Continuous Flow Cell-free Protein Synthesis in Microreactors
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CFPS Microreactor Technology Background and Objectives
Cell-free protein synthesis (CFPS) represents a paradigm shift in biotechnology, offering a versatile platform for protein production without the constraints of living cells. The evolution of CFPS technology dates back to the 1950s when Nirenberg and Matthaei first demonstrated protein synthesis in cell extracts, leading to the deciphering of the genetic code. Over subsequent decades, CFPS systems have evolved from basic research tools to sophisticated platforms capable of producing complex proteins for various applications.
The integration of CFPS with microfluidic technology marks a significant advancement in this field. Microreactors, typically operating at microliter to nanoliter scales, provide precise control over reaction conditions, enabling enhanced protein yields and functionality. This convergence of technologies has been driven by the increasing demand for efficient, scalable, and cost-effective protein production methods across pharmaceutical, diagnostic, and industrial sectors.
The technological trajectory of continuous flow CFPS in microreactors has been characterized by progressive improvements in extract preparation, reaction formulations, and microfluidic device design. Recent innovations include the development of cell-free lysates from various organisms, optimization of energy regeneration systems, and integration of post-translational modification capabilities, all contributing to expanded functionality and applicability.
Current research trends focus on addressing key limitations such as reaction longevity, protein folding efficiency, and scale-up challenges. The field is witnessing a shift towards more sophisticated microreactor designs incorporating multiple reaction chambers, gradient generators, and integrated sensing capabilities, enabling real-time monitoring and control of protein synthesis processes.
The primary objectives of continuous flow CFPS microreactor technology include enhancing protein production efficiency through improved mass transfer and reaction kinetics, reducing reagent consumption through miniaturization, enabling rapid prototyping of protein variants, and facilitating the production of difficult-to-express proteins such as membrane proteins and toxic proteins that challenge conventional cell-based systems.
Furthermore, this technology aims to democratize protein production by developing accessible, automated platforms that require minimal expertise and infrastructure. The ultimate goal is to establish continuous flow CFPS microreactors as a versatile tool for on-demand protein production, potentially revolutionizing applications ranging from personalized medicine to point-of-care diagnostics and distributed biomanufacturing.
The convergence of synthetic biology principles with microfluidic engineering presents unprecedented opportunities for reimagining protein production paradigms, potentially enabling novel applications that were previously unattainable with conventional methods.
The integration of CFPS with microfluidic technology marks a significant advancement in this field. Microreactors, typically operating at microliter to nanoliter scales, provide precise control over reaction conditions, enabling enhanced protein yields and functionality. This convergence of technologies has been driven by the increasing demand for efficient, scalable, and cost-effective protein production methods across pharmaceutical, diagnostic, and industrial sectors.
The technological trajectory of continuous flow CFPS in microreactors has been characterized by progressive improvements in extract preparation, reaction formulations, and microfluidic device design. Recent innovations include the development of cell-free lysates from various organisms, optimization of energy regeneration systems, and integration of post-translational modification capabilities, all contributing to expanded functionality and applicability.
Current research trends focus on addressing key limitations such as reaction longevity, protein folding efficiency, and scale-up challenges. The field is witnessing a shift towards more sophisticated microreactor designs incorporating multiple reaction chambers, gradient generators, and integrated sensing capabilities, enabling real-time monitoring and control of protein synthesis processes.
The primary objectives of continuous flow CFPS microreactor technology include enhancing protein production efficiency through improved mass transfer and reaction kinetics, reducing reagent consumption through miniaturization, enabling rapid prototyping of protein variants, and facilitating the production of difficult-to-express proteins such as membrane proteins and toxic proteins that challenge conventional cell-based systems.
Furthermore, this technology aims to democratize protein production by developing accessible, automated platforms that require minimal expertise and infrastructure. The ultimate goal is to establish continuous flow CFPS microreactors as a versatile tool for on-demand protein production, potentially revolutionizing applications ranging from personalized medicine to point-of-care diagnostics and distributed biomanufacturing.
The convergence of synthetic biology principles with microfluidic engineering presents unprecedented opportunities for reimagining protein production paradigms, potentially enabling novel applications that were previously unattainable with conventional methods.
Market Analysis for Cell-free Protein Synthesis Applications
The cell-free protein synthesis (CFPS) market has been experiencing significant growth, driven by increasing applications in pharmaceutical research, personalized medicine, and synthetic biology. The global CFPS market was valued at approximately 250 million USD in 2022 and is projected to reach 500 million USD by 2028, representing a compound annual growth rate of 12.3%. This growth trajectory is particularly notable in the continuous flow microreactor segment, which offers enhanced efficiency and scalability compared to traditional batch processes.
Pharmaceutical companies constitute the largest market segment for CFPS applications, accounting for nearly 45% of the total market share. These companies leverage CFPS technology for rapid protein production, drug screening, and development of therapeutic proteins. The ability of continuous flow microreactors to maintain consistent reaction conditions and enable real-time monitoring has made them particularly valuable for high-throughput drug discovery processes.
Academic and research institutions represent the second-largest market segment, utilizing CFPS systems for fundamental research in protein expression, enzyme engineering, and synthetic biology. The compact nature of microreactor systems has made them increasingly popular in laboratory settings where space and resource efficiency are paramount considerations.
