Microfluidic technology has evolved significantly over the past three decades, transforming from a conceptual laboratory technique to a commercially viable platform with applications spanning healthcare, pharmaceuticals, environmental monitoring, and chemical synthesis. The miniaturization of fluid handling systems has enabled unprecedented control over small sample volumes, offering advantages in efficiency, speed, and cost-effectiveness compared to traditional analytical methods.
The safety and compliance aspects of microfluidic chips have become increasingly important as these devices transition from research tools to commercial products that directly impact human health and environmental safety. Early microfluidic systems focused primarily on functionality, with less emphasis on safety considerations. However, as applications expanded into clinical diagnostics, drug delivery, and food safety testing, regulatory scrutiny has intensified.
Recent technological advancements have introduced new materials, fabrication techniques, and integration methods that present novel safety challenges. The incorporation of nanomaterials, biological components, and complex electronic elements into microfluidic platforms has created multifaceted safety considerations that extend beyond traditional biocompatibility concerns to include electrical safety, chemical stability, and long-term reliability.
The global regulatory landscape for microfluidic devices remains fragmented, with different regions implementing varying standards and requirements. This inconsistency creates challenges for manufacturers seeking international market access and complicates the establishment of universal safety benchmarks. The FDA, EMA, and NMPA have each developed distinct approaches to evaluating microfluidic technologies, particularly those intended for diagnostic or therapeutic applications.
The primary objective of this technical research is to comprehensively identify and analyze the factors that influence the safety and compliance of microfluidic chips across their entire lifecycle. This includes examining material selection considerations, fabrication process controls, design principles that enhance safety, testing methodologies, and regulatory pathways across major markets.
Additionally, this research aims to establish a framework for evaluating emerging safety concerns as microfluidic technology continues to evolve, particularly regarding novel materials, increased integration complexity, and expanded application domains. By anticipating future challenges, we can develop proactive strategies to address potential safety issues before they manifest in commercial products.
The ultimate goal is to provide actionable insights that can guide the development of safer microfluidic platforms while maintaining the innovative potential that makes this technology so promising for addressing critical challenges in healthcare, environmental monitoring, and industrial applications.
The safety and compliance aspects of microfluidic chips have become increasingly important as these devices transition from research tools to commercial products that directly impact human health and environmental safety. Early microfluidic systems focused primarily on functionality, with less emphasis on safety considerations. However, as applications expanded into clinical diagnostics, drug delivery, and food safety testing, regulatory scrutiny has intensified.
Recent technological advancements have introduced new materials, fabrication techniques, and integration methods that present novel safety challenges. The incorporation of nanomaterials, biological components, and complex electronic elements into microfluidic platforms has created multifaceted safety considerations that extend beyond traditional biocompatibility concerns to include electrical safety, chemical stability, and long-term reliability.
The global regulatory landscape for microfluidic devices remains fragmented, with different regions implementing varying standards and requirements. This inconsistency creates challenges for manufacturers seeking international market access and complicates the establishment of universal safety benchmarks. The FDA, EMA, and NMPA have each developed distinct approaches to evaluating microfluidic technologies, particularly those intended for diagnostic or therapeutic applications.
The primary objective of this technical research is to comprehensively identify and analyze the factors that influence the safety and compliance of microfluidic chips across their entire lifecycle. This includes examining material selection considerations, fabrication process controls, design principles that enhance safety, testing methodologies, and regulatory pathways across major markets.
Additionally, this research aims to establish a framework for evaluating emerging safety concerns as microfluidic technology continues to evolve, particularly regarding novel materials, increased integration complexity, and expanded application domains. By anticipating future challenges, we can develop proactive strategies to address potential safety issues before they manifest in commercial products.
The ultimate goal is to provide actionable insights that can guide the development of safer microfluidic platforms while maintaining the innovative potential that makes this technology so promising for addressing critical challenges in healthcare, environmental monitoring, and industrial applications.
Market Demand Analysis for Compliant Microfluidic Solutions
The global market for microfluidic chips has experienced significant growth in recent years, driven by increasing applications in healthcare, pharmaceuticals, and life sciences. The demand for compliant microfluidic solutions is particularly robust, with the market valued at approximately $16 billion in 2022 and projected to reach $42 billion by 2027, representing a compound annual growth rate of 21.3%.
