Atomic Force Microscopy Vs Laser Scanning Microscopy: Clarity, Depth
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microscopy Technologies Background and Objectives
Microscopy has evolved significantly over the past century, transforming from simple optical systems to sophisticated technologies capable of visualizing structures at the nanoscale. Atomic Force Microscopy (AFM) and Laser Scanning Microscopy (LSM) represent two distinct approaches that have revolutionized our ability to examine surfaces and biological specimens with unprecedented clarity and depth resolution.
AFM emerged in the 1980s as an extension of scanning tunneling microscopy, utilizing a physical probe to map surface topography through mechanical interactions. This technology marked a paradigm shift by enabling researchers to visualize non-conductive samples in various environments, including ambient conditions, liquids, and vacuum. The evolution of AFM has been characterized by continuous improvements in probe design, force detection sensitivity, and scanning speeds.
In parallel, Laser Scanning Microscopy has developed along a different technological trajectory. Beginning with confocal microscopy in the 1950s and advancing to multiphoton techniques in the 1990s, LSM leverages focused laser beams and optical sectioning to generate three-dimensional images of specimens. The development of fluorescent markers and super-resolution techniques has further enhanced the capabilities of LSM systems.
The primary objective of comparing these technologies is to establish a comprehensive understanding of their respective strengths and limitations in terms of image clarity and depth resolution. AFM excels in providing topographical information with nanometer-scale resolution in the z-axis, while LSM offers superior optical sectioning capabilities for volumetric imaging of transparent specimens.
Current technological trends indicate convergence and complementarity between these approaches. Correlative microscopy, combining AFM with various optical techniques, represents an emerging field that aims to leverage the strengths of both methodologies. Additionally, advancements in computational image processing and artificial intelligence are enhancing the resolution and information content obtainable from both platforms.
The ultimate goal of this technical assessment is to evaluate how these technologies can be optimally deployed across different application domains, including materials science, semiconductor manufacturing, biological research, and medical diagnostics. By understanding the fundamental principles, current capabilities, and future development trajectories of AFM and LSM, we can identify opportunities for technological innovation and strategic investment in microscopy infrastructure.
AFM emerged in the 1980s as an extension of scanning tunneling microscopy, utilizing a physical probe to map surface topography through mechanical interactions. This technology marked a paradigm shift by enabling researchers to visualize non-conductive samples in various environments, including ambient conditions, liquids, and vacuum. The evolution of AFM has been characterized by continuous improvements in probe design, force detection sensitivity, and scanning speeds.
In parallel, Laser Scanning Microscopy has developed along a different technological trajectory. Beginning with confocal microscopy in the 1950s and advancing to multiphoton techniques in the 1990s, LSM leverages focused laser beams and optical sectioning to generate three-dimensional images of specimens. The development of fluorescent markers and super-resolution techniques has further enhanced the capabilities of LSM systems.
The primary objective of comparing these technologies is to establish a comprehensive understanding of their respective strengths and limitations in terms of image clarity and depth resolution. AFM excels in providing topographical information with nanometer-scale resolution in the z-axis, while LSM offers superior optical sectioning capabilities for volumetric imaging of transparent specimens.
Current technological trends indicate convergence and complementarity between these approaches. Correlative microscopy, combining AFM with various optical techniques, represents an emerging field that aims to leverage the strengths of both methodologies. Additionally, advancements in computational image processing and artificial intelligence are enhancing the resolution and information content obtainable from both platforms.
The ultimate goal of this technical assessment is to evaluate how these technologies can be optimally deployed across different application domains, including materials science, semiconductor manufacturing, biological research, and medical diagnostics. By understanding the fundamental principles, current capabilities, and future development trajectories of AFM and LSM, we can identify opportunities for technological innovation and strategic investment in microscopy infrastructure.
