Automation of Cell-free Protein Synthesis in Microfluidic Devices
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CFPS Microfluidic Automation Background & Objectives
Cell-free protein synthesis (CFPS) has evolved significantly since its inception in the 1960s, transitioning from a fundamental research tool to a versatile platform for various biotechnological applications. This technology eliminates the need for intact cells by utilizing cellular extracts containing the necessary machinery for transcription and translation, offering unprecedented flexibility in protein production. The integration of CFPS with microfluidic technologies represents a revolutionary advancement, enabling precise control over reaction conditions in miniaturized environments.
The evolution of CFPS automation in microfluidic devices has been driven by increasing demands for high-throughput protein production with minimal resource consumption. Early systems focused primarily on basic proof-of-concept demonstrations, while contemporary approaches emphasize scalability, reproducibility, and integration with downstream applications. This technological convergence addresses critical limitations in traditional protein synthesis methods, including inefficient resource utilization and limited parallelization capabilities.
Recent advancements in microfluidic fabrication techniques, including soft lithography, 3D printing, and laser ablation, have significantly expanded the design possibilities for CFPS platforms. Concurrently, improvements in cell extract preparation protocols and reaction formulations have enhanced protein yields and reduced costs, making automated CFPS increasingly accessible to researchers across various disciplines.
The primary objective of automating CFPS in microfluidic devices is to establish a robust, versatile platform capable of on-demand protein production with minimal human intervention. This encompasses several specific goals: maximizing reaction efficiency through precise control of environmental parameters; enabling multiplexed synthesis of diverse proteins simultaneously; reducing reagent consumption through miniaturization; and facilitating rapid prototyping of novel proteins for research and therapeutic applications.
Additionally, this technology aims to bridge the gap between laboratory-scale protein production and industrial applications by providing scalable solutions that maintain consistency across different production volumes. The development of standardized interfaces between automated CFPS systems and downstream processing technologies represents another crucial objective, potentially revolutionizing the protein production pipeline.
From a broader perspective, the automation of CFPS in microfluidic devices aligns with the emerging trend toward decentralized biomanufacturing, where production facilities can be smaller, more adaptable, and geographically distributed. This paradigm shift could dramatically reduce the time and resources required to develop and deploy new protein-based therapeutics, diagnostics, and industrial enzymes, ultimately accelerating innovation in biotechnology and healthcare.
The evolution of CFPS automation in microfluidic devices has been driven by increasing demands for high-throughput protein production with minimal resource consumption. Early systems focused primarily on basic proof-of-concept demonstrations, while contemporary approaches emphasize scalability, reproducibility, and integration with downstream applications. This technological convergence addresses critical limitations in traditional protein synthesis methods, including inefficient resource utilization and limited parallelization capabilities.
Recent advancements in microfluidic fabrication techniques, including soft lithography, 3D printing, and laser ablation, have significantly expanded the design possibilities for CFPS platforms. Concurrently, improvements in cell extract preparation protocols and reaction formulations have enhanced protein yields and reduced costs, making automated CFPS increasingly accessible to researchers across various disciplines.
The primary objective of automating CFPS in microfluidic devices is to establish a robust, versatile platform capable of on-demand protein production with minimal human intervention. This encompasses several specific goals: maximizing reaction efficiency through precise control of environmental parameters; enabling multiplexed synthesis of diverse proteins simultaneously; reducing reagent consumption through miniaturization; and facilitating rapid prototyping of novel proteins for research and therapeutic applications.
Additionally, this technology aims to bridge the gap between laboratory-scale protein production and industrial applications by providing scalable solutions that maintain consistency across different production volumes. The development of standardized interfaces between automated CFPS systems and downstream processing technologies represents another crucial objective, potentially revolutionizing the protein production pipeline.
From a broader perspective, the automation of CFPS in microfluidic devices aligns with the emerging trend toward decentralized biomanufacturing, where production facilities can be smaller, more adaptable, and geographically distributed. This paradigm shift could dramatically reduce the time and resources required to develop and deploy new protein-based therapeutics, diagnostics, and industrial enzymes, ultimately accelerating innovation in biotechnology and healthcare.