Healthcare applications dominate the market demand, accounting for nearly 60% of the total market share. Within this segment, point-of-care diagnostics represents the fastest-growing application area, driven by the need for rapid, accurate, and portable testing solutions. The COVID-19 pandemic has further accelerated this trend, highlighting the critical importance of compliant microfluidic technologies in addressing global health challenges.
Pharmaceutical companies are increasingly adopting microfluidic technologies for drug discovery and development processes. The demand for compliant microfluidic solutions in this sector is driven by the need for high-throughput screening, precise control over experimental conditions, and reduced sample volumes. Industry reports indicate that pharmaceutical companies can reduce drug development costs by up to 30% through the implementation of microfluidic technologies.
Regulatory requirements are significantly shaping market demand for compliant microfluidic solutions. The FDA in the United States and the EMA in Europe have established stringent guidelines for medical devices incorporating microfluidic technologies. These regulations focus on biocompatibility, material safety, manufacturing consistency, and performance reliability. Consequently, there is growing demand for microfluidic chips that meet these regulatory standards from the design phase through to production.
Environmental considerations are also influencing market demand. The push toward sustainable healthcare solutions has created a market segment for eco-friendly microfluidic chips made from biodegradable materials. This trend is particularly strong in European markets, where environmental regulations are more stringent.
From a geographical perspective, North America currently leads the market for compliant microfluidic solutions, accounting for approximately 40% of global demand. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by increasing healthcare expenditure, expanding research infrastructure, and growing awareness of advanced diagnostic technologies.
The industrial sector represents an emerging market for compliant microfluidic solutions, particularly in applications related to process monitoring and quality control. This sector values microfluidic technologies that can withstand harsh operating conditions while maintaining compliance with industry-specific regulations.
Healthcare applications dominate the market demand, accounting for nearly 60% of the total market share. Within this segment, point-of-care diagnostics represents the fastest-growing application area, driven by the need for rapid, accurate, and portable testing solutions. The COVID-19 pandemic has further accelerated this trend, highlighting the critical importance of compliant microfluidic technologies in addressing global health challenges.
Pharmaceutical companies are increasingly adopting microfluidic technologies for drug discovery and development processes. The demand for compliant microfluidic solutions in this sector is driven by the need for high-throughput screening, precise control over experimental conditions, and reduced sample volumes. Industry reports indicate that pharmaceutical companies can reduce drug development costs by up to 30% through the implementation of microfluidic technologies.
Regulatory requirements are significantly shaping market demand for compliant microfluidic solutions. The FDA in the United States and the EMA in Europe have established stringent guidelines for medical devices incorporating microfluidic technologies. These regulations focus on biocompatibility, material safety, manufacturing consistency, and performance reliability. Consequently, there is growing demand for microfluidic chips that meet these regulatory standards from the design phase through to production.
Environmental considerations are also influencing market demand. The push toward sustainable healthcare solutions has created a market segment for eco-friendly microfluidic chips made from biodegradable materials. This trend is particularly strong in European markets, where environmental regulations are more stringent.
From a geographical perspective, North America currently leads the market for compliant microfluidic solutions, accounting for approximately 40% of global demand. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by increasing healthcare expenditure, expanding research infrastructure, and growing awareness of advanced diagnostic technologies.
The industrial sector represents an emerging market for compliant microfluidic solutions, particularly in applications related to process monitoring and quality control. This sector values microfluidic technologies that can withstand harsh operating conditions while maintaining compliance with industry-specific regulations.
Current Safety Challenges in Microfluidic Technology
Microfluidic technology faces several critical safety challenges that must be addressed to ensure reliable and compliant operation in various applications. Material biocompatibility represents a primary concern, as microfluidic chips often interact directly with biological samples. The materials used in chip fabrication—typically polymers like PDMS, PMMA, or COC—may leach chemicals or absorb small molecules from samples, potentially compromising experimental results or introducing toxicity in diagnostic applications.