Market Applications and Demand Analysis
The market for advanced microscopy technologies has witnessed significant growth in recent years, driven by increasing demand for high-resolution imaging across various scientific and industrial applications. Atomic Force Microscopy (AFM) and Laser Scanning Microscopy (LSM) represent two distinct approaches that address different market needs while occasionally competing in overlapping application spaces.
In the life sciences sector, LSM technologies have established a dominant position, with the global confocal microscopy market valued at approximately $1.1 billion in 2022 and projected to grow at a CAGR of 7.2% through 2030. This growth is primarily fueled by increasing research activities in cell biology, neuroscience, and developmental biology, where the non-invasive optical sectioning capabilities of LSM provide critical advantages for studying living specimens.
Conversely, the AFM market, valued at around $570 million in 2022, is experiencing faster growth at nearly 8.5% CAGR, driven by expanding applications in materials science, semiconductor manufacturing, and nanotechnology. The superior resolution capabilities of AFM, reaching atomic-level detail, have made it indispensable for industries requiring precise surface characterization and nanoscale measurements.
Healthcare and pharmaceutical research represent significant growth sectors for both technologies. LSM dominates in clinical pathology and diagnostic applications due to its speed and compatibility with fluorescent labeling techniques. Meanwhile, AFM is gaining traction in drug delivery research and biomaterial development, where nanoscale surface interactions critically influence functionality.
Regional market analysis reveals distinct adoption patterns, with North America leading in both technologies due to substantial research funding and established life science industries. Asia-Pacific represents the fastest-growing market, particularly for AFM systems, driven by expanding semiconductor manufacturing and materials research facilities in China, South Korea, and Taiwan.
Customer demand increasingly focuses on integrated solutions that combine multiple imaging modalities. This trend has spurred the development of correlative microscopy approaches that leverage the complementary strengths of AFM (superior spatial resolution) and LSM (superior depth imaging and molecular specificity through fluorescence). Market research indicates that systems offering such integration command premium pricing and are experiencing growth rates exceeding those of standalone systems.
End-user surveys highlight divergent priorities, with academic research institutions prioritizing versatility and cost-effectiveness, while industrial users emphasize throughput, reliability, and automation capabilities. This bifurcation has led to market segmentation with specialized instruments targeting specific application niches, alongside more flexible platforms designed for multi-purpose research environments.
In the life sciences sector, LSM technologies have established a dominant position, with the global confocal microscopy market valued at approximately $1.1 billion in 2022 and projected to grow at a CAGR of 7.2% through 2030. This growth is primarily fueled by increasing research activities in cell biology, neuroscience, and developmental biology, where the non-invasive optical sectioning capabilities of LSM provide critical advantages for studying living specimens.
Conversely, the AFM market, valued at around $570 million in 2022, is experiencing faster growth at nearly 8.5% CAGR, driven by expanding applications in materials science, semiconductor manufacturing, and nanotechnology. The superior resolution capabilities of AFM, reaching atomic-level detail, have made it indispensable for industries requiring precise surface characterization and nanoscale measurements.
Healthcare and pharmaceutical research represent significant growth sectors for both technologies. LSM dominates in clinical pathology and diagnostic applications due to its speed and compatibility with fluorescent labeling techniques. Meanwhile, AFM is gaining traction in drug delivery research and biomaterial development, where nanoscale surface interactions critically influence functionality.
Regional market analysis reveals distinct adoption patterns, with North America leading in both technologies due to substantial research funding and established life science industries. Asia-Pacific represents the fastest-growing market, particularly for AFM systems, driven by expanding semiconductor manufacturing and materials research facilities in China, South Korea, and Taiwan.
Customer demand increasingly focuses on integrated solutions that combine multiple imaging modalities. This trend has spurred the development of correlative microscopy approaches that leverage the complementary strengths of AFM (superior spatial resolution) and LSM (superior depth imaging and molecular specificity through fluorescence). Market research indicates that systems offering such integration command premium pricing and are experiencing growth rates exceeding those of standalone systems.