Market Analysis for Cell-free Protein Synthesis Applications
The global market for cell-free protein synthesis (CFPS) applications is experiencing robust growth, driven by increasing demand in pharmaceutical research, diagnostics, and synthetic biology. Current market valuation stands at approximately 250 million USD, with projections indicating a compound annual growth rate of 8-10% over the next five years, potentially reaching 400 million USD by 2028.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for nearly 60% of the total CFPS market. These industries leverage CFPS for rapid protein production, drug screening, and therapeutic development. The ability to quickly synthesize proteins without maintaining living cells provides significant advantages in research efficiency and cost reduction.
Academic research institutions constitute the second-largest market segment at roughly 25%, utilizing CFPS systems for fundamental research in protein structure, function, and interaction studies. The remaining market share is distributed among diagnostic applications, agricultural biotechnology, and emerging industrial applications.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, South Korea, and Singapore, demonstrates the fastest growth rate due to increasing investment in biotechnology infrastructure and research capabilities.
The integration of CFPS with microfluidic devices represents a particularly promising market opportunity. This combination offers advantages in miniaturization, automation, and high-throughput capabilities that align with industry trends toward more efficient, cost-effective research tools. The market for microfluidic-based CFPS systems is currently valued at around 50 million USD but shows potential for 15-18% annual growth.
Key market drivers include the rising demand for personalized medicine, increasing research in synthetic biology, and the need for rapid protein production platforms in vaccine development. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid protein synthesis capabilities for diagnostic and therapeutic applications.
Market barriers include high initial setup costs, technical complexity requiring specialized expertise, and regulatory challenges for commercial applications. Additionally, standardization issues and reproducibility concerns present obstacles to wider adoption in certain sectors.
Customer segments show distinct needs: pharmaceutical companies prioritize scalability and regulatory compliance; academic institutions value flexibility and cost-effectiveness; while diagnostic developers focus on speed and integration capabilities. Understanding these differentiated requirements is essential for successful market penetration of automated CFPS microfluidic technologies.
Pharmaceutical and biotechnology sectors represent the largest market segments, accounting for nearly 60% of the total CFPS market. These industries leverage CFPS for rapid protein production, drug screening, and therapeutic development. The ability to quickly synthesize proteins without maintaining living cells provides significant advantages in research efficiency and cost reduction.
Academic research institutions constitute the second-largest market segment at roughly 25%, utilizing CFPS systems for fundamental research in protein structure, function, and interaction studies. The remaining market share is distributed among diagnostic applications, agricultural biotechnology, and emerging industrial applications.
Regionally, North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, South Korea, and Singapore, demonstrates the fastest growth rate due to increasing investment in biotechnology infrastructure and research capabilities.
The integration of CFPS with microfluidic devices represents a particularly promising market opportunity. This combination offers advantages in miniaturization, automation, and high-throughput capabilities that align with industry trends toward more efficient, cost-effective research tools. The market for microfluidic-based CFPS systems is currently valued at around 50 million USD but shows potential for 15-18% annual growth.
Key market drivers include the rising demand for personalized medicine, increasing research in synthetic biology, and the need for rapid protein production platforms in vaccine development. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid protein synthesis capabilities for diagnostic and therapeutic applications.
Market barriers include high initial setup costs, technical complexity requiring specialized expertise, and regulatory challenges for commercial applications. Additionally, standardization issues and reproducibility concerns present obstacles to wider adoption in certain sectors.
Customer segments show distinct needs: pharmaceutical companies prioritize scalability and regulatory compliance; academic institutions value flexibility and cost-effectiveness; while diagnostic developers focus on speed and integration capabilities. Understanding these differentiated requirements is essential for successful market penetration of automated CFPS microfluidic technologies.
