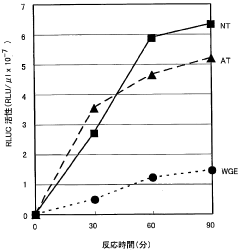
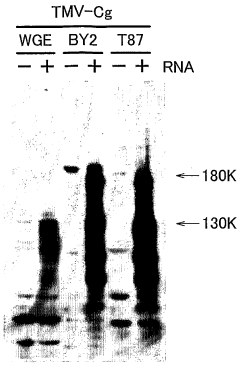
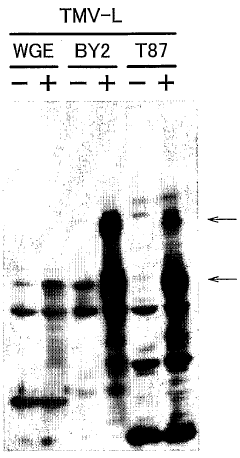
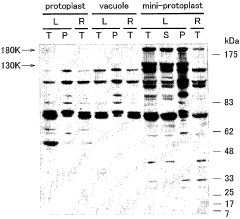
Cell-free Protein Synthesis for Cell-Mimicking Protocells
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis Background and Objectives
Cell-free protein synthesis (CFPS) has evolved significantly since its inception in the 1960s, transitioning from a fundamental research tool to a versatile platform for synthetic biology applications. This technology extracts cellular machinery necessary for protein production while eliminating cellular boundaries, allowing for direct manipulation of the transcription-translation environment. The historical trajectory shows a shift from crude cell extracts to highly optimized systems with enhanced yields, stability, and functionality.
Recent advancements have positioned CFPS as a cornerstone technology for creating cell-mimicking protocells—artificial constructs that replicate specific cellular functions without being fully living entities. These protocells represent a bridge between non-living chemical systems and biological cells, offering unique advantages for both fundamental research and practical applications.
The integration of CFPS with protocell development aims to create biomimetic systems capable of performing complex biological functions in controlled environments. This convergence addresses limitations of traditional cell-based approaches, particularly regarding customization, scalability, and deployment in non-physiological conditions. The field has witnessed exponential growth in publications over the past decade, indicating increasing scientific interest and investment.
Current technical objectives focus on enhancing the efficiency and longevity of CFPS reactions within protocell architectures. This includes optimizing energy regeneration systems, improving translation efficiency, and developing mechanisms for continuous protein production. Additionally, researchers aim to incorporate post-translational modification capabilities and complex metabolic pathways to more accurately mimic cellular functionality.
Another critical objective involves the development of responsive protocells that can sense and react to environmental stimuli through programmed protein synthesis. This capability would enable applications in biosensing, targeted drug delivery, and environmental remediation. The field also seeks to establish standardized protocols and modular components to facilitate broader adoption across scientific disciplines.
Long-term goals encompass the creation of self-sustaining protocells with dynamic protein expression profiles and the ability to evolve functionality over time. This represents a step toward minimal artificial cells that maintain core living characteristics while being engineered for specific purposes. The technology trajectory suggests potential convergence with fields like microfluidics, nanotechnology, and artificial intelligence to create increasingly sophisticated cell-mimicking systems.
The ultimate objective extends beyond technological development to address fundamental questions about the nature of life itself, exploring the minimal requirements for cellular function and the emergence of complex behaviors from simple components. This positions CFPS-based protocells at the intersection of applied biotechnology and foundational life science research.
Recent advancements have positioned CFPS as a cornerstone technology for creating cell-mimicking protocells—artificial constructs that replicate specific cellular functions without being fully living entities. These protocells represent a bridge between non-living chemical systems and biological cells, offering unique advantages for both fundamental research and practical applications.
The integration of CFPS with protocell development aims to create biomimetic systems capable of performing complex biological functions in controlled environments. This convergence addresses limitations of traditional cell-based approaches, particularly regarding customization, scalability, and deployment in non-physiological conditions. The field has witnessed exponential growth in publications over the past decade, indicating increasing scientific interest and investment.
Current technical objectives focus on enhancing the efficiency and longevity of CFPS reactions within protocell architectures. This includes optimizing energy regeneration systems, improving translation efficiency, and developing mechanisms for continuous protein production. Additionally, researchers aim to incorporate post-translational modification capabilities and complex metabolic pathways to more accurately mimic cellular functionality.