End-user surveys highlight divergent priorities, with academic research institutions prioritizing versatility and cost-effectiveness, while industrial users emphasize throughput, reliability, and automation capabilities. This bifurcation has led to market segmentation with specialized instruments targeting specific application niches, alongside more flexible platforms designed for multi-purpose research environments.
Current Capabilities and Technical Limitations
Atomic Force Microscopy (AFM) and Laser Scanning Microscopy (LSM) represent two distinct approaches to high-resolution imaging, each with specific capabilities and limitations that define their applicability across scientific and industrial domains. AFM offers exceptional resolution capabilities, routinely achieving sub-nanometer resolution in the vertical dimension and 1-5 nm laterally under optimal conditions. This remarkable precision allows for atomic-level surface characterization that remains unmatched by optical techniques.
AFM excels in providing three-dimensional topographical information of surfaces with minimal sample preparation, operating effectively in various environments including ambient conditions, liquid, and vacuum. The technique can measure mechanical properties such as elasticity, adhesion, and friction through force spectroscopy, offering valuable insights into material characteristics beyond mere topography.
However, AFM faces significant speed limitations, with typical scan rates ranging from several minutes to hours per image, making it unsuitable for dynamic processes requiring real-time observation. The technique is fundamentally restricted to surface or near-surface measurements, with a maximum depth penetration of only a few nanometers, preventing analysis of subsurface or internal structures.
In contrast, Laser Scanning Microscopy, particularly confocal LSM, offers optical sectioning capabilities with resolution approaching the diffraction limit (approximately 200-300 nm laterally and 500-700 nm axially). LSM enables rapid imaging with frame rates of several images per second, facilitating the observation of dynamic biological processes and time-dependent phenomena.
LSM's ability to penetrate samples to depths of several hundred micrometers represents a significant advantage over AFM, allowing for non-invasive examination of internal structures in transparent and semi-transparent specimens. The technique also offers versatile contrast mechanisms through fluorescence, reflectance, and transmitted light modalities, enabling specific targeting of structures through fluorescent labeling.
Nevertheless, LSM remains fundamentally limited by the diffraction barrier, preventing resolution of features smaller than approximately half the wavelength of light used. While super-resolution techniques have pushed beyond this limit, they often require specialized sample preparation, extended acquisition times, or introduce artifacts.
Both technologies face challenges with certain sample types: AFM struggles with highly rough surfaces or deeply recessed features due to tip geometry constraints, while LSM performs poorly with opaque samples or in environments with significant light scattering. Temperature stability affects both techniques, with AFM particularly sensitive to thermal drift that can distort measurements during long acquisition periods.
The complementary nature of these technologies has driven recent developments in correlative microscopy approaches, combining the surface precision of AFM with the depth capabilities of LSM to provide more comprehensive sample characterization across multiple scales and dimensions.
AFM excels in providing three-dimensional topographical information of surfaces with minimal sample preparation, operating effectively in various environments including ambient conditions, liquid, and vacuum. The technique can measure mechanical properties such as elasticity, adhesion, and friction through force spectroscopy, offering valuable insights into material characteristics beyond mere topography.
However, AFM faces significant speed limitations, with typical scan rates ranging from several minutes to hours per image, making it unsuitable for dynamic processes requiring real-time observation. The technique is fundamentally restricted to surface or near-surface measurements, with a maximum depth penetration of only a few nanometers, preventing analysis of subsurface or internal structures.
In contrast, Laser Scanning Microscopy, particularly confocal LSM, offers optical sectioning capabilities with resolution approaching the diffraction limit (approximately 200-300 nm laterally and 500-700 nm axially). LSM enables rapid imaging with frame rates of several images per second, facilitating the observation of dynamic biological processes and time-dependent phenomena.
LSM's ability to penetrate samples to depths of several hundred micrometers represents a significant advantage over AFM, allowing for non-invasive examination of internal structures in transparent and semi-transparent specimens. The technique also offers versatile contrast mechanisms through fluorescence, reflectance, and transmitted light modalities, enabling specific targeting of structures through fluorescent labeling.