Cross-contamination presents another significant challenge, particularly in diagnostic and analytical applications where sample integrity is paramount. The microscale channels in these devices can retain residual materials from previous runs, leading to false results and compromised safety. This issue becomes especially critical in point-of-care diagnostics where cleaning between uses may be limited.
Sterilization compatibility poses unique difficulties for microfluidic devices. Many conventional sterilization methods such as autoclaving can damage polymer-based chips, while chemical sterilization agents may be absorbed by porous materials, later leaching into samples. This creates a complex balance between ensuring sterility and maintaining device integrity.
Bubble formation during operation represents an often-overlooked safety concern. Air bubbles can disrupt fluid flow, cause pressure fluctuations, and lead to unpredictable behavior in critical applications. In medical devices, such disruptions could lead to inaccurate dosing or diagnostic errors with potentially serious consequences.
Regulatory compliance frameworks for microfluidic technology remain fragmented and evolving. Devices intended for clinical applications must navigate complex approval pathways through agencies like the FDA or EMA, which have not fully adapted their guidelines to address the unique characteristics of microfluidic systems. This regulatory uncertainty creates challenges for manufacturers seeking to validate safety claims.
Manufacturing consistency presents another challenge, as microscale fabrication requires extremely tight tolerances. Variations in channel dimensions, surface properties, or bonding quality can lead to unpredictable fluid behavior and compromise safety. Current quality control methods struggle to efficiently detect these microscale defects at production scale.
Long-term stability and shelf-life considerations further complicate safety assessments. Many microfluidic devices experience material degradation over time, potentially releasing particles or changing surface properties. Understanding and predicting these aging effects remains difficult, particularly for devices intended for long-term storage before use in critical applications.
Cross-contamination presents another significant challenge, particularly in diagnostic and analytical applications where sample integrity is paramount. The microscale channels in these devices can retain residual materials from previous runs, leading to false results and compromised safety. This issue becomes especially critical in point-of-care diagnostics where cleaning between uses may be limited.
Sterilization compatibility poses unique difficulties for microfluidic devices. Many conventional sterilization methods such as autoclaving can damage polymer-based chips, while chemical sterilization agents may be absorbed by porous materials, later leaching into samples. This creates a complex balance between ensuring sterility and maintaining device integrity.
Bubble formation during operation represents an often-overlooked safety concern. Air bubbles can disrupt fluid flow, cause pressure fluctuations, and lead to unpredictable behavior in critical applications. In medical devices, such disruptions could lead to inaccurate dosing or diagnostic errors with potentially serious consequences.
Regulatory compliance frameworks for microfluidic technology remain fragmented and evolving. Devices intended for clinical applications must navigate complex approval pathways through agencies like the FDA or EMA, which have not fully adapted their guidelines to address the unique characteristics of microfluidic systems. This regulatory uncertainty creates challenges for manufacturers seeking to validate safety claims.
Manufacturing consistency presents another challenge, as microscale fabrication requires extremely tight tolerances. Variations in channel dimensions, surface properties, or bonding quality can lead to unpredictable fluid behavior and compromise safety. Current quality control methods struggle to efficiently detect these microscale defects at production scale.
Long-term stability and shelf-life considerations further complicate safety assessments. Many microfluidic devices experience material degradation over time, potentially releasing particles or changing surface properties. Understanding and predicting these aging effects remains difficult, particularly for devices intended for long-term storage before use in critical applications.
Existing Safety and Compliance Solutions
01 Biocompatibility and material safety standards
Microfluidic chips must be manufactured using biocompatible materials that do not release harmful substances when in contact with biological samples. These materials need to comply with specific safety standards to ensure they don't interfere with test results or cause adverse reactions. The selection of polymers, glass, or silicon substrates must consider their interaction with biological fluids, cells, and reagents. Manufacturers must perform biocompatibility testing and adhere to material safety regulations to ensure the chips are safe for their intended applications.- Biocompatibility and material safety standards: Microfluidic chips must be manufactured using biocompatible materials that do not release harmful substances when in contact with biological samples. These materials need to comply with international safety standards to ensure they don't cause adverse reactions in biological testing. The selection of appropriate polymers, glass, or silicon substrates is critical for maintaining sample integrity and preventing contamination that could compromise test results or pose health risks.