Another critical objective involves the development of responsive protocells that can sense and react to environmental stimuli through programmed protein synthesis. This capability would enable applications in biosensing, targeted drug delivery, and environmental remediation. The field also seeks to establish standardized protocols and modular components to facilitate broader adoption across scientific disciplines.
Long-term goals encompass the creation of self-sustaining protocells with dynamic protein expression profiles and the ability to evolve functionality over time. This represents a step toward minimal artificial cells that maintain core living characteristics while being engineered for specific purposes. The technology trajectory suggests potential convergence with fields like microfluidics, nanotechnology, and artificial intelligence to create increasingly sophisticated cell-mimicking systems.
The ultimate objective extends beyond technological development to address fundamental questions about the nature of life itself, exploring the minimal requirements for cellular function and the emergence of complex behaviors from simple components. This positions CFPS-based protocells at the intersection of applied biotechnology and foundational life science research.
Market Analysis for Cell-free Biotechnology Applications
The cell-free protein synthesis (CFPS) market is experiencing significant growth, driven by increasing applications in synthetic biology, pharmaceuticals, and biomaterials. The global market for cell-free technologies was valued at approximately $208 million in 2021 and is projected to reach $310 million by 2026, growing at a CAGR of 8.3%. This growth trajectory reflects the expanding utility of CFPS systems across multiple industries.
Pharmaceutical applications currently dominate the market landscape, accounting for nearly 40% of the total market share. The ability to rapidly produce therapeutic proteins and vaccines without the constraints of living cells has positioned CFPS as a valuable tool for drug discovery and development. Several major pharmaceutical companies have integrated CFPS platforms into their R&D pipelines, recognizing the potential for accelerated development timelines and reduced costs.
The diagnostic sector represents another significant market segment, with CFPS-based biosensors and point-of-care testing devices gaining traction. These applications leverage the rapid protein production capabilities of cell-free systems to detect pathogens, biomarkers, and environmental contaminants with high sensitivity and specificity. The COVID-19 pandemic has further accelerated interest in this area, with several CFPS-based diagnostic tools receiving emergency use authorization.
Emerging applications in biomaterials and protocell development are expected to drive future market growth. The ability to create cell-mimicking structures with functional protein components opens new possibilities for tissue engineering, drug delivery systems, and bioremediation. Industry analysts predict this segment could grow at a CAGR of 12-15% over the next five years, outpacing the overall market growth rate.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing investments in biotechnology infrastructure and research capabilities in countries like China, Japan, and South Korea.
Key market challenges include high production costs, scalability issues, and regulatory uncertainties. The cost of cell-free reaction components remains a significant barrier to widespread commercial adoption, though recent advances in manufacturing processes have begun to address this limitation. Additionally, standardization of CFPS protocols and regulatory frameworks will be essential for market expansion, particularly for applications in healthcare and food production.
Consumer acceptance and ethical considerations surrounding synthetic biology applications may influence market dynamics, especially for products intended for direct human use or environmental release. Industry stakeholders are increasingly engaging with regulatory bodies and public communication initiatives to address these concerns proactively.
Pharmaceutical applications currently dominate the market landscape, accounting for nearly 40% of the total market share. The ability to rapidly produce therapeutic proteins and vaccines without the constraints of living cells has positioned CFPS as a valuable tool for drug discovery and development. Several major pharmaceutical companies have integrated CFPS platforms into their R&D pipelines, recognizing the potential for accelerated development timelines and reduced costs.
The diagnostic sector represents another significant market segment, with CFPS-based biosensors and point-of-care testing devices gaining traction. These applications leverage the rapid protein production capabilities of cell-free systems to detect pathogens, biomarkers, and environmental contaminants with high sensitivity and specificity. The COVID-19 pandemic has further accelerated interest in this area, with several CFPS-based diagnostic tools receiving emergency use authorization.
Emerging applications in biomaterials and protocell development are expected to drive future market growth. The ability to create cell-mimicking structures with functional protein components opens new possibilities for tissue engineering, drug delivery systems, and bioremediation. Industry analysts predict this segment could grow at a CAGR of 12-15% over the next five years, outpacing the overall market growth rate.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing investments in biotechnology infrastructure and research capabilities in countries like China, Japan, and South Korea.