Technical Challenges in Microfluidic CFPS Integration
The integration of Cell-free Protein Synthesis (CFPS) with microfluidic platforms presents significant technical challenges that require innovative solutions. One of the primary obstacles is the miniaturization of reaction volumes while maintaining protein yield and quality. Traditional CFPS reactions typically operate at microliter to milliliter scales, whereas microfluidic systems function optimally at nanoliter volumes. This scale disparity creates difficulties in adapting established CFPS protocols to microfluidic environments without compromising efficiency.
Surface-to-volume ratio effects become pronounced at microfluidic scales, leading to increased protein adsorption to device surfaces. This non-specific binding can deplete essential components from the reaction mixture and potentially inhibit protein synthesis. Various surface treatments and passivation strategies have been explored, including BSA coating, PEG-silane derivatization, and dynamic passivation with Pluronic surfactants, yet a universally effective solution remains elusive.
Temperature control represents another critical challenge. CFPS reactions typically require precise temperature maintenance (usually 30-37°C) for optimal enzyme activity. Microfluidic devices, due to their small thermal mass and high surface area, experience rapid heat dissipation and temperature gradients that can significantly impact reaction kinetics and protein yield. Integrated heating elements and temperature sensors add complexity to device fabrication and operation.
Oxygen transfer limitations constitute a substantial hurdle, particularly for energy-intensive protein synthesis. CFPS reactions often require adequate oxygen supply for ATP regeneration, yet the limited gas permeability of common microfluidic materials (PDMS, glass, thermoplastics) restricts oxygen availability. This limitation becomes more pronounced in continuous-flow systems where reaction times extend beyond several hours.
Component stability presents ongoing challenges, as CFPS extracts contain numerous enzymes and cofactors with varying stability profiles. The microfluidic environment, characterized by high surface interactions and potential material incompatibilities, can accelerate component degradation. This necessitates careful material selection and potentially requires supplementation strategies to maintain reaction viability over extended periods.
Automation and integration complexities arise when attempting to incorporate multiple reaction steps, including transcription, translation, and post-translational modifications, into a single microfluidic workflow. The precise control of reagent additions, mixing efficiency, and reaction timing requires sophisticated fluidic control systems that can reliably operate at microliter to nanoliter scales without introducing bubbles or flow irregularities that disrupt the sensitive biochemical processes.
Analytical detection and monitoring capabilities within microfluidic CFPS systems remain limited, with most current approaches relying on fluorescent reporter proteins that provide only indirect measures of synthesis efficiency. Real-time monitoring of complex reaction parameters such as ATP consumption, amino acid utilization, and formation of functional proteins presents significant technical barriers that impede process optimization and control.
Surface-to-volume ratio effects become pronounced at microfluidic scales, leading to increased protein adsorption to device surfaces. This non-specific binding can deplete essential components from the reaction mixture and potentially inhibit protein synthesis. Various surface treatments and passivation strategies have been explored, including BSA coating, PEG-silane derivatization, and dynamic passivation with Pluronic surfactants, yet a universally effective solution remains elusive.
Temperature control represents another critical challenge. CFPS reactions typically require precise temperature maintenance (usually 30-37°C) for optimal enzyme activity. Microfluidic devices, due to their small thermal mass and high surface area, experience rapid heat dissipation and temperature gradients that can significantly impact reaction kinetics and protein yield. Integrated heating elements and temperature sensors add complexity to device fabrication and operation.
Oxygen transfer limitations constitute a substantial hurdle, particularly for energy-intensive protein synthesis. CFPS reactions often require adequate oxygen supply for ATP regeneration, yet the limited gas permeability of common microfluidic materials (PDMS, glass, thermoplastics) restricts oxygen availability. This limitation becomes more pronounced in continuous-flow systems where reaction times extend beyond several hours.
Component stability presents ongoing challenges, as CFPS extracts contain numerous enzymes and cofactors with varying stability profiles. The microfluidic environment, characterized by high surface interactions and potential material incompatibilities, can accelerate component degradation. This necessitates careful material selection and potentially requires supplementation strategies to maintain reaction viability over extended periods.