- Regulatory compliance frameworks for microfluidic devices: Microfluidic chips used in diagnostic or medical applications must adhere to regulatory frameworks such as FDA requirements in the US or CE marking in Europe. These regulations govern the validation, verification, and quality control processes required for approval. Manufacturers must demonstrate that their microfluidic devices meet performance specifications, safety requirements, and intended use criteria through documented testing and risk assessment procedures.
- Contamination prevention and cross-reactivity control: Effective microfluidic chip design must incorporate features that prevent cross-contamination between samples and reagents. This includes appropriate channel geometries, surface treatments, and barrier structures that maintain sample isolation. Advanced manufacturing techniques ensure that channels are properly sealed and that materials do not leach compounds that might interfere with analytical results. Quality control protocols must verify the absence of carryover between sequential analyses.
- Safety monitoring and failure detection systems: Integrated safety monitoring systems in microfluidic platforms can detect potential failures before they lead to hazardous conditions. These systems may include pressure sensors, flow monitors, and temperature controls that trigger alerts or automatic shutdown procedures when parameters exceed safe thresholds. Real-time monitoring capabilities ensure that microfluidic operations remain within validated safety parameters and provide documentation for compliance verification.
- Validation protocols and quality assurance: Comprehensive validation protocols are essential for ensuring microfluidic chip safety and compliance. These include performance qualification, installation qualification, and operational qualification procedures that verify chip functionality across intended operating conditions. Quality assurance measures involve statistical process control, batch testing, and documentation of manufacturing processes. Traceability systems track materials and production parameters to facilitate recall procedures if safety issues are identified after distribution.
02 Regulatory compliance and certification requirements
Microfluidic chips used in diagnostic or medical applications must comply with various regulatory frameworks including FDA regulations in the US, CE marking in Europe, and other international standards. This involves validation of manufacturing processes, quality control systems, and performance testing. Manufacturers need to maintain documentation of compliance testing, risk assessments, and quality management systems. Certification may require clinical validation studies and demonstration of consistent performance across production batches to ensure reliability and safety in clinical settings.Expand Specific Solutions03 Contamination prevention and quality control
Ensuring microfluidic chips remain free from contamination during manufacturing, storage, and use is critical for safety and reliability. This involves implementing clean room manufacturing processes, sterile packaging, and quality control testing protocols. Manufacturers must establish procedures to detect and prevent cross-contamination between samples and ensure the integrity of microchannels and reaction chambers. Quality control measures include visual inspections, functional testing, and validation of sterilization methods to maintain the purity of samples and accuracy of results.Expand Specific Solutions04 Safety monitoring and failure detection systems
Advanced microfluidic chips incorporate safety monitoring features and failure detection systems to prevent hazardous conditions or inaccurate results. These systems may include pressure sensors to detect blockages, temperature monitors to prevent overheating, and leak detection mechanisms. Integrated safety protocols can automatically shut down operations if abnormal conditions are detected. Real-time monitoring capabilities allow for immediate identification of potential safety issues, reducing risks to users and ensuring the reliability of test results.Expand Specific Solutions05 Environmental impact and disposal considerations
The environmental safety aspects of microfluidic chips include considerations for proper disposal, recyclability of materials, and reduction of hazardous waste. Manufacturers are developing biodegradable or recyclable chip materials to minimize environmental impact. Proper disposal protocols must be established for chips that have been in contact with biological samples or hazardous reagents. Some designs incorporate features that neutralize or contain potentially harmful substances before disposal. Compliance with environmental regulations requires documentation of material composition and guidance for end-users on appropriate disposal methods.Expand Specific Solutions
Key Industry Players in Microfluidic Compliance
The microfluidic chip safety and compliance landscape is currently in a growth phase, with the market expected to reach significant expansion as applications diversify across healthcare, diagnostics, and research sectors. While technical maturity varies considerably among key players, companies like BOE Technology Group and Koninklijke Philips have established advanced manufacturing capabilities and quality control systems. Research institutions including Tsinghua University, Fudan University, and the Industrial Technology Research Institute are driving innovation in biocompatibility and standardization. Companies such as Lansion Biotechnology and Guangzhou Wondfo Biotech are focusing on clinical applications with rigorous compliance frameworks, while IBM and SRI International contribute advanced materials science expertise to address safety challenges in this evolving field.