Key market challenges include high production costs, scalability issues, and regulatory uncertainties. The cost of cell-free reaction components remains a significant barrier to widespread commercial adoption, though recent advances in manufacturing processes have begun to address this limitation. Additionally, standardization of CFPS protocols and regulatory frameworks will be essential for market expansion, particularly for applications in healthcare and food production.
Consumer acceptance and ethical considerations surrounding synthetic biology applications may influence market dynamics, especially for products intended for direct human use or environmental release. Industry stakeholders are increasingly engaging with regulatory bodies and public communication initiatives to address these concerns proactively.
Current Challenges in Protocell Development
Despite significant advancements in protocell development, several critical challenges continue to impede progress in creating fully functional cell-mimicking systems using cell-free protein synthesis (CFPS). The primary obstacle remains the limited stability of protocells, with most current designs maintaining functionality for only hours to days, far shorter than required for practical applications. This instability stems from degradation of essential components, including enzymes and nucleic acids, as well as membrane destabilization over time.
Energy supply represents another fundamental challenge, as CFPS systems rapidly deplete ATP and other energy-rich molecules. While regeneration systems have been developed, they often introduce additional complexity and potential incompatibilities with other protocell components. The efficiency of continuous energy production without external intervention remains elusive, limiting the self-sustainability of protocell systems.
Spatial organization within protocells presents significant difficulties, as natural cells utilize complex compartmentalization to regulate biochemical processes. Current protocell designs struggle to replicate this sophisticated architecture, resulting in reduced efficiency and control over protein synthesis pathways. The lack of proper spatial organization also impacts reaction kinetics and molecular interactions essential for complex cellular functions.
Scalability issues persist in protocell production, with most successful demonstrations limited to laboratory scales. Transitioning to industrial production faces challenges in maintaining consistency, quality control, and cost-effectiveness. The complex nature of CFPS components makes standardization particularly difficult, hampering widespread adoption and commercialization efforts.
Regulatory and safety concerns also present significant hurdles. The potential environmental impact of synthetic protocells, including horizontal gene transfer and ecological disruption, remains inadequately addressed. Additionally, regulatory frameworks for these novel entities are underdeveloped, creating uncertainty for research and commercial applications.
The integration of sensing and response mechanisms represents another major challenge. While natural cells can detect environmental changes and adapt accordingly, protocells typically lack sophisticated feedback systems. Developing protocells with dynamic responsiveness requires complex genetic circuits and signaling pathways that are difficult to implement in simplified cell-free systems.
Finally, achieving reproducibility across different laboratories and production batches remains problematic. Variations in extract preparation, component quality, and environmental conditions lead to inconsistent performance, complicating both fundamental research and potential applications. Standardized protocols and quality control measures are urgently needed to address this challenge.
Energy supply represents another fundamental challenge, as CFPS systems rapidly deplete ATP and other energy-rich molecules. While regeneration systems have been developed, they often introduce additional complexity and potential incompatibilities with other protocell components. The efficiency of continuous energy production without external intervention remains elusive, limiting the self-sustainability of protocell systems.
Spatial organization within protocells presents significant difficulties, as natural cells utilize complex compartmentalization to regulate biochemical processes. Current protocell designs struggle to replicate this sophisticated architecture, resulting in reduced efficiency and control over protein synthesis pathways. The lack of proper spatial organization also impacts reaction kinetics and molecular interactions essential for complex cellular functions.
Scalability issues persist in protocell production, with most successful demonstrations limited to laboratory scales. Transitioning to industrial production faces challenges in maintaining consistency, quality control, and cost-effectiveness. The complex nature of CFPS components makes standardization particularly difficult, hampering widespread adoption and commercialization efforts.
Regulatory and safety concerns also present significant hurdles. The potential environmental impact of synthetic protocells, including horizontal gene transfer and ecological disruption, remains inadequately addressed. Additionally, regulatory frameworks for these novel entities are underdeveloped, creating uncertainty for research and commercial applications.
The integration of sensing and response mechanisms represents another major challenge. While natural cells can detect environmental changes and adapt accordingly, protocells typically lack sophisticated feedback systems. Developing protocells with dynamic responsiveness requires complex genetic circuits and signaling pathways that are difficult to implement in simplified cell-free systems.
Finally, achieving reproducibility across different laboratories and production batches remains problematic. Variations in extract preparation, component quality, and environmental conditions lead to inconsistent performance, complicating both fundamental research and potential applications. Standardized protocols and quality control measures are urgently needed to address this challenge.
