Atomic Force Microscopy Vs Light Sheet Microscopy: Detail Comparison
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microscopy Technologies Background and Objectives
Microscopy has evolved significantly over the past century, transforming from simple optical systems to sophisticated technologies capable of visualizing structures at nanometer scales. Atomic Force Microscopy (AFM) and Light Sheet Microscopy (LSM) represent two distinct approaches in advanced microscopy that have revolutionized different aspects of scientific imaging. AFM emerged in the 1980s as an extension of scanning tunneling microscopy, while LSM evolved from confocal microscopy techniques in the early 2000s, with both technologies addressing specific limitations in conventional imaging methods.
The development trajectory of these technologies reflects broader trends in microscopy evolution—moving from purely optical approaches toward hybrid systems that combine multiple physical principles to achieve enhanced resolution and functionality. AFM leverages mechanical interactions between a probe and sample surface, whereas LSM utilizes specialized illumination strategies to minimize photodamage while maintaining optical sectioning capabilities. This divergence in fundamental operating principles has led to complementary applications across scientific disciplines.
Current technological trends indicate a convergence of multiple imaging modalities, with researchers increasingly seeking to combine the strengths of different microscopy techniques. The integration of AFM's superior surface resolution capabilities with LSM's ability to image living specimens with minimal photodamage represents a frontier in microscopy development. This integration aims to overcome the inherent limitations of each individual technology while capitalizing on their respective strengths.
The primary objective of comparing these technologies is to establish a comprehensive understanding of their relative advantages, limitations, and optimal application scenarios. This comparison seeks to identify potential synergies between AFM and LSM that could lead to novel hybrid systems capable of addressing current imaging challenges across biological, materials, and medical research fields.
Additionally, this analysis aims to map the technological evolution pathways for both AFM and LSM, identifying key innovation milestones and projecting future development directions. Understanding these trajectories is crucial for anticipating emerging capabilities and applications, particularly as these technologies continue to advance in resolution, speed, and sample compatibility.
The ultimate goal is to provide strategic insights for research institutions and technology developers regarding investment priorities, collaboration opportunities, and potential market applications. By thoroughly examining the technical foundations, current capabilities, and future potential of AFM and LSM, this analysis will serve as a roadmap for navigating the complex landscape of advanced microscopy technologies and their evolving role in scientific discovery and industrial applications.
The development trajectory of these technologies reflects broader trends in microscopy evolution—moving from purely optical approaches toward hybrid systems that combine multiple physical principles to achieve enhanced resolution and functionality. AFM leverages mechanical interactions between a probe and sample surface, whereas LSM utilizes specialized illumination strategies to minimize photodamage while maintaining optical sectioning capabilities. This divergence in fundamental operating principles has led to complementary applications across scientific disciplines.
Current technological trends indicate a convergence of multiple imaging modalities, with researchers increasingly seeking to combine the strengths of different microscopy techniques. The integration of AFM's superior surface resolution capabilities with LSM's ability to image living specimens with minimal photodamage represents a frontier in microscopy development. This integration aims to overcome the inherent limitations of each individual technology while capitalizing on their respective strengths.
The primary objective of comparing these technologies is to establish a comprehensive understanding of their relative advantages, limitations, and optimal application scenarios. This comparison seeks to identify potential synergies between AFM and LSM that could lead to novel hybrid systems capable of addressing current imaging challenges across biological, materials, and medical research fields.
Additionally, this analysis aims to map the technological evolution pathways for both AFM and LSM, identifying key innovation milestones and projecting future development directions. Understanding these trajectories is crucial for anticipating emerging capabilities and applications, particularly as these technologies continue to advance in resolution, speed, and sample compatibility.
The ultimate goal is to provide strategic insights for research institutions and technology developers regarding investment priorities, collaboration opportunities, and potential market applications. By thoroughly examining the technical foundations, current capabilities, and future potential of AFM and LSM, this analysis will serve as a roadmap for navigating the complex landscape of advanced microscopy technologies and their evolving role in scientific discovery and industrial applications.
Market Applications and Research Demand Analysis
The market for advanced microscopy technologies has witnessed significant growth in recent years, driven by increasing demand for high-resolution imaging in life sciences, materials science, and nanotechnology. Atomic Force Microscopy (AFM) and Light Sheet Microscopy (LSM) represent two distinct approaches that address different market needs and research requirements.
In the life sciences sector, Light Sheet Microscopy has gained substantial traction due to its ability to image living specimens with minimal phototoxicity. This capability has created a robust demand in developmental biology, where researchers need to observe embryonic development and cellular processes in real-time without damaging the specimen. The global market for light sheet microscopy is expanding rapidly, particularly in neuroscience research where whole-brain imaging at cellular resolution has become increasingly important for understanding neural networks and disease mechanisms.