Nevertheless, LSM remains fundamentally limited by the diffraction barrier, preventing resolution of features smaller than approximately half the wavelength of light used. While super-resolution techniques have pushed beyond this limit, they often require specialized sample preparation, extended acquisition times, or introduce artifacts.
Both technologies face challenges with certain sample types: AFM struggles with highly rough surfaces or deeply recessed features due to tip geometry constraints, while LSM performs poorly with opaque samples or in environments with significant light scattering. Temperature stability affects both techniques, with AFM particularly sensitive to thermal drift that can distort measurements during long acquisition periods.
The complementary nature of these technologies has driven recent developments in correlative microscopy approaches, combining the surface precision of AFM with the depth capabilities of LSM to provide more comprehensive sample characterization across multiple scales and dimensions.
Comparative Analysis of AFM and LSM Solutions
01 AFM imaging techniques for enhanced clarity
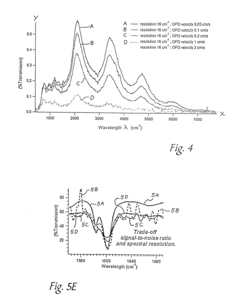
Advanced atomic force microscopy (AFM) imaging techniques have been developed to improve clarity and resolution of surface measurements. These techniques include specialized probe designs, signal processing algorithms, and feedback control systems that minimize noise and artifacts. Enhanced AFM imaging allows for clearer visualization of nanoscale features and structures, providing more accurate topographical data and material property measurements at the atomic level.- Atomic Force Microscopy (AFM) imaging techniques and resolution enhancement: Various techniques have been developed to enhance the clarity and resolution of atomic force microscopy imaging. These include specialized probe designs, vibration isolation systems, and signal processing algorithms that improve the quality of topographical data. Advanced AFM methods can achieve nanometer-scale resolution while providing detailed surface characterization with improved depth perception and reduced artifacts.
- Laser Scanning Microscopy (LSM) depth imaging capabilities: Laser scanning microscopy techniques offer superior depth imaging capabilities through optical sectioning methods. These systems can produce clear three-dimensional reconstructions of samples by capturing multiple focal planes and combining them into volumetric datasets. Innovations in laser scanning technology have improved penetration depth in biological samples while maintaining high resolution, enabling detailed visualization of internal structures without physical sectioning.
- Combined AFM and optical microscopy systems: Integrated systems combining atomic force microscopy with optical microscopy techniques provide complementary data about sample properties. These hybrid approaches allow simultaneous acquisition of topographical information from AFM with spectroscopic or fluorescence data from optical methods. The correlation between mechanical and optical properties enhances sample characterization, providing more comprehensive analysis with improved clarity across different depth scales.
- Sample preparation and environmental control for microscopy: Specialized sample preparation techniques and environmental control systems have been developed to optimize imaging clarity and depth in both AFM and laser scanning microscopy. These include methods for surface modification, humidity control, temperature regulation, and vibration isolation. Proper sample preparation significantly enhances image quality by reducing artifacts and improving signal-to-noise ratios, allowing for more accurate depth measurements and clearer visualization.
- Data processing and image enhancement algorithms: Advanced computational methods have been developed to process and enhance microscopy data from both AFM and laser scanning systems. These algorithms include deconvolution techniques, noise reduction filters, contrast enhancement, and 3D reconstruction methods that significantly improve image clarity and depth perception. Machine learning approaches are increasingly being applied to extract meaningful information from complex microscopy datasets, enabling more accurate quantitative analysis of surface topography and internal structures.