Current Methodologies for Protocell Engineering
01 Cell-free protein synthesis systems and components
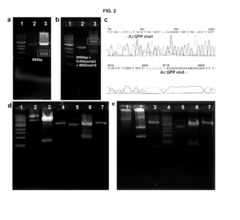
Cell-free protein synthesis systems utilize cellular extracts containing the necessary machinery for protein production without intact cells. These systems typically include ribosomes, tRNAs, aminoacyl-tRNA synthetases, translation factors, and energy regeneration components. Various extracts can be derived from different organisms such as E. coli, wheat germ, rabbit reticulocytes, or insect cells, each with specific advantages for different applications. These systems allow for rapid protein production and are particularly useful for proteins that may be toxic to living cells.- Cell-free protein synthesis systems and components: Cell-free protein synthesis systems utilize cellular extracts containing the necessary machinery for protein translation without intact cells. These systems typically include ribosomes, translation factors, tRNAs, aminoacyl-tRNA synthetases, and energy regeneration components. Various extracts can be derived from different organisms such as E. coli, wheat germ, rabbit reticulocytes, or insect cells, each with specific advantages for different applications. These systems enable rapid protein production without the constraints of cell viability or growth.
- Energy regeneration systems for sustained protein synthesis: Efficient energy regeneration is crucial for prolonged and high-yield cell-free protein synthesis. Advanced systems incorporate ATP regeneration mechanisms, phosphoenolpyruvate-based energy sources, creatine phosphate/creatine kinase systems, or glucose-based energy regeneration pathways. These energy systems maintain ATP levels and remove inhibitory byproducts, allowing for extended reaction times and increased protein yields compared to conventional methods. Optimized energy regeneration can significantly enhance the efficiency and productivity of cell-free protein synthesis reactions.
- Continuous-exchange cell-free protein synthesis: Continuous-exchange cell-free protein synthesis systems utilize dialysis membranes or microfluidic devices to continuously supply fresh substrates while removing inhibitory byproducts. This approach overcomes limitations of batch reactions by maintaining optimal reaction conditions over extended periods. The continuous exchange of small molecules while retaining larger translation machinery components allows for dramatically increased protein yields. These systems can be miniaturized for high-throughput applications or scaled up for industrial production of proteins.
- Cell-free protein synthesis for therapeutic applications: Cell-free protein synthesis offers advantages for producing therapeutic proteins, including difficult-to-express proteins, cytotoxic proteins, and proteins requiring specific modifications. The open nature of the system allows for incorporation of non-natural amino acids, site-specific modifications, and direct production of conjugated therapeutics. This approach enables rapid production of personalized medicines, vaccines, and antibodies with reduced contamination risks compared to cell-based systems. The technology has been applied to produce various biopharmaceuticals including growth factors, cytokines, and antibody fragments.
- Enhancing cell-free protein synthesis with additives and modifications: Various additives and system modifications can significantly enhance cell-free protein synthesis performance. These include molecular crowding agents like PEG, chaperones to assist protein folding, protease inhibitors to prevent product degradation, and redox-controlling compounds for proper disulfide bond formation. Additionally, supplementation with specific lipids, detergents, or nanodiscs can facilitate membrane protein production. Genetic modifications of the source organisms used for extract preparation can also optimize the system by removing inhibitory pathways or enhancing beneficial components.