Vanderbilt University
Technical Solution: Vanderbilt University has developed a pioneering approach to microfluidic chip safety and compliance through their "BioSafeChip" framework. Their methodology begins with comprehensive material biocompatibility assessment using both standard ISO 10993 protocols and advanced in vitro tissue models that more accurately predict human biological responses. Vanderbilt researchers have created specialized surface treatment processes that minimize protein adsorption and cellular adhesion to prevent sample contamination and analytical interference. Their microfluidic designs incorporate fail-safe containment features including redundant sealing mechanisms and pressure-activated isolation valves that prevent cross-contamination between sample chambers. Vanderbilt's compliance strategy includes standardized documentation templates that address FDA, EU MDR, and ISO requirements through systematic risk assessment and mitigation planning. They've pioneered advanced imaging techniques for non-destructive quality control inspection, allowing 100% verification of critical safety features without compromising chip integrity. Their research has established correlation models between accelerated aging tests and real-world performance, enabling more accurate predictions of long-term safety and stability.
Strengths: Vanderbilt's academic research orientation enables innovative approaches to safety challenges that may not be pursued in commercial settings. Their extensive biological expertise provides deeper insights into biocompatibility issues than many engineering-focused organizations. Weaknesses: As an academic institution, their solutions sometimes prioritize scientific novelty over practical implementation considerations. Commercialization pathways for their technologies may be less developed than industry competitors.
International Business Machines Corp.
Technical Solution: IBM has developed advanced microfluidic chip technologies with integrated safety protocols focusing on biocompatibility and contamination prevention. Their approach includes using medical-grade polymers (PDMS, COC) that meet ISO 10993 standards for biocompatibility. IBM's microfluidic platforms incorporate closed-loop monitoring systems that continuously assess fluid dynamics and detect potential leakages or cross-contamination events in real-time. Their compliance framework addresses both FDA and EU MDR requirements through comprehensive documentation and validation protocols. IBM has pioneered automated quality control processes using machine vision and AI algorithms to inspect microfluidic channels for manufacturing defects that could compromise safety. Additionally, they've implemented standardized testing protocols for chemical compatibility between reagents and chip materials to prevent degradation or unwanted reactions during operation.
Strengths: IBM's integration of AI-based monitoring systems provides superior real-time safety assessment capabilities. Their extensive experience with regulatory frameworks across multiple industries enables comprehensive compliance strategies. Weaknesses: Their solutions tend to be more expensive and complex than competitors, potentially limiting accessibility for smaller research institutions or startups. Implementation often requires specialized technical expertise.
Critical Patents and Literature on Microfluidic Safety
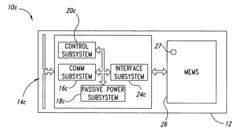
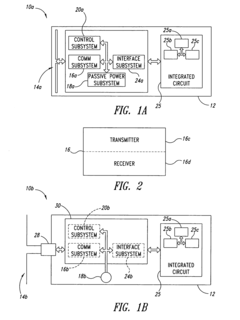
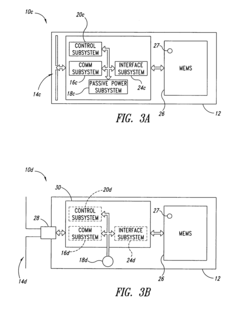
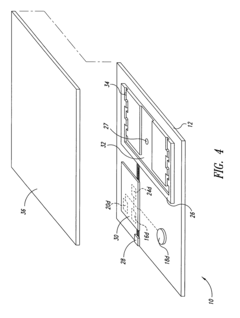
Integrated test-on-chip system and method and apparatus for manufacturing and operating same
PatentInactiveUS7249302B2
Innovation
- A microchip system comprising a substrate with a control subsystem for self-testing, a communications subsystem for wireless data transmission, and a passive power subsystem powered by an electromagnetic field, enabling self-check tests during manufacturing and operation, and facilitating fault detection and reduction of manufacturing costs.