Automation and integration complexities arise when attempting to incorporate multiple reaction steps, including transcription, translation, and post-translational modifications, into a single microfluidic workflow. The precise control of reagent additions, mixing efficiency, and reaction timing requires sophisticated fluidic control systems that can reliably operate at microliter to nanoliter scales without introducing bubbles or flow irregularities that disrupt the sensitive biochemical processes.
Analytical detection and monitoring capabilities within microfluidic CFPS systems remain limited, with most current approaches relying on fluorescent reporter proteins that provide only indirect measures of synthesis efficiency. Real-time monitoring of complex reaction parameters such as ATP consumption, amino acid utilization, and formation of functional proteins presents significant technical barriers that impede process optimization and control.
Current Microfluidic Platforms for CFPS Automation
01 Microfluidic device design for cell-free protein synthesis
Specialized microfluidic devices designed specifically for cell-free protein synthesis applications. These devices incorporate features such as reaction chambers, flow channels, and integrated sensors to optimize protein production in a controlled environment. The designs focus on miniaturization while maintaining or improving synthesis efficiency compared to conventional methods, allowing for precise control over reaction conditions and reagent delivery.- Microfluidic device design for cell-free protein synthesis: Specialized microfluidic devices designed specifically for cell-free protein synthesis applications. These devices incorporate features such as reaction chambers, mixing channels, and temperature control elements to optimize protein production. The designs focus on miniaturization while maintaining or improving synthesis efficiency compared to conventional methods, allowing for precise control over reaction conditions in microscale environments.
- Automation systems for cell-free protein synthesis workflows: Integrated automation systems that control the entire cell-free protein synthesis process in microfluidic platforms. These systems incorporate robotics, fluid handling mechanisms, and software control to automate sample preparation, reagent addition, reaction monitoring, and product collection. Automation reduces human error, increases throughput, and enables continuous or semi-continuous operation for extended protein production runs.
- Real-time monitoring and control techniques: Methods for real-time monitoring and feedback control of cell-free protein synthesis in microfluidic devices. These techniques incorporate sensors for measuring parameters such as pH, temperature, oxygen levels, and protein concentration during synthesis. The monitoring systems allow for dynamic adjustment of reaction conditions to optimize protein yield and quality, with data analysis capabilities for process optimization.
- High-throughput parallel synthesis platforms: Microfluidic platforms designed for parallel cell-free protein synthesis, enabling high-throughput production of multiple proteins simultaneously. These systems feature arrays of reaction chambers or droplet-based approaches that allow for testing different conditions or producing different proteins in parallel. The platforms incorporate multiplexed control systems and often include methods for rapid screening and analysis of the synthesized proteins.
- Integration of purification and analysis systems: Microfluidic systems that integrate cell-free protein synthesis with downstream purification and analysis capabilities. These integrated platforms incorporate on-chip purification methods such as chromatography, filtration, or electrophoresis to process synthesized proteins directly. Some systems also include analytical capabilities for protein characterization, enabling a complete workflow from synthesis to purified, analyzed product within a single automated platform.