02 Enhanced efficiency and yield optimization
Improving the efficiency and yield of cell-free protein synthesis involves optimizing reaction conditions and supplementing with additional components. Strategies include adding energy regeneration systems, optimizing ion concentrations, incorporating chaperones for proper protein folding, and developing continuous-exchange cell-free systems. These enhancements can significantly increase protein yields and extend the duration of protein synthesis reactions, making the technology more commercially viable for various applications.Expand Specific Solutions03 Applications in therapeutic protein production
Cell-free protein synthesis offers advantages for producing therapeutic proteins and vaccines. The open nature of the system allows for incorporation of non-natural amino acids and direct production of proteins with specific modifications. This technology enables rapid production of personalized medicines, antibodies, and vaccine candidates, particularly valuable during pandemic responses. The absence of cellular contaminants also simplifies downstream purification processes for therapeutic applications.Expand Specific Solutions04 Microfluidic and miniaturized systems
Miniaturized cell-free protein synthesis platforms utilize microfluidic technologies to reduce reaction volumes and increase throughput. These systems enable high-throughput screening of protein variants, on-demand protein production, and integration with detection methods for rapid analysis. Microfluidic approaches also allow for precise control of reaction conditions and can be integrated with downstream applications, making them valuable for point-of-care diagnostics and personalized medicine applications.Expand Specific Solutions05 Novel extract preparation and preservation methods
Innovations in extract preparation and preservation have improved the stability and activity of cell-free protein synthesis systems. These include optimized cell lysis techniques, extract fractionation methods, lyophilization processes for long-term storage, and the addition of stabilizing agents. Such advancements have made cell-free systems more accessible, cost-effective, and suitable for field applications where cold chain storage may not be available, expanding their utility in various research and industrial settings.Expand Specific Solutions
Leading Research Groups and Companies in CFPS
Cell-free Protein Synthesis (CFPS) for cell-mimicking protocells is emerging as a transformative technology in synthetic biology, currently in its early growth phase. The market is expanding rapidly with an estimated value of $100-150 million, driven by applications in pharmaceuticals, diagnostics, and materials science. The technology is advancing from experimental to commercial readiness, with key players demonstrating varying levels of specialization. Companies like Cellfree Sciences and Kangma Biological Technology have developed proprietary CFPS platforms, while research institutions including Tsinghua University, Cornell University, and RIKEN are pioneering fundamental advances. Major corporations such as Toyota Motor Corp. and Samsung Electronics are exploring industrial applications, indicating growing commercial interest in this technology's potential to revolutionize biomanufacturing.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed a sophisticated cell-free protein synthesis platform for creating biomimetic protocells. Their approach combines elements of both extract-based and PURE systems, utilizing selectively purified components from various cellular sources to create optimized reaction environments. CNRS researchers have pioneered the development of "minimal cells" by encapsulating cell-free protein synthesis machinery within phospholipid vesicles, creating self-sustaining systems capable of expressing genes and synthesizing proteins within confined compartments. Their technology incorporates engineered pore proteins that allow selective permeability across the vesicle membrane, enabling continuous supply of substrates while retaining synthesized proteins and genetic material. A key innovation from CNRS is the development of light-activated gene expression systems within protocells, allowing spatial and temporal control over protein synthesis through external stimuli. Their recent work has focused on creating protocell networks that can communicate through chemical signals, mimicking intercellular communication in natural biological systems. Additionally, CNRS has developed methods to incorporate cytoskeletal proteins into protocells, creating dynamic internal structures that can change shape and potentially enable protocell motility.
Strengths: Highly customizable system allowing precise control over reaction components; excellent for studying fundamental biological processes; capable of producing complex multi-domain proteins; supports incorporation of non-canonical amino acids. Weaknesses: Requires significant expertise in biochemistry and biophysics; lower throughput compared to commercial systems; complex setup procedures; higher variability between experiments due to custom preparation methods.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed the WEPRO® system, a wheat germ extract-based cell-free protein synthesis (CFPS) platform specifically optimized for protocell applications. Their technology utilizes a bilayer reaction format where translation components are separated from transcription components, allowing for extended reaction times and higher protein yields (up to 10mg/ml). The company has engineered their extracts to minimize nuclease and protease activity, resulting in stable protein production for up to 72 hours. Their CFPS system incorporates liposome encapsulation techniques to create cell-mimicking protocells with functional membrane proteins and metabolic pathways. Recent advancements include the development of continuous-exchange cell-free (CECF) formats that extend reaction lifetimes by continuously supplying substrates and removing inhibitory byproducts, enabling the synthesis of complex multi-domain proteins essential for protocell functionality.
Strengths: Superior protein yield compared to other plant-based systems; exceptional stability allowing for longer reaction times; highly scalable from microliter to liter scale; reduced endogenous nuclease activity. Weaknesses: Higher cost compared to E. coli-based systems; limited post-translational modifications compared to insect or mammalian systems; requires specialized equipment for optimal performance.
Key Technologies in Cell-Mimicking Systems
Cell-free protein synthesis solution, process for producing the same and utilization of the same
PatentWO2004027077A1
Innovation
- A cell-free protein synthesis solution is developed using devesiculated protoplasts from plant cells, where intact protoplasts are recovered using density gradient centrifugation with Percoll solutions of varying concentrations, retaining intracellular membrane components to facilitate post-translational modifications and enhance high-molecular-weight protein synthesis.