02 Laser scanning microscopy depth enhancement methods
Various methods have been developed to enhance the depth capabilities of laser scanning microscopy. These include advanced optical configurations, multi-photon excitation techniques, and specialized scanning algorithms that allow for deeper tissue penetration and improved signal collection from subsurface structures. These enhancements enable three-dimensional imaging with better clarity at increased depths, which is particularly valuable for biological samples and thick specimen analysis.Expand Specific Solutions03 Combined AFM and laser scanning microscopy systems
Integrated systems that combine atomic force microscopy with laser scanning microscopy leverage the strengths of both techniques. These hybrid systems allow for simultaneous or correlative imaging, providing both high-resolution surface topography from AFM and optical information from laser scanning microscopy. The combination enables researchers to obtain complementary data sets that offer more comprehensive sample characterization with enhanced clarity and depth information.Expand Specific Solutions04 Probe and cantilever innovations for improved microscopy
Innovations in probe and cantilever design have significantly improved microscopy performance. These include specialized tip geometries, novel materials with enhanced mechanical properties, and functionalized probes that increase sensitivity and specificity. Advanced cantilever designs with optimized spring constants and resonance frequencies reduce noise and increase measurement precision, resulting in clearer images with better depth resolution in both AFM and certain laser scanning applications.Expand Specific Solutions05 Signal processing and image enhancement algorithms
Advanced signal processing and image enhancement algorithms have been developed to improve clarity and depth information in microscopy data. These computational methods include noise reduction techniques, deconvolution algorithms, and machine learning approaches that extract meaningful information from raw microscopy data. By applying these algorithms, researchers can achieve higher contrast, better feature discrimination, and improved visualization of three-dimensional structures in both AFM and laser scanning microscopy.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Atomic Force Microscopy (AFM) and Laser Scanning Microscopy (LSM) compete in the advanced imaging market, currently in a growth phase with an estimated market size of $3-4 billion annually. AFM technology offers superior resolution at the nanoscale level but with limited depth capabilities, while LSM provides better depth penetration with slightly lower resolution. Key industry players like Bruker Nano, Park Systems, and JPKinstruments lead AFM development, while Carl Zeiss, Hitachi, and Agilent Technologies dominate the LSM sector. Research institutions including Beihang University and CNRS are advancing both technologies, with applications expanding across semiconductor, materials science, and biomedical fields as these complementary technologies mature.
Bruker Nano, Inc.
Technical Solution: Bruker Nano has developed advanced Atomic Force Microscopy (AFM) solutions that integrate multimodal capabilities for comprehensive surface analysis. Their PeakForce Tapping technology allows simultaneous acquisition of nanomechanical properties and topography with minimal sample damage[1]. Bruker's BioScope Resolve AFM system specifically addresses biological imaging challenges by combining high-resolution AFM with optical microscopy techniques, enabling correlative imaging across scales[2]. Their FastScan technology achieves imaging speeds up to 20 times faster than conventional AFMs while maintaining nanometer resolution[3]. For comparative analysis against Laser Scanning Microscopy (LSM), Bruker has implemented proprietary algorithms that enhance AFM depth perception and clarity through advanced signal processing and noise reduction techniques, achieving vertical resolution below 0.1 nm in optimal conditions compared to the diffraction-limited resolution of conventional LSM[4].
Strengths: Superior spatial resolution beyond optical diffraction limits; ability to measure physical properties (elasticity, adhesion) simultaneously with topography; no sample labeling required; works with non-transparent samples. Weaknesses: Slower image acquisition compared to LSM; more complex sample preparation; limited to surface or near-surface measurements; smaller field of view than LSM systems.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed the 9500 AFM system that incorporates their exclusive "scanning microwave microscopy" technology, bridging the gap between AFM's nanoscale resolution and electrical characterization capabilities[1]. Their patented MAC Mode (Magnetic Alternating Current) AFM enables gentle imaging of soft samples in liquid environments with minimal deformation, particularly valuable for biological specimens where traditional AFM techniques might cause damage[2]. For comparative analysis with Laser Scanning Microscopy, Agilent has implemented Quick Scan technology that increases imaging speed while maintaining resolution, addressing one of AFM's traditional disadvantages compared to optical techniques[3]. Their systems feature integrated optical microscopy capabilities allowing seamless correlation between AFM and optical data, including compatibility with confocal laser scanning microscopy for multimodal imaging of the same sample area[4]. Agilent's PicoView software platform enables advanced image processing algorithms that enhance depth perception in AFM data through sophisticated 3D rendering techniques comparable to confocal LSM depth reconstructions[5].