02 Automation systems for cell-free protein synthesis
Automated systems that integrate multiple steps of the cell-free protein synthesis process, including reagent handling, reaction monitoring, and product purification. These systems employ robotics, programmable controllers, and software interfaces to minimize manual intervention and increase throughput. Automation enables consistent results, reduces human error, and allows for continuous operation of synthesis reactions in microfluidic platforms.Expand Specific Solutions03 Continuous-flow cell-free protein synthesis methods
Methods for performing cell-free protein synthesis in continuous-flow microfluidic systems rather than batch reactions. These approaches allow for constant replenishment of substrates and removal of inhibitory byproducts, extending reaction lifetimes and improving yields. Continuous-flow systems can be designed with multiple stages for sequential processing steps, enabling more efficient protein production and real-time monitoring of synthesis progress.Expand Specific Solutions04 Integration of analysis and purification in microfluidic CFPS systems
Microfluidic platforms that combine cell-free protein synthesis with downstream analysis and purification steps in a single integrated device. These systems incorporate components for protein characterization (such as fluorescence detection or mass spectrometry) and purification methods (such as chromatography or electrophoresis) directly within the microfluidic architecture. Integration reduces sample loss, contamination risks, and processing time while enabling real-time quality control.Expand Specific Solutions05 Energy-efficient and portable CFPS microfluidic systems
Development of energy-efficient and portable microfluidic systems for cell-free protein synthesis that can operate outside traditional laboratory settings. These systems utilize low-power components, alternative energy sources, and miniaturized control electronics to enable field deployment. Portable systems are designed with simplified user interfaces and robust operation parameters to facilitate use in resource-limited environments or point-of-need applications.Expand Specific Solutions
Leading Companies in Microfluidic CFPS Development
The cell-free protein synthesis (CFPS) in microfluidic devices market is in an early growth phase, with increasing adoption driven by advantages in rapid prototyping and high-throughput applications. The global market is expanding as automation technologies mature, estimated to reach significant value within the bioprocessing sector. Leading players include specialized companies like Cellfree Sciences and Swiftscale Biologics focusing exclusively on CFPS technologies, alongside diversified corporations such as Shimadzu and New England Biolabs that integrate microfluidic CFPS into broader portfolios. Academic institutions including Northwestern University, Tsinghua University, and ETH Zurich are driving innovation through fundamental research, while emerging companies like Nuclera and Kangma are developing commercial applications combining CFPS with microfluidic automation for point-of-need protein production.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed a proprietary WEPRO® system for cell-free protein synthesis automation in microfluidic platforms. Their technology utilizes wheat germ extract-based cell-free protein expression systems integrated with microfluidic chips for high-throughput protein production. The company's automated platform incorporates continuous-exchange cell-free (CECF) reaction formats that allow for extended reaction times and higher protein yields compared to batch reactions. Their microfluidic devices feature multiple reaction chambers with integrated temperature control systems, enabling parallel synthesis of different proteins simultaneously. The system includes automated sample handling, reagent dispensing, and real-time monitoring capabilities, making it suitable for both research and commercial applications in pharmaceutical development and protein engineering.
Strengths: Superior protein yield using wheat germ extract system; extended reaction times through CECF format; high-throughput parallel processing capabilities. Weaknesses: Higher cost compared to bacterial cell-free systems; requires specialized reagents; system complexity may present maintenance challenges.
Nuclera Ltd.
Technical Solution: Nuclera has pioneered the eProtein™ Desktop Bioprinter, an integrated microfluidic platform that automates cell-free protein synthesis from digital DNA sequences to purified proteins within 24 hours. Their technology combines digital microfluidics with proprietary enzymatic DNA synthesis and cell-free expression systems. The platform utilizes electrowetting-on-dielectric (EWOD) technology to precisely manipulate nanoliter-scale droplets containing reaction components across a microfluidic chip surface. This approach enables automated DNA template generation, transcription, translation, and protein purification in a single integrated device. The system incorporates multiple temperature zones for optimal reaction conditions and features in-line quality control monitoring. Nuclera's technology significantly reduces sample volumes and reagent costs while maintaining high protein yields and functionality.
Strengths: End-to-end automation from DNA sequence to purified protein; reduced reagent consumption through nanoliter-scale reactions; rapid turnaround time (24 hours). Weaknesses: Limited scalability for large-volume protein production; relatively new technology with less established track record; higher initial capital investment.
Key Patents in Automated Cell-free Protein Synthesis
Methods for cell-free protein expression
PatentActiveUS11821018B2
Innovation
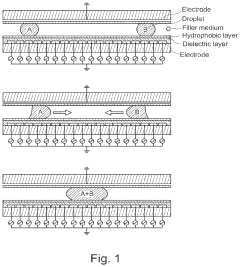
- The method involves using an oil-filled digital microfluidic device with electrokinesis to move droplets containing nucleic acid templates and cell-free protein synthesis components, allowing for extended protein expression while supplying oxygen, and using supplemental oxygen sources and humidification to prevent evaporation and biofouling.