Development of nucleic acid gel matrix for cell-free protein synthesis of cell nucleus replicate, and method for producing same
PatentInactiveUS20150050698A1
Innovation
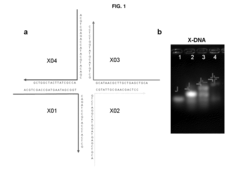
- A nucleic acid gel matrix is developed, comprising an X-type nucleic acid nano structure, an expression plasmid with a DNA fragment, transcription and translation factors, and lipid membrane components, which mimics the nucleus structure to enhance protein production capacity and stability.
Biosafety and Regulatory Considerations
The biosafety and regulatory landscape for cell-free protein synthesis (CFPS) in protocell development presents unique challenges that differ from traditional genetic engineering approaches. Unlike living organisms, cell-mimicking protocells utilizing CFPS technology do not replicate or evolve independently, potentially reducing certain biosafety concerns. However, their biomimetic properties and potential environmental interactions necessitate careful consideration within existing regulatory frameworks.
Current regulatory frameworks worldwide are not specifically designed for cell-free systems, creating a gray area for protocell technologies. The European Union's precautionary principle approach may classify some protocell applications under genetically modified organism (GMO) regulations despite their non-living status. In contrast, the United States FDA and EPA employ a more product-based regulatory approach, focusing on intended use rather than production method, which may provide more flexibility for CFPS protocell development.
Biosafety considerations for CFPS protocells include potential environmental persistence, interaction with biological systems, and horizontal gene transfer possibilities. Although CFPS systems lack reproductive capabilities, the stability of synthetic proteins and nucleic acids in environmental settings remains inadequately characterized. Research indicates that cell-free reaction components generally degrade within hours to days, but encapsulation within protocell membranes may extend persistence significantly.
Containment strategies represent a critical aspect of CFPS protocell biosafety. Physical containment through laboratory protocols provides immediate protection, while engineered biological safeguards offer additional security layers. These include designing protocells with limited environmental stability, incorporating nucleic acid degradation mechanisms, and developing "kill-switch" functionalities that respond to specific environmental triggers.
Standardization efforts are emerging to address regulatory challenges, with organizations like the International Organization for Standardization (ISO) developing technical specifications for synthetic biology products. The establishment of clear risk assessment frameworks specifically for cell-free systems would significantly advance regulatory clarity. Industry stakeholders and academic researchers are increasingly collaborating with regulatory bodies to develop appropriate governance models that balance innovation with safety considerations.
Public perception and ethical considerations also influence the regulatory landscape. Transparent communication about the non-living nature of CFPS protocells, their contained functionality, and built-in safety mechanisms is essential for public acceptance and appropriate regulatory treatment. Stakeholder engagement throughout the development process can help identify concerns and shape responsible innovation pathways.
Current regulatory frameworks worldwide are not specifically designed for cell-free systems, creating a gray area for protocell technologies. The European Union's precautionary principle approach may classify some protocell applications under genetically modified organism (GMO) regulations despite their non-living status. In contrast, the United States FDA and EPA employ a more product-based regulatory approach, focusing on intended use rather than production method, which may provide more flexibility for CFPS protocell development.
Biosafety considerations for CFPS protocells include potential environmental persistence, interaction with biological systems, and horizontal gene transfer possibilities. Although CFPS systems lack reproductive capabilities, the stability of synthetic proteins and nucleic acids in environmental settings remains inadequately characterized. Research indicates that cell-free reaction components generally degrade within hours to days, but encapsulation within protocell membranes may extend persistence significantly.
Containment strategies represent a critical aspect of CFPS protocell biosafety. Physical containment through laboratory protocols provides immediate protection, while engineered biological safeguards offer additional security layers. These include designing protocells with limited environmental stability, incorporating nucleic acid degradation mechanisms, and developing "kill-switch" functionalities that respond to specific environmental triggers.
Standardization efforts are emerging to address regulatory challenges, with organizations like the International Organization for Standardization (ISO) developing technical specifications for synthetic biology products. The establishment of clear risk assessment frameworks specifically for cell-free systems would significantly advance regulatory clarity. Industry stakeholders and academic researchers are increasingly collaborating with regulatory bodies to develop appropriate governance models that balance innovation with safety considerations.