Strengths: Exceptional versatility with modular design allowing multiple measurement modes; superior electrical characterization capabilities; excellent stability for long-duration experiments; comprehensive software for data analysis. Weaknesses: Complex user interface with steeper learning curve; larger footprint than some competing systems; higher sensitivity to environmental vibrations; more expensive consumables (probes) for specialized modes.
Key Patents and Technical Innovations
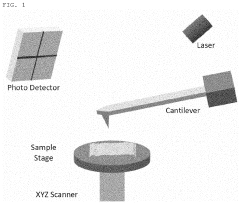
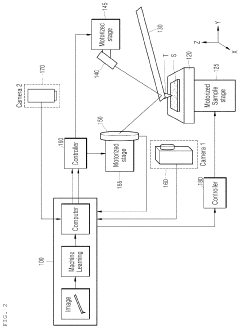
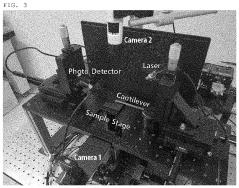
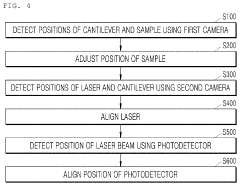
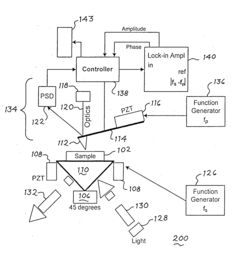
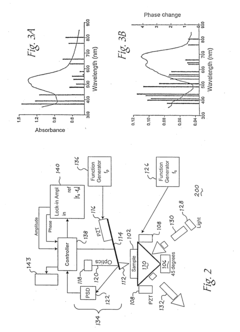
Atomic force microscope using artificial intelligence object recognition technology and operation method thereof
PatentActiveUS20220373575A1
Innovation
- An automated atomic force microscope employing artificial intelligence object recognition technology, including cameras and a processor to automatically align the cantilever, sample, laser beam, and photodetector, using machine-learning programs to detect and correct positions for precise adjustments.
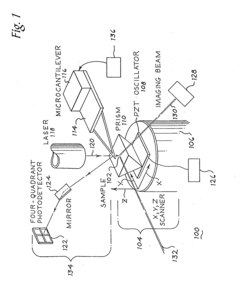
Scanning probe microscopy with spectroscopic molecular recognition
PatentInactiveUS20110231966A1
Innovation
- The system combines Scanning Near-field Acoustic Photothermal/photoacoustic Spectroscopy (SNAPS) by applying two independent acoustic waves and electromagnetic energy to a sample, using a microcantilever probe to detect phase changes and generate signals representative of chemical characteristics, enabling simultaneous acquisition of spatial and chemical information.
Cost-Benefit Analysis of Microscopy Technologies
When evaluating microscopy technologies for research or industrial applications, cost-benefit analysis becomes a critical decision-making factor. Atomic Force Microscopy (AFM) and Laser Scanning Microscopy (LSM) represent significant investments with distinct financial implications that extend beyond initial acquisition costs.
The capital expenditure for AFM systems typically ranges from $100,000 to $500,000, depending on capabilities and specifications. These systems require specialized environments with vibration isolation and temperature control, adding $20,000-50,000 in infrastructure costs. Conversely, LSM systems generally cost between $150,000 and $700,000, with confocal variants at the higher end of this spectrum. LSM installations require less stringent environmental controls but often need dedicated dark rooms and stable power supplies.