Methods for cell free protein synthesis and post translational modification of the expressed proteins
PatentPendingUS20250146041A1
Innovation
- The development of optimized cell-free protein synthesis reagents and methods that combine cell lysates with purified recombinant elements, allowing for enhanced protein expression yields and post-translational modifications, while minimizing biofouling on microfluidic devices.
Scalability and Manufacturing Considerations
The scalability of cell-free protein synthesis (CFPS) in microfluidic devices represents a critical challenge for transitioning from laboratory-scale demonstrations to industrial applications. Current microfluidic CFPS systems typically operate at microliter to nanoliter volumes, which are suitable for research purposes but insufficient for commercial production demands. Scaling these systems requires parallel processing architectures that maintain the advantages of microfluidics while increasing throughput.
Manufacturing considerations for automated CFPS microfluidic platforms must address both hardware and biochemical components. The fabrication of microfluidic devices traditionally relies on soft lithography techniques using polydimethylsiloxane (PDMS), which presents limitations for mass production. Alternative materials such as thermoplastics (polycarbonate, PMMA) and glass offer better durability and manufacturing compatibility, though they require different fabrication approaches like injection molding or hot embossing.
Integration of control systems presents another manufacturing challenge. Automated CFPS platforms require precise coordination of multiple components including pumps, valves, sensors, and thermal regulators. Standardization of these interfaces would significantly reduce manufacturing complexity and cost, enabling more widespread adoption of these technologies. Recent advances in modular microfluidic design show promise for addressing these integration challenges.
Supply chain considerations for CFPS reagents also impact scalability. The cell extracts and biochemical components required for CFPS have limited shelf life and specific storage requirements. Developing lyophilized or otherwise stabilized reagent formulations would enhance manufacturing practicality by extending shelf life and reducing cold chain dependencies. Companies like Sutro Biopharma and Greenlight Biosciences have made progress in this area, developing proprietary stabilization methods for their CFPS platforms.
Economic viability remains a key consideration for scaled manufacturing. The cost per unit protein must be competitive with traditional bioreactor-based expression systems. Analysis suggests that for high-value therapeutic proteins and personalized medicine applications, microfluidic CFPS can achieve favorable economics through reduced capital expenditure and increased flexibility, despite potentially higher reagent costs.
Regulatory pathways for manufacturing automated CFPS systems must also be considered. As these platforms move toward clinical applications, compliance with Good Manufacturing Practice (GMP) standards becomes essential. Designing systems with built-in quality control monitoring and validation capabilities will facilitate regulatory approval and commercial deployment.
Manufacturing considerations for automated CFPS microfluidic platforms must address both hardware and biochemical components. The fabrication of microfluidic devices traditionally relies on soft lithography techniques using polydimethylsiloxane (PDMS), which presents limitations for mass production. Alternative materials such as thermoplastics (polycarbonate, PMMA) and glass offer better durability and manufacturing compatibility, though they require different fabrication approaches like injection molding or hot embossing.
Integration of control systems presents another manufacturing challenge. Automated CFPS platforms require precise coordination of multiple components including pumps, valves, sensors, and thermal regulators. Standardization of these interfaces would significantly reduce manufacturing complexity and cost, enabling more widespread adoption of these technologies. Recent advances in modular microfluidic design show promise for addressing these integration challenges.
Supply chain considerations for CFPS reagents also impact scalability. The cell extracts and biochemical components required for CFPS have limited shelf life and specific storage requirements. Developing lyophilized or otherwise stabilized reagent formulations would enhance manufacturing practicality by extending shelf life and reducing cold chain dependencies. Companies like Sutro Biopharma and Greenlight Biosciences have made progress in this area, developing proprietary stabilization methods for their CFPS platforms.
Economic viability remains a key consideration for scaled manufacturing. The cost per unit protein must be competitive with traditional bioreactor-based expression systems. Analysis suggests that for high-value therapeutic proteins and personalized medicine applications, microfluidic CFPS can achieve favorable economics through reduced capital expenditure and increased flexibility, despite potentially higher reagent costs.
Regulatory pathways for manufacturing automated CFPS systems must also be considered. As these platforms move toward clinical applications, compliance with Good Manufacturing Practice (GMP) standards becomes essential. Designing systems with built-in quality control monitoring and validation capabilities will facilitate regulatory approval and commercial deployment.
Regulatory Pathway for CFPS Diagnostic Applications
The regulatory landscape for Cell-free Protein Synthesis (CFPS) diagnostic applications presents a complex pathway that developers must navigate carefully. Currently, CFPS-based diagnostics fall primarily under in vitro diagnostic (IVD) regulations in most jurisdictions. In the United States, the Food and Drug Administration (FDA) regulates these technologies through the Center for Devices and Radiological Health (CDRH), with classification depending on intended use and risk profile. Most CFPS diagnostic applications would likely be classified as Class II devices, requiring 510(k) clearance demonstrating substantial equivalence to predicate devices.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVDD directive in May 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Under this framework, CFPS-based diagnostics may fall into different risk classes (A through D) depending on their intended purpose and inherent risks, with most novel applications likely classified as Class C.
Regulatory considerations specific to CFPS microfluidic diagnostics include validation of protein expression efficiency, demonstration of analytical performance (sensitivity, specificity, reproducibility), and stability studies under various environmental conditions. Developers must establish quality control parameters for cell-free lysates and reaction components, which presents unique challenges compared to traditional diagnostic platforms.
A critical regulatory hurdle involves demonstrating that the automated microfluidic CFPS system maintains consistent performance across different manufacturing lots and operating conditions. This includes validation of the automated fluid handling systems, reaction chambers, and detection modules. The integration of multiple components increases regulatory complexity as each element must meet applicable standards.
For point-of-care applications, additional considerations include usability testing, shelf-life determination, and performance in resource-limited settings. Regulatory bodies increasingly require evidence that diagnostic tests perform consistently across diverse user groups and environmental conditions, particularly for tests intended for global health applications.
Emerging regulatory frameworks for novel diagnostic technologies are evolving to address CFPS-specific concerns. The FDA's Pre-Cert program and similar initiatives may offer accelerated pathways for innovative diagnostic platforms with appropriate quality management systems. International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to standardize requirements across major markets, potentially simplifying the global regulatory process for CFPS diagnostic developers.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR 2017/746), which replaced the previous IVDD directive in May 2022, introducing more stringent requirements for clinical evidence, post-market surveillance, and risk classification. Under this framework, CFPS-based diagnostics may fall into different risk classes (A through D) depending on their intended purpose and inherent risks, with most novel applications likely classified as Class C.
Regulatory considerations specific to CFPS microfluidic diagnostics include validation of protein expression efficiency, demonstration of analytical performance (sensitivity, specificity, reproducibility), and stability studies under various environmental conditions. Developers must establish quality control parameters for cell-free lysates and reaction components, which presents unique challenges compared to traditional diagnostic platforms.
A critical regulatory hurdle involves demonstrating that the automated microfluidic CFPS system maintains consistent performance across different manufacturing lots and operating conditions. This includes validation of the automated fluid handling systems, reaction chambers, and detection modules. The integration of multiple components increases regulatory complexity as each element must meet applicable standards.
For point-of-care applications, additional considerations include usability testing, shelf-life determination, and performance in resource-limited settings. Regulatory bodies increasingly require evidence that diagnostic tests perform consistently across diverse user groups and environmental conditions, particularly for tests intended for global health applications.
Emerging regulatory frameworks for novel diagnostic technologies are evolving to address CFPS-specific concerns. The FDA's Pre-Cert program and similar initiatives may offer accelerated pathways for innovative diagnostic platforms with appropriate quality management systems. International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to standardize requirements across major markets, potentially simplifying the global regulatory process for CFPS diagnostic developers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!