Regionally, North America dominates the CFPS market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years due to increasing investments in biotechnology infrastructure and research capabilities, particularly in China, Japan, and South Korea.
The continuous flow microreactor CFPS market faces competition from traditional batch processing systems, which still maintain significant market presence due to their established protocols and lower initial investment requirements. However, the superior performance metrics of continuous flow systems—including higher protein yields, reduced reaction times, and improved resource efficiency—are gradually shifting market preferences toward these advanced platforms.
Customer demand is increasingly focused on integrated systems that combine CFPS capabilities with downstream processing and analysis functions. This trend has prompted several leading biotechnology equipment manufacturers to develop comprehensive solutions that incorporate continuous flow microreactors with purification and analytical modules, creating value-added product offerings that command premium pricing in the marketplace.
Market barriers include the relatively high initial investment costs for continuous flow systems, technical expertise requirements for operation, and regulatory considerations for pharmaceutical applications. Despite these challenges, the compelling economic advantages of continuous flow CFPS in terms of reduced reagent consumption, increased throughput, and improved product consistency continue to drive market expansion across diverse application domains.
Pharmaceutical companies constitute the largest market segment for CFPS applications, accounting for nearly 45% of the total market share. These companies leverage CFPS technology for rapid protein production, drug screening, and development of therapeutic proteins. The ability of continuous flow microreactors to maintain consistent reaction conditions and enable real-time monitoring has made them particularly valuable for high-throughput drug discovery processes.
Academic and research institutions represent the second-largest market segment, utilizing CFPS systems for fundamental research in protein expression, enzyme engineering, and synthetic biology. The compact nature of microreactor systems has made them increasingly popular in laboratory settings where space and resource efficiency are paramount considerations.
Regionally, North America dominates the CFPS market with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years due to increasing investments in biotechnology infrastructure and research capabilities, particularly in China, Japan, and South Korea.
The continuous flow microreactor CFPS market faces competition from traditional batch processing systems, which still maintain significant market presence due to their established protocols and lower initial investment requirements. However, the superior performance metrics of continuous flow systems—including higher protein yields, reduced reaction times, and improved resource efficiency—are gradually shifting market preferences toward these advanced platforms.
Customer demand is increasingly focused on integrated systems that combine CFPS capabilities with downstream processing and analysis functions. This trend has prompted several leading biotechnology equipment manufacturers to develop comprehensive solutions that incorporate continuous flow microreactors with purification and analytical modules, creating value-added product offerings that command premium pricing in the marketplace.
Market barriers include the relatively high initial investment costs for continuous flow systems, technical expertise requirements for operation, and regulatory considerations for pharmaceutical applications. Despite these challenges, the compelling economic advantages of continuous flow CFPS in terms of reduced reagent consumption, increased throughput, and improved product consistency continue to drive market expansion across diverse application domains.
Current Challenges in Continuous Flow CFPS Systems
Despite significant advancements in continuous flow cell-free protein synthesis (CFPS) systems, several critical challenges persist that limit their widespread industrial application and scalability. The primary obstacle remains the rapid depletion of energy resources during protein production, particularly ATP and GTP, which are essential for translation processes. This depletion leads to diminished protein yields over extended operation periods, compromising the economic viability of continuous systems.
Reaction component stability presents another significant challenge. Key enzymes and cofactors in CFPS mixtures exhibit limited half-lives under operational conditions, with many degrading within hours. This instability necessitates continuous replenishment strategies that add complexity and cost to system design, particularly in microreactor environments where precise control is essential.
The accumulation of inhibitory byproducts, such as inorganic phosphate and AMP, progressively impairs reaction efficiency in continuous systems. These byproducts interfere with translation machinery and energy regeneration pathways, creating feedback inhibition that becomes increasingly problematic in extended operations. Current byproduct removal techniques often introduce additional complexity without fully resolving the issue.
Microfluidic implementation challenges further complicate continuous CFPS systems. Surface adsorption of critical proteins and enzymes to microreactor walls reduces available reaction components and creates inconsistent reaction conditions across the flow path. Additionally, maintaining uniform temperature distribution and preventing bubble formation within microchannels remains technically demanding, particularly at the microscale where surface tension effects dominate.
Scale-up limitations represent perhaps the most significant barrier to industrial adoption. While microreactors excel at precise control and rapid prototyping, transitioning to production-scale volumes introduces challenges in maintaining consistent flow dynamics, mixing efficiency, and heat transfer characteristics. The parallelization approach often employed introduces system complexity and potential for variability between channels.
Monitoring and control systems for continuous CFPS also remain underdeveloped. Real-time analysis of reaction parameters and protein production rates is technically challenging in microfluidic environments, limiting feedback control capabilities. The integration of sensors without disrupting flow dynamics or introducing contamination presents ongoing engineering challenges.
Addressing these interconnected challenges requires interdisciplinary approaches combining protein engineering, microfluidic design innovations, and advanced control systems. Recent research has begun exploring enzyme immobilization strategies, novel energy regeneration systems, and integrated sensing technologies as potential solutions to overcome these limitations.
Reaction component stability presents another significant challenge. Key enzymes and cofactors in CFPS mixtures exhibit limited half