Public perception and ethical considerations also influence the regulatory landscape. Transparent communication about the non-living nature of CFPS protocells, their contained functionality, and built-in safety mechanisms is essential for public acceptance and appropriate regulatory treatment. Stakeholder engagement throughout the development process can help identify concerns and shape responsible innovation pathways.
Scalability and Industrial Implementation
The transition of cell-free protein synthesis (CFPS) systems from laboratory-scale research to industrial implementation presents significant challenges and opportunities. Currently, most CFPS applications for protocell development remain confined to small-scale experimental settings, typically in the microliter to milliliter range. Scaling these systems to industrial volumes requires addressing several critical factors that impact both technical feasibility and economic viability.
Production scale-up demands optimization of reaction conditions across larger volumes while maintaining consistency in protein yield and quality. The primary challenge lies in ensuring uniform distribution of components, stable temperature control, and effective oxygen transfer in larger reaction vessels. Several bioreactor designs have been proposed specifically for CFPS applications, including continuous-flow systems that allow for replenishment of energy sources and removal of inhibitory byproducts, potentially extending reaction durations from hours to days.
Cost considerations represent another significant barrier to industrial implementation. The high cost of energy-rich compounds (ATP, GTP) and enzymes required for CFPS currently limits large-scale applications. Recent advances in cost reduction strategies include the development of alternative energy regeneration systems and the use of crude cell extracts rather than purified components. These approaches have demonstrated potential to reduce production costs by 5-10 fold, bringing CFPS closer to economic viability for industrial applications.
Stability and shelf-life of CFPS components present additional challenges for commercialization. Lyophilization and freeze-drying techniques have shown promise in preserving activity of cell extracts for extended periods, with some formulations maintaining over 70% activity after six months of storage. These preservation methods are essential for developing commercially viable CFPS kits and reagents for protocell applications.
Regulatory frameworks for CFPS-based protocell technologies remain underdeveloped, particularly for applications in medicine and food production. Establishing clear guidelines for safety assessment, quality control, and standardization will be crucial for industrial adoption. Several companies, including Sutro Biopharma and Greenlight Biosciences, have made significant progress in navigating these regulatory challenges for their CFPS-based pharmaceutical and agricultural products.
The emergence of automated, high-throughput CFPS platforms represents a promising direction for industrial implementation. These systems integrate microfluidics, robotics, and real-time monitoring capabilities to optimize reaction conditions and increase throughput. Such platforms could potentially bridge the gap between laboratory research and industrial production by enabling rapid prototyping and optimization of protocell designs before full-scale implementation.
Production scale-up demands optimization of reaction conditions across larger volumes while maintaining consistency in protein yield and quality. The primary challenge lies in ensuring uniform distribution of components, stable temperature control, and effective oxygen transfer in larger reaction vessels. Several bioreactor designs have been proposed specifically for CFPS applications, including continuous-flow systems that allow for replenishment of energy sources and removal of inhibitory byproducts, potentially extending reaction durations from hours to days.
Cost considerations represent another significant barrier to industrial implementation. The high cost of energy-rich compounds (ATP, GTP) and enzymes required for CFPS currently limits large-scale applications. Recent advances in cost reduction strategies include the development of alternative energy regeneration systems and the use of crude cell extracts rather than purified components. These approaches have demonstrated potential to reduce production costs by 5-10 fold, bringing CFPS closer to economic viability for industrial applications.
Stability and shelf-life of CFPS components present additional challenges for commercialization. Lyophilization and freeze-drying techniques have shown promise in preserving activity of cell extracts for extended periods, with some formulations maintaining over 70% activity after six months of storage. These preservation methods are essential for developing commercially viable CFPS kits and reagents for protocell applications.
Regulatory frameworks for CFPS-based protocell technologies remain underdeveloped, particularly for applications in medicine and food production. Establishing clear guidelines for safety assessment, quality control, and standardization will be crucial for industrial adoption. Several companies, including Sutro Biopharma and Greenlight Biosciences, have made significant progress in navigating these regulatory challenges for their CFPS-based pharmaceutical and agricultural products.
The emergence of automated, high-throughput CFPS platforms represents a promising direction for industrial implementation. These systems integrate microfluidics, robotics, and real-time monitoring capabilities to optimize reaction conditions and increase throughput. Such platforms could potentially bridge the gap between laboratory research and industrial production by enabling rapid prototyping and optimization of protocell designs before full-scale implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!