Regulatory Framework for Microfluidic Applications
The regulatory landscape for microfluidic chips spans multiple jurisdictions and frameworks, creating a complex compliance environment for manufacturers and researchers. In the United States, the FDA oversees microfluidic devices through various pathways depending on their intended use, with diagnostic applications falling under the Clinical Laboratory Improvement Amendments (CLIA) and medical devices regulated through the 510(k) or Premarket Approval (PMA) processes. The classification of these devices—ranging from Class I to Class III—significantly impacts the regulatory burden and time-to-market.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR) and Medical Device Regulation (MDR), which impose stringent requirements for clinical validation, post-market surveillance, and technical documentation. These regulations emphasize risk-based approaches and require manufacturers to demonstrate both safety and performance through comprehensive clinical evidence.
In Asia, regulatory frameworks vary considerably. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific pathways for innovative medical technologies, while China's National Medical Products Administration (NMPA) has recently updated its regulations to accelerate approval processes for certain microfluidic applications, particularly those addressing public health priorities.
International standards play a crucial role in harmonizing these diverse regulatory requirements. ISO 13485 for quality management systems and IEC 62304 for medical device software are particularly relevant for microfluidic chip manufacturers. Additionally, the International Medical Device Regulators Forum (IMDRF) has been working to establish globally recognized principles for microfluidic technology evaluation.
Material biocompatibility represents a critical regulatory consideration, with standards such as ISO 10993 providing frameworks for biological evaluation. For microfluidic chips used in pharmaceutical development, Good Manufacturing Practice (GMP) compliance becomes essential, particularly regarding material selection and manufacturing processes that might introduce leachables or extractables.
Data privacy regulations, including GDPR in Europe and HIPAA in the US, introduce additional compliance requirements for microfluidic applications that generate or process patient data. These considerations are especially relevant for point-of-care diagnostic platforms and personalized medicine applications utilizing microfluidic technology.
Emerging regulatory trends indicate a shift toward adaptive licensing pathways that allow for iterative development and conditional approvals based on real-world performance data. This approach may prove particularly beneficial for novel microfluidic applications where traditional regulatory paradigms may impede innovation while still ensuring patient safety.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR) and Medical Device Regulation (MDR), which impose stringent requirements for clinical validation, post-market surveillance, and technical documentation. These regulations emphasize risk-based approaches and require manufacturers to demonstrate both safety and performance through comprehensive clinical evidence.
In Asia, regulatory frameworks vary considerably. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific pathways for innovative medical technologies, while China's National Medical Products Administration (NMPA) has recently updated its regulations to accelerate approval processes for certain microfluidic applications, particularly those addressing public health priorities.
International standards play a crucial role in harmonizing these diverse regulatory requirements. ISO 13485 for quality management systems and IEC 62304 for medical device software are particularly relevant for microfluidic chip manufacturers. Additionally, the International Medical Device Regulators Forum (IMDRF) has been working to establish globally recognized principles for microfluidic technology evaluation.
Material biocompatibility represents a critical regulatory consideration, with standards such as ISO 10993 providing frameworks for biological evaluation. For microfluidic chips used in pharmaceutical development, Good Manufacturing Practice (GMP) compliance becomes essential, particularly regarding material selection and manufacturing processes that might introduce leachables or extractables.
Data privacy regulations, including GDPR in Europe and HIPAA in the US, introduce additional compliance requirements for microfluidic applications that generate or process patient data. These considerations are especially relevant for point-of-care diagnostic platforms and personalized medicine applications utilizing microfluidic technology.
Emerging regulatory trends indicate a shift toward adaptive licensing pathways that allow for iterative development and conditional approvals based on real-world performance data. This approach may prove particularly beneficial for novel microfluidic applications where traditional regulatory paradigms may impede innovation while still ensuring patient safety.