Operational expenses differ significantly between these technologies. AFM probes require regular replacement at $20-100 per tip, with specialized tips costing up to $300 each. Annual maintenance contracts for AFM systems typically run 8-12% of the initial purchase price. LSM systems incur lower consumable costs but higher energy consumption, with laser replacements potentially costing $5,000-15,000 every 3-5 years. Software licensing and updates represent ongoing expenses for both technologies, averaging $2,000-8,000 annually.
Personnel requirements constitute another significant cost factor. AFM operation demands specialized training with a steeper learning curve, often requiring dedicated technicians commanding salaries of $60,000-90,000 annually. LSM systems, while still requiring expertise, typically have more intuitive interfaces and broader user accessibility, potentially allowing for shared operator responsibilities.
Return on investment calculations must consider sample throughput capabilities. LSM excels in rapid imaging, processing 20-30 samples daily in routine applications, while AFM typically handles 5-10 samples per day due to more complex sample preparation and longer acquisition times. This throughput differential significantly impacts cost-per-sample metrics in high-volume environments.
Versatility provides additional value considerations. AFM offers mechanical property measurements and nanomanipulation capabilities that extend its utility beyond imaging. LSM systems, particularly multiphoton variants, enable live cell imaging and deep tissue penetration that AFM cannot match. These complementary capabilities may justify maintaining both technologies in comprehensive research facilities.
Ultimately, the optimal cost-benefit ratio depends on specific application requirements, sample characteristics, and institutional priorities. Organizations with diverse research portfolios may find greater value in the versatility of maintaining both technologies, while specialized applications may favor the unique capabilities of either AFM or LSM despite higher initial or operational costs.
The capital expenditure for AFM systems typically ranges from $100,000 to $500,000, depending on capabilities and specifications. These systems require specialized environments with vibration isolation and temperature control, adding $20,000-50,000 in infrastructure costs. Conversely, LSM systems generally cost between $150,000 and $700,000, with confocal variants at the higher end of this spectrum. LSM installations require less stringent environmental controls but often need dedicated dark rooms and stable power supplies.
Operational expenses differ significantly between these technologies. AFM probes require regular replacement at $20-100 per tip, with specialized tips costing up to $300 each. Annual maintenance contracts for AFM systems typically run 8-12% of the initial purchase price. LSM systems incur lower consumable costs but higher energy consumption, with laser replacements potentially costing $5,000-15,000 every 3-5 years. Software licensing and updates represent ongoing expenses for both technologies, averaging $2,000-8,000 annually.
Personnel requirements constitute another significant cost factor. AFM operation demands specialized training with a steeper learning curve, often requiring dedicated technicians commanding salaries of $60,000-90,000 annually. LSM systems, while still requiring expertise, typically have more intuitive interfaces and broader user accessibility, potentially allowing for shared operator responsibilities.
Return on investment calculations must consider sample throughput capabilities. LSM excels in rapid imaging, processing 20-30 samples daily in routine applications, while AFM typically handles 5-10 samples per day due to more complex sample preparation and longer acquisition times. This throughput differential significantly impacts cost-per-sample metrics in high-volume environments.
Versatility provides additional value considerations. AFM offers mechanical property measurements and nanomanipulation capabilities that extend its utility beyond imaging. LSM systems, particularly multiphoton variants, enable live cell imaging and deep tissue penetration that AFM cannot match. These complementary capabilities may justify maintaining both technologies in comprehensive research facilities.
Ultimately, the optimal cost-benefit ratio depends on specific application requirements, sample characteristics, and institutional priorities. Organizations with diverse research portfolios may find greater value in the versatility of maintaining both technologies, while specialized applications may favor the unique capabilities of either AFM or LSM despite higher initial or operational costs.
Integration Possibilities with AI and Machine Learning
The integration of artificial intelligence and machine learning with microscopy technologies represents a transformative frontier in scientific imaging. For both Atomic Force Microscopy (AFM) and Laser Scanning Microscopy (LSM), AI integration offers unprecedented opportunities to enhance image acquisition, processing, and interpretation capabilities.