Atomic Force Microscopy, conversely, has established a strong market presence in materials science, semiconductor industry, and nanotechnology. The ability to achieve atomic-level resolution and measure surface properties has made AFM indispensable for quality control in semiconductor manufacturing, where feature sizes continue to shrink below 10nm. Additionally, the pharmaceutical industry increasingly relies on AFM for drug formulation studies and characterization of nanoparticle-based drug delivery systems.
Research institutions represent a significant market segment for both technologies, with different departments often requiring complementary imaging capabilities. Universities and government laboratories typically invest in both technologies to support diverse research portfolios, while specialized research centers may prioritize one technology based on their specific focus areas.
The healthcare sector demonstrates growing interest in both technologies, albeit for different applications. AFM finds applications in pathology for analyzing tissue mechanics related to disease states, while LSM is increasingly utilized for 3D imaging of tissue samples and organoids in personalized medicine approaches. This diversification of applications has expanded the total addressable market for advanced microscopy.
Geographically, North America and Europe currently represent the largest markets for both technologies, driven by substantial research funding and established life science and materials science industries. However, the Asia-Pacific region is experiencing the fastest growth rate, particularly in China, Japan, and South Korea, where significant investments in research infrastructure and semiconductor manufacturing are creating new demand centers.
The COVID-19 pandemic has further accelerated market demand for advanced imaging technologies, as researchers worldwide sought to understand viral structures and infection mechanisms at the cellular and molecular levels. This trend has highlighted the complementary nature of these technologies in comprehensive research approaches.
In the life sciences sector, Light Sheet Microscopy has gained substantial traction due to its ability to image living specimens with minimal phototoxicity. This capability has created a robust demand in developmental biology, where researchers need to observe embryonic development and cellular processes in real-time without damaging the specimen. The global market for light sheet microscopy is expanding rapidly, particularly in neuroscience research where whole-brain imaging at cellular resolution has become increasingly important for understanding neural networks and disease mechanisms.
Atomic Force Microscopy, conversely, has established a strong market presence in materials science, semiconductor industry, and nanotechnology. The ability to achieve atomic-level resolution and measure surface properties has made AFM indispensable for quality control in semiconductor manufacturing, where feature sizes continue to shrink below 10nm. Additionally, the pharmaceutical industry increasingly relies on AFM for drug formulation studies and characterization of nanoparticle-based drug delivery systems.
Research institutions represent a significant market segment for both technologies, with different departments often requiring complementary imaging capabilities. Universities and government laboratories typically invest in both technologies to support diverse research portfolios, while specialized research centers may prioritize one technology based on their specific focus areas.
The healthcare sector demonstrates growing interest in both technologies, albeit for different applications. AFM finds applications in pathology for analyzing tissue mechanics related to disease states, while LSM is increasingly utilized for 3D imaging of tissue samples and organoids in personalized medicine approaches. This diversification of applications has expanded the total addressable market for advanced microscopy.
Geographically, North America and Europe currently represent the largest markets for both technologies, driven by substantial research funding and established life science and materials science industries. However, the Asia-Pacific region is experiencing the fastest growth rate, particularly in China, Japan, and South Korea, where significant investments in research infrastructure and semiconductor manufacturing are creating new demand centers.
The COVID-19 pandemic has further accelerated market demand for advanced imaging technologies, as researchers worldwide sought to understand viral structures and infection mechanisms at the cellular and molecular levels. This trend has highlighted the complementary nature of these technologies in comprehensive research approaches.
Current Capabilities and Technical Limitations
Atomic Force Microscopy (AFM) currently offers unparalleled spatial resolution down to the atomic level, enabling researchers to visualize and manipulate individual atoms and molecules on surfaces. This capability makes it invaluable for nanotechnology, materials science, and semiconductor research. AFM operates effectively in various environments including ambient conditions, vacuum, and liquid media, providing versatility across research domains. Modern AFM systems have achieved scan rates of up to several frames per second for high-speed imaging of dynamic processes, though this remains significantly slower than optical techniques.
Despite these strengths, AFM faces substantial limitations. The technique is inherently surface-restricted, typically imaging only the topmost layers of a sample with limited penetration depth. Scan speeds, while improved, remain a bottleneck for capturing rapid biological processes occurring at millisecond timescales. Sample preparation can be challenging, as specimens must be immobilized on substrates, potentially altering their natural state. Additionally, AFM probe-sample interactions may introduce artifacts or damage delicate biological structures during imaging.
Light Sheet Microscopy (LSM), in contrast, excels at imaging living specimens with minimal photodamage. Current systems achieve subcellular resolution (typically 300-500 nm laterally and 1-2 μm axially) while maintaining high acquisition speeds of up to hundreds of frames per second. This enables real-time visualization of cellular dynamics across entire organisms. Modern LSM configurations can image samples up to several millimeters in size with penetration depths reaching hundreds of micrometers in transparent tissues.
However, LSM confronts its own technical challenges. Resolution remains diffraction-limited, preventing visualization of molecular-scale structures that AFM can readily resolve. Sample opacity presents a significant barrier, as light scattering in non-transparent tissues degrades image quality with increasing depth. While clearing techniques can mitigate this issue for fixed samples, they are incompatible with live imaging applications. Additionally, LSM typically requires specialized sample mounting configurations that may constrain experimental design.
The complementary capabilities of these technologies highlight their distinct application domains. AFM provides superior resolution for surface analysis but with limited temporal resolution and sample accessibility. LSM offers excellent volumetric imaging of living specimens with high temporal resolution but cannot match AFM's spatial precision. Recent technological advances are addressing these limitations, with super-resolution LSM techniques pushing beyond the diffraction limit and high-speed AFM improving temporal resolution, gradually narrowing the performance gap between these complementary imaging modalities.
Despite these strengths, AFM faces substantial limitations. The technique is inherently surface-restricted, typically imaging only the topmost layers of a sample with limited penetration depth. Scan speeds, while improved, remain a bottleneck for capturing rapid biological processes occurring at millisecond timescales. Sample preparation can be challenging, as specimens must be immobilized on substrates, potentially altering their natural state. Additionally, AFM probe-sample interactions may introduce artifacts or damage delicate biological structures during imaging.
Light Sheet Microscopy (LSM), in contrast, excels at imaging living specimens with minimal photodamage. Current systems achieve subcellular resolution (typically 300-500 nm laterally and 1-2 μm axially) while maintaining high acquisition speeds of up to hundreds of frames per second. This enables real-time visualization of cellular dynamics across entire organisms. Modern LSM configurations can image samples up to several millimeters in size with penetration depths reaching hundreds of micrometers in transparent tissues.
However, LSM confronts its own technical challenges. Resolution remains diffraction-limited, preventing visualization of molecular-scale structures that AFM can readily resolve. Sample opacity presents a significant barrier, as light scattering in non-transparent tissues degrades image quality with increasing depth. While clearing techniques can mitigate this issue for fixed samples, they are incompatible with live imaging applications. Additionally, LSM typically requires specialized sample mounting configurations that may constrain experimental design.
The complementary capabilities of these technologies highlight their distinct application domains. AFM provides superior resolution for surface analysis but with limited temporal resolution and sample accessibility. LSM offers excellent volumetric imaging of living specimens with high temporal resolution but cannot match AFM's spatial precision. Recent technological advances are addressing these limitations, with super-resolution LSM techniques pushing beyond the diffraction limit and high-speed AFM improving temporal resolution, gradually narrowing the performance gap between these complementary imaging modalities.
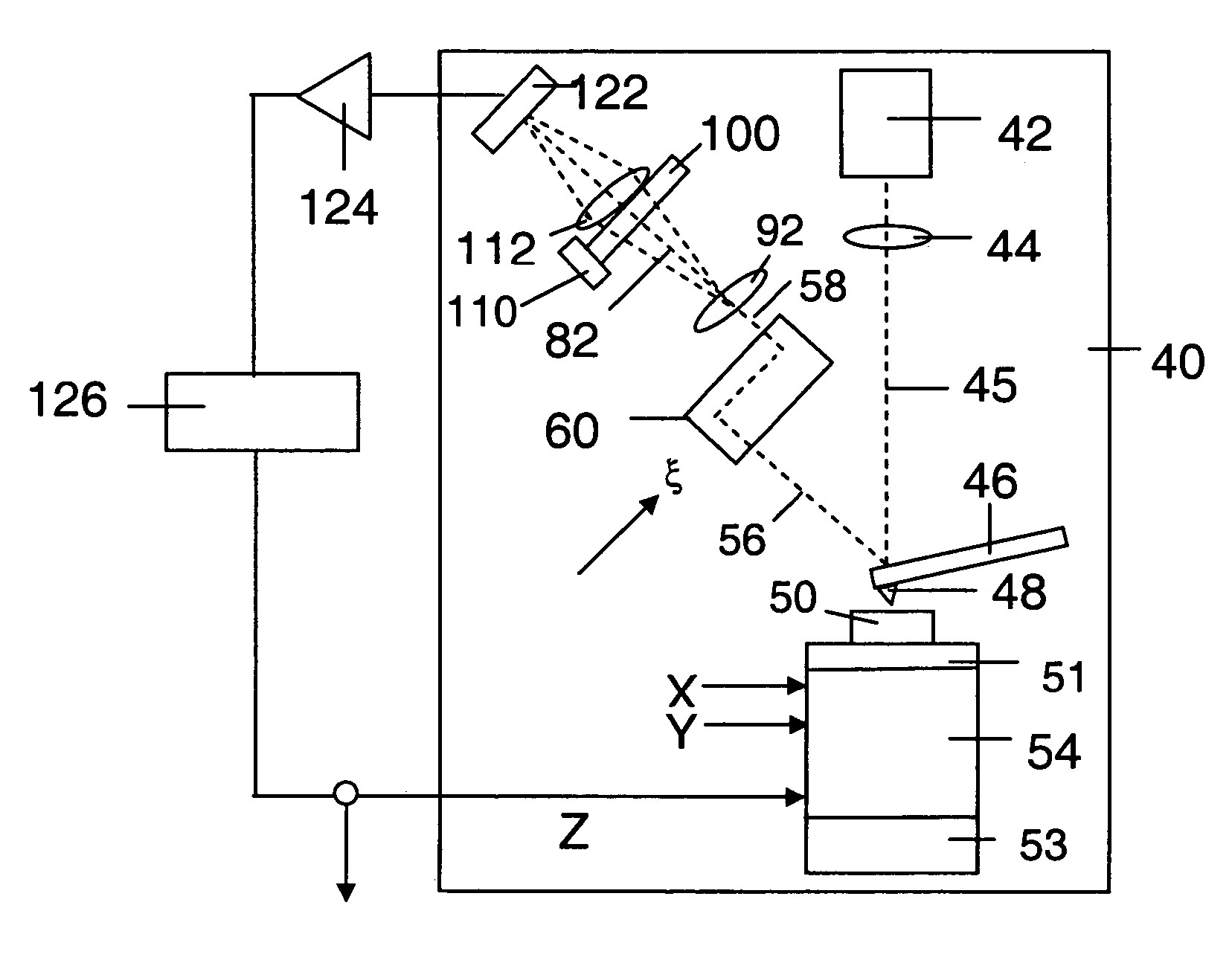
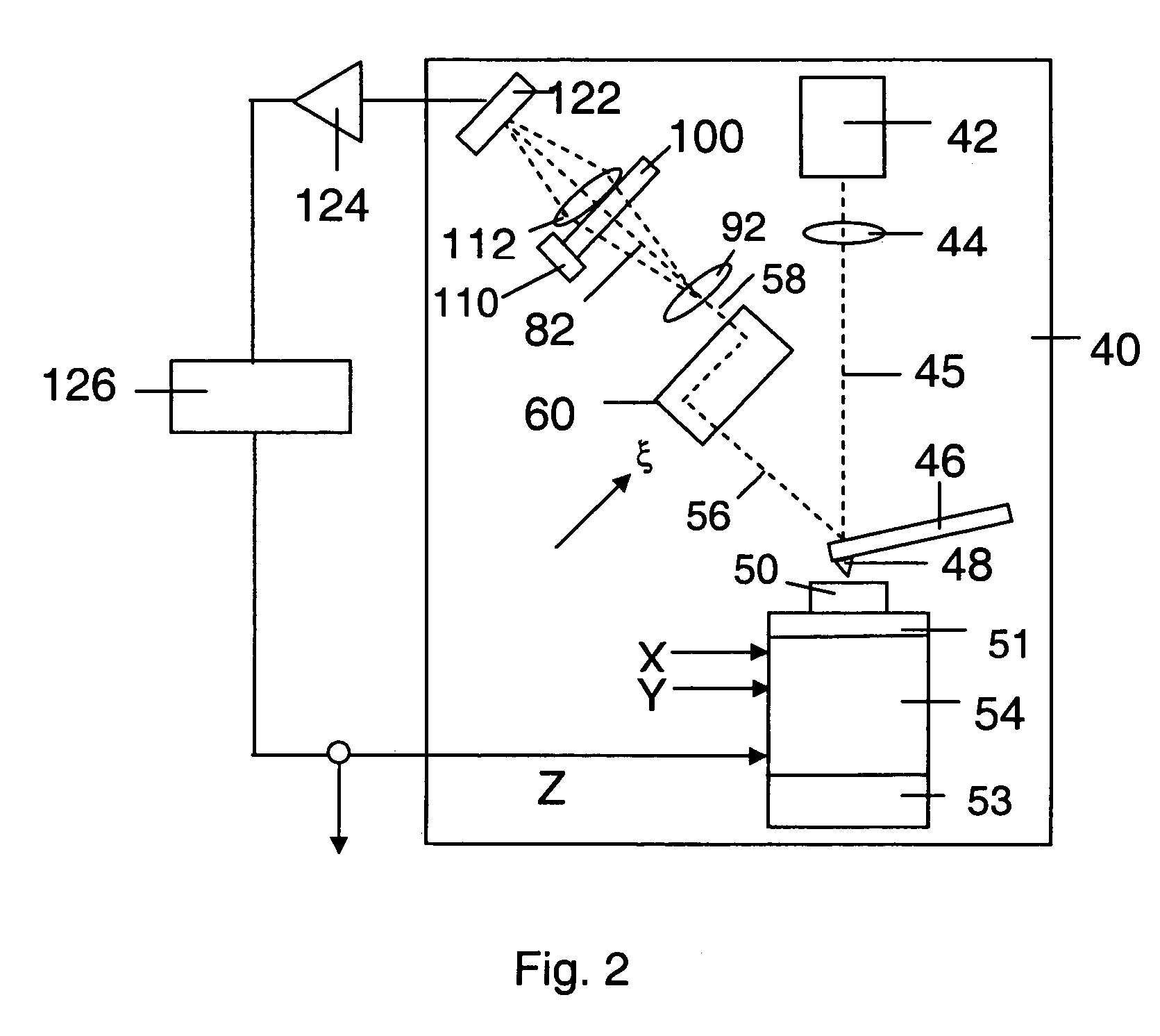
Technical Specifications and Operational Principles
01 Imaging resolution comparison between AFM and Light Sheet Microscopy
Atomic Force Microscopy (AFM) provides nanometer-scale resolution for surface imaging, allowing visualization of molecular structures and topographical features at the atomic level. In contrast, Light Sheet Microscopy offers optical resolution in the sub-micron range, enabling 3D imaging of larger biological samples with less photodamage. The resolution difference makes these techniques complementary, with AFM excelling at surface detail and Light Sheet Microscopy providing better volumetric imaging of living specimens.- Imaging resolution comparison between AFM and Light Sheet Microscopy: Atomic Force Microscopy (AFM) provides nanometer-scale resolution for surface imaging, allowing for detailed topographical analysis at the molecular level. In contrast, Light Sheet Microscopy offers optical resolution in the sub-micron range, enabling 3D visualization of larger biological specimens with minimal photodamage. The resolution capabilities of these techniques are complementary, with AFM excelling at surface detail and Light Sheet Microscopy providing superior volumetric imaging of living samples.
- Sample preparation requirements and considerations: Sample preparation differs significantly between these microscopy techniques. AFM typically requires samples to be immobilized on flat substrates, with minimal preparation for hard materials but more complex protocols for biological specimens including fixation or specialized imaging media. Light Sheet Microscopy generally requires transparent samples, often embedded in agarose or similar matrices to maintain position while allowing optical access from multiple angles. Specialized sample holders and mounting techniques have been developed to optimize imaging conditions while maintaining sample viability.
- Imaging speed and throughput capabilities: Light Sheet Microscopy offers significantly faster acquisition speeds compared to AFM, enabling real-time imaging of dynamic biological processes across entire specimens. AFM scanning is inherently slower due to its mechanical nature, with typical scan rates ranging from seconds to minutes per frame depending on scan size and resolution requirements. Recent technological advances have improved the temporal resolution of both techniques, with high-speed AFM variants and optimized Light Sheet configurations allowing for faster data acquisition while maintaining acceptable signal-to-noise ratios.
- Combined and correlative microscopy approaches: Integrating AFM with Light Sheet Microscopy enables correlative imaging that combines nanoscale surface information with volumetric fluorescence data. These hybrid approaches allow researchers to connect molecular-scale surface features with cellular and tissue-level structures and functions. Specialized sample stages, alignment procedures, and software tools have been developed to facilitate correlative workflows, enabling researchers to examine the same region of interest with complementary techniques and merge the resulting datasets for comprehensive analysis.
- Environmental control and live specimen imaging: Both microscopy techniques have been adapted for live specimen imaging through environmental control systems. Light Sheet Microscopy excels at long-term imaging of living samples with minimal phototoxicity, utilizing temperature control, gas exchange, and perfusion systems to maintain physiological conditions. AFM can operate in liquid environments with temperature control, though with more constraints on sample movement and imaging duration. Advanced implementations incorporate incubation chambers, perfusion systems, and specialized sample holders to maintain sample viability during extended imaging sessions.
02 Sample preparation requirements for microscopy techniques
Sample preparation for Atomic Force Microscopy typically requires samples to be immobilized on flat substrates like mica or glass, with minimal liquid layers for optimal imaging. Samples often need to be chemically fixed or dried depending on the imaging mode. For Light Sheet Microscopy, specimens are usually suspended in transparent media or embedded in low-melting agarose to allow optical access from multiple angles. Biological samples may require fluorescent labeling and must be optically transparent for light sheet penetration, with specialized mounting techniques to minimize movement during imaging.Expand Specific Solutions03 Imaging speed and throughput capabilities
Light Sheet Microscopy offers significantly faster imaging speeds compared to Atomic Force Microscopy, capable of capturing volumetric data of living specimens at video rates. This high-speed capability makes it suitable for dynamic biological processes and high-throughput screening. AFM typically operates at slower scanning rates, with conventional imaging taking minutes to hours per frame, though recent high-speed AFM developments have improved temporal resolution for certain applications. The speed difference reflects the fundamental trade-off between resolution and imaging rate in these complementary techniques.Expand Specific Solutions04 Combined microscopy approaches and multimodal imaging
Integrating Atomic Force Microscopy with Light Sheet Microscopy creates powerful multimodal imaging platforms that combine nanoscale surface information with volumetric fluorescence data. These hybrid systems enable correlation between mechanical properties and biological function in living samples. Technical innovations include specialized sample holders, synchronized data acquisition systems, and software for correlative analysis. Combined approaches address limitations of individual techniques, providing comprehensive structural and functional information across different spatial scales and physical properties.Expand Specific Solutions05 Environmental control and live specimen imaging
Both microscopy techniques have been adapted for live specimen imaging through environmental control systems. For Light Sheet Microscopy, temperature-controlled chambers, perfusion systems, and gas exchange modules enable long-term imaging of living organisms with minimal phototoxicity. AFM requires specialized liquid cells and temperature control for maintaining viable biological samples during imaging. Innovations in both fields focus on reducing invasiveness while maintaining physiological conditions, with particular emphasis on minimizing mechanical disturbance in AFM and photodamage in Light Sheet Microscopy.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The atomic force microscopy (AFM) and light sheet microscopy (LSM) market is currently in a growth phase, with an expanding global market estimated at approximately $1.5 billion and projected to grow at 6-8% annually. The competitive landscape features established industry leaders like IBM, Bruker Nano, and Olympus alongside emerging specialized players such as Infinitesima and Artidis AG. Technologically, AFM has reached higher maturity with robust commercial applications, while LSM is experiencing rapid innovation particularly in biomedical research. Academic institutions including National University of Singapore, University of Bristol, and Zhejiang University are driving fundamental research advances, while commercial entities like Keysight Technologies, Hitachi, and Samsung Electronics focus on industrial applications and integration with complementary technologies. The convergence of these microscopy techniques represents a significant opportunity for cross-platform innovations.
Infinitesima Ltd.
Technical Solution: Infinitesima has developed the Rapid Probe Microscopy (RPM) technology, a revolutionary approach to atomic force microscopy that dramatically increases imaging speed while maintaining nanometer resolution. Their proprietary technology uses resonating probes that scan surfaces at speeds up to 100 times faster than conventional AFM systems. This breakthrough enables real-time imaging of dynamic processes at the nanoscale, addressing one of the traditional limitations of AFM technology. Infinitesima's systems incorporate advanced feedback control algorithms that maintain consistent tip-sample interactions during high-speed scanning, preserving image quality and measurement accuracy. Their technology has been particularly valuable for semiconductor metrology applications, where high-throughput inspection is essential. The company has also developed specialized probes with enhanced durability for sustained high-speed operation, significantly improving system reliability and reducing consumable costs[6].
Strengths: Exceptional scanning speed compared to traditional AFM systems; maintains nanometer resolution at high scan rates; robust design suitable for industrial applications; reduced thermal drift effects due to faster acquisition. Weaknesses: More specialized for industrial metrology than biological applications; higher initial investment cost; limited integration with light microscopy techniques; requires specialized training for operation and maintenance.
Bruker Nano, Inc.
Technical Solution: Bruker Nano has developed advanced Atomic Force Microscopy (AFM) solutions with their proprietary PeakForce Tapping technology that enables simultaneous acquisition of multiple sample properties while protecting both tip and sample. Their systems achieve nanometer-scale resolution by measuring forces between a sharp probe and sample surface. Bruker's BioScope Resolve AFM system specifically integrates with optical microscopy platforms, allowing correlative imaging between AFM and fluorescence techniques. This integration provides mechanical property mapping at the nanoscale while maintaining the context of fluorescence imaging[1]. Their FastScan technology enables high-speed AFM imaging at rates up to 20 frames per second, significantly faster than conventional AFM systems, making it suitable for capturing dynamic biological processes[2].
Strengths: Superior spatial resolution (sub-nanometer) allowing visualization of individual molecules and atomic structures; provides quantitative mechanical property measurements; operates in various environments including liquids. Weaknesses: Limited imaging depth compared to light sheet microscopy; smaller field of view; slower acquisition speed for large areas; more complex sample preparation requirements.
Key Patents and Scientific Breakthroughs
High sensitivity scanning probe system
PatentInactiveUS7230719B2
Innovation
- The system employs a scanning probe microscope with an interferometer that splits and recombines light beams to form an interference pattern, using a transmission grating and actuators to modulate the fringe pattern and cancel noise, allowing for enhanced sensitivity and independent measurement of bending and torsion modes without the need for complex alignment or expensive manufacturing.
High sensitivity scanning probe system
PatentWO2005055245A1
Innovation
- The system employs a method of splitting a light beam into two beams with different path lengths to form an interference beam, which is then processed to enhance sensitivity and noise cancellation using a transmission grating and actuators to adjust the fringe pattern, allowing for independent measurement of bending and torsion modes without requiring photodetector position adjustments.
Sample Preparation Requirements and Considerations
Sample preparation represents a critical differentiating factor between Atomic Force Microscopy (AFM) and Light Sheet Microscopy (LSM), with each technique imposing distinct requirements that significantly impact experimental design and outcomes. AFM typically requires samples to be immobilized on flat, rigid substrates such as glass, mica, or silicon wafers. The surface must be exceptionally clean and smooth at the nanoscale to avoid artifacts in imaging. For biological specimens, chemical fixation protocols using glutaraldehyde or formaldehyde are common, though these may introduce structural alterations that must be considered during data interpretation.
In contrast, Light Sheet Microscopy accommodates larger, three-dimensional specimens and often requires optical clearing techniques to enhance transparency. Samples are typically mounted in low-melting-point agarose or similar hydrogels that provide stability while maintaining the specimen in a hydrated state. For developmental biology applications, LSM frequently employs specialized sample holders that allow for precise positioning and rotation of embryos or organoids during extended time-lapse imaging.
Environmental conditions during imaging also differ substantially between these techniques. AFM can operate in various environments including ambient air, controlled gas atmospheres, liquids, and vacuum, with each environment imposing specific sample preparation considerations. LSM typically requires samples to be immersed in appropriate imaging media with controlled temperature and sometimes gas exchange systems for live-cell applications.
The time investment for sample preparation varies significantly between these modalities. AFM sample preparation can be relatively quick for simple specimens but may require hours of optimization for complex biological samples to achieve proper immobilization without compromising structural integrity. LSM sample preparation often involves multi-step protocols including fluorescent labeling, clearing, and mounting that may extend over several days, particularly for thick tissue specimens.
Resolution considerations also drive preparation requirements. AFM's nanometer-scale resolution demands meticulous attention to sample cleanliness and stability, with even minor contamination potentially obscuring features of interest. LSM's micron-scale resolution is more forgiving regarding sample purity but requires careful consideration of fluorophore selection, labeling density, and potential photobleaching effects during extended imaging sessions.
For correlative microscopy approaches combining both techniques, specialized preparation protocols have been developed that satisfy the requirements of both modalities, typically involving reversible immobilization strategies and compatible fluorescent markers that withstand the mechanical probing inherent to AFM while maintaining optical properties suitable for LSM.
In contrast, Light Sheet Microscopy accommodates larger, three-dimensional specimens and often requires optical clearing techniques to enhance transparency. Samples are typically mounted in low-melting-point agarose or similar hydrogels that provide stability while maintaining the specimen in a hydrated state. For developmental biology applications, LSM frequently employs specialized sample holders that allow for precise positioning and rotation of embryos or organoids during extended time-lapse imaging.
Environmental conditions during imaging also differ substantially between these techniques. AFM can operate in various environments including ambient air, controlled gas atmospheres, liquids, and vacuum, with each environment imposing specific sample preparation considerations. LSM typically requires samples to be immersed in appropriate imaging media with controlled temperature and sometimes gas exchange systems for live-cell applications.
The time investment for sample preparation varies significantly between these modalities. AFM sample preparation can be relatively quick for simple specimens but may require hours of optimization for complex biological samples to achieve proper immobilization without compromising structural integrity. LSM sample preparation often involves multi-step protocols including fluorescent labeling, clearing, and mounting that may extend over several days, particularly for thick tissue specimens.
Resolution considerations also drive preparation requirements. AFM's nanometer-scale resolution demands meticulous attention to sample cleanliness and stability, with even minor contamination potentially obscuring features of interest. LSM's micron-scale resolution is more forgiving regarding sample purity but requires careful consideration of fluorophore selection, labeling density, and potential photobleaching effects during extended imaging sessions.
For correlative microscopy approaches combining both techniques, specialized preparation protocols have been developed that satisfy the requirements of both modalities, typically involving reversible immobilization strategies and compatible fluorescent markers that withstand the mechanical probing inherent to AFM while maintaining optical properties suitable for LSM.
Cost-Benefit Analysis and ROI Assessment
When evaluating the financial implications of adopting either Atomic Force Microscopy (AFM) or Light Sheet Microscopy (LSM) technologies, organizations must conduct a comprehensive cost-benefit analysis to determine the most appropriate investment strategy. The initial acquisition costs for AFM systems typically range from $100,000 to $500,000, while LSM systems generally cost between $200,000 and $700,000, depending on specifications and capabilities.
Operational expenses present significant differences between these technologies. AFM requires specialized probes that need regular replacement (approximately $100-300 per probe), with each probe lasting for limited scanning sessions. Conversely, LSM has lower consumable costs but higher energy consumption due to laser systems and cooling requirements. Maintenance costs for AFM average 5-8% of the initial investment annually, while LSM maintenance typically runs 7-10% annually due to the complexity of optical components and laser systems.
Training requirements represent another critical cost factor. AFM operation demands extensive technical expertise, with training periods ranging from 2-6 months for proficient operation, translating to approximately $10,000-$20,000 in personnel costs. LSM typically requires 1-3 months of training, with costs ranging from $5,000-$15,000, offering a faster return on training investment.
The return on investment calculations reveal distinctive patterns between these technologies. AFM provides superior ROI for materials science applications, with payback periods typically ranging from 2-3 years when utilized at optimal capacity. The high-resolution capabilities justify the investment for specialized research requiring atomic-level imaging. LSM demonstrates stronger ROI metrics for biological research, particularly for live cell imaging, with typical payback periods of 1.5-2.5 years due to higher throughput capabilities and reduced sample preparation time.
Productivity gains must be factored into ROI assessments. LSM offers significant advantages in imaging speed, capable of capturing dynamic biological processes at rates 10-20 times faster than AFM. This translates to higher sample throughput and accelerated research timelines. AFM, while slower, provides unmatched precision for certain applications that cannot be achieved through alternative methods, creating value through unique capabilities rather than throughput.
Long-term value assessment indicates that both technologies maintain relatively strong resale values, with AFM systems typically retaining 40-50% of their value after 5 years, while LSM systems retain approximately 30-40%. The total cost of ownership over a 5-year period, including acquisition, maintenance, training, and consumables, averages $250,000-$650,000 for AFM and $350,000-$800,000 for LSM, depending on usage intensity and configuration.
Operational expenses present significant differences between these technologies. AFM requires specialized probes that need regular replacement (approximately $100-300 per probe), with each probe lasting for limited scanning sessions. Conversely, LSM has lower consumable costs but higher energy consumption due to laser systems and cooling requirements. Maintenance costs for AFM average 5-8% of the initial investment annually, while LSM maintenance typically runs 7-10% annually due to the complexity of optical components and laser systems.
Training requirements represent another critical cost factor. AFM operation demands extensive technical expertise, with training periods ranging from 2-6 months for proficient operation, translating to approximately $10,000-$20,000 in personnel costs. LSM typically requires 1-3 months of training, with costs ranging from $5,000-$15,000, offering a faster return on training investment.
The return on investment calculations reveal distinctive patterns between these technologies. AFM provides superior ROI for materials science applications, with payback periods typically ranging from 2-3 years when utilized at optimal capacity. The high-resolution capabilities justify the investment for specialized research requiring atomic-level imaging. LSM demonstrates stronger ROI metrics for biological research, particularly for live cell imaging, with typical payback periods of 1.5-2.5 years due to higher throughput capabilities and reduced sample preparation time.
Productivity gains must be factored into ROI assessments. LSM offers significant advantages in imaging speed, capable of capturing dynamic biological processes at rates 10-20 times faster than AFM. This translates to higher sample throughput and accelerated research timelines. AFM, while slower, provides unmatched precision for certain applications that cannot be achieved through alternative methods, creating value through unique capabilities rather than throughput.
Long-term value assessment indicates that both technologies maintain relatively strong resale values, with AFM systems typically retaining 40-50% of their value after 5 years, while LSM systems retain approximately 30-40%. The total cost of ownership over a 5-year period, including acquisition, maintenance, training, and consumables, averages $250,000-$650,000 for AFM and $350,000-$800,000 for LSM, depending on usage intensity and configuration.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!