Material Biocompatibility and Risk Assessment
Material biocompatibility represents a critical factor in microfluidic chip safety and compliance, particularly for applications involving biological samples or medical diagnostics. The materials used in chip fabrication must not leach harmful substances into samples, alter biological processes, or trigger immune responses when in contact with human tissues. Common materials such as polydimethylsiloxane (PDMS), polymethyl methacrylate (PMMA), and glass each present distinct biocompatibility profiles that must be thoroughly evaluated before implementation in clinical or biological research settings.
Risk assessment frameworks for microfluidic devices typically follow a structured approach that includes hazard identification, exposure assessment, dose-response assessment, and risk characterization. These assessments must consider both direct risks (such as cytotoxicity or inflammatory responses) and indirect risks (such as interference with analytical results or sample degradation). International standards like ISO 10993 provide guidelines for biological evaluation of medical devices, which can be adapted for microfluidic applications.
Material degradation over time presents another significant concern, as environmental factors, sample chemistry, and operational conditions can alter material properties. For instance, PDMS may absorb small hydrophobic molecules from samples, potentially compromising analytical accuracy and introducing unexpected biological effects. Systematic stability testing under various conditions is therefore essential to predict device performance throughout its intended lifecycle.
Surface modifications and coatings often employed to enhance chip functionality must undergo separate biocompatibility evaluations. Treatments designed to alter hydrophobicity, prevent protein adsorption, or enable specific molecular interactions introduce additional chemical components that require thorough safety assessment. The interaction between these surface treatments and biological systems must be characterized across various timeframes and exposure scenarios.
Regulatory compliance for microfluidic chips varies significantly across global markets, with particularly stringent requirements for clinical applications. In the United States, the FDA classifies many microfluidic devices as medical devices subject to premarket approval processes, while the European Union applies CE marking requirements under the Medical Device Regulation (MDR). Manufacturers must navigate these complex regulatory landscapes by implementing comprehensive quality management systems and generating robust biocompatibility data through standardized testing protocols.
Emerging approaches to biocompatibility assessment include organ-on-chip models that simulate tissue responses more accurately than traditional cell culture methods, and computational toxicology tools that predict material-biological interactions. These advanced methodologies promise more relevant safety data while potentially reducing animal testing requirements in compliance with ethical considerations and regulatory trends toward alternative testing methods.
Risk assessment frameworks for microfluidic devices typically follow a structured approach that includes hazard identification, exposure assessment, dose-response assessment, and risk characterization. These assessments must consider both direct risks (such as cytotoxicity or inflammatory responses) and indirect risks (such as interference with analytical results or sample degradation). International standards like ISO 10993 provide guidelines for biological evaluation of medical devices, which can be adapted for microfluidic applications.
Material degradation over time presents another significant concern, as environmental factors, sample chemistry, and operational conditions can alter material properties. For instance, PDMS may absorb small hydrophobic molecules from samples, potentially compromising analytical accuracy and introducing unexpected biological effects. Systematic stability testing under various conditions is therefore essential to predict device performance throughout its intended lifecycle.
Surface modifications and coatings often employed to enhance chip functionality must undergo separate biocompatibility evaluations. Treatments designed to alter hydrophobicity, prevent protein adsorption, or enable specific molecular interactions introduce additional chemical components that require thorough safety assessment. The interaction between these surface treatments and biological systems must be characterized across various timeframes and exposure scenarios.
Regulatory compliance for microfluidic chips varies significantly across global markets, with particularly stringent requirements for clinical applications. In the United States, the FDA classifies many microfluidic devices as medical devices subject to premarket approval processes, while the European Union applies CE marking requirements under the Medical Device Regulation (MDR). Manufacturers must navigate these complex regulatory landscapes by implementing comprehensive quality management systems and generating robust biocompatibility data through standardized testing protocols.
Emerging approaches to biocompatibility assessment include organ-on-chip models that simulate tissue responses more accurately than traditional cell culture methods, and computational toxicology tools that predict material-biological interactions. These advanced methodologies promise more relevant safety data while potentially reducing animal testing requirements in compliance with ethical considerations and regulatory trends toward alternative testing methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!