Machine learning algorithms can significantly improve AFM image quality by addressing common challenges such as noise reduction, artifact removal, and tip-sample interaction corrections. Neural networks trained on extensive datasets of AFM images can learn to distinguish between actual surface features and measurement artifacts, resulting in more accurate topographical representations. Similarly, for LSM, AI can enhance contrast, resolution, and depth perception through advanced image processing techniques.
Real-time image analysis represents another promising integration area. AI systems can process microscopy data streams as they are generated, enabling immediate feedback for experimental adjustments. This capability is particularly valuable in dynamic biological studies where rapid decision-making based on cellular changes can be critical. For industrial applications, this translates to faster quality control processes and more efficient materials characterization.
Deep learning approaches have demonstrated remarkable success in automating feature identification across both microscopy platforms. Convolutional neural networks can identify specific structures, defects, or cellular components that might be overlooked by human operators. This automation not only increases throughput but also improves consistency in analysis across large datasets, reducing operator bias and fatigue-related errors.
The combination of AFM's superior spatial resolution with LSM's optical sectioning capabilities creates complementary datasets that AI can fuse into comprehensive multi-dimensional representations. Machine learning algorithms excel at integrating these heterogeneous data types, extracting correlations between mechanical properties from AFM and fluorescence information from LSM to provide deeper insights into sample characteristics.
Predictive modeling represents perhaps the most advanced integration possibility. By analyzing patterns in microscopy data, AI systems can predict sample behaviors under different conditions or forecast how materials might evolve over time. This predictive capability could revolutionize fields ranging from materials science to drug development by reducing experimental iterations and accelerating discovery timelines.
As these technologies continue to converge, we can anticipate the development of increasingly autonomous microscopy systems capable of self-optimization. Such systems would dynamically adjust scanning parameters based on sample characteristics and research objectives, potentially discovering novel imaging approaches beyond current human-designed protocols.
Machine learning algorithms can significantly improve AFM image quality by addressing common challenges such as noise reduction, artifact removal, and tip-sample interaction corrections. Neural networks trained on extensive datasets of AFM images can learn to distinguish between actual surface features and measurement artifacts, resulting in more accurate topographical representations. Similarly, for LSM, AI can enhance contrast, resolution, and depth perception through advanced image processing techniques.
Real-time image analysis represents another promising integration area. AI systems can process microscopy data streams as they are generated, enabling immediate feedback for experimental adjustments. This capability is particularly valuable in dynamic biological studies where rapid decision-making based on cellular changes can be critical. For industrial applications, this translates to faster quality control processes and more efficient materials characterization.
Deep learning approaches have demonstrated remarkable success in automating feature identification across both microscopy platforms. Convolutional neural networks can identify specific structures, defects, or cellular components that might be overlooked by human operators. This automation not only increases throughput but also improves consistency in analysis across large datasets, reducing operator bias and fatigue-related errors.
The combination of AFM's superior spatial resolution with LSM's optical sectioning capabilities creates complementary datasets that AI can fuse into comprehensive multi-dimensional representations. Machine learning algorithms excel at integrating these heterogeneous data types, extracting correlations between mechanical properties from AFM and fluorescence information from LSM to provide deeper insights into sample characteristics.
Predictive modeling represents perhaps the most advanced integration possibility. By analyzing patterns in microscopy data, AI systems can predict sample behaviors under different conditions or forecast how materials might evolve over time. This predictive capability could revolutionize fields ranging from materials science to drug development by reducing experimental iterations and accelerating discovery timelines.
As these technologies continue to converge, we can anticipate the development of increasingly autonomous microscopy systems capable of self-optimization. Such systems would dynamically adjust scanning parameters based on sample characteristics and research objectives, potentially discovering novel imaging approaches beyond current human-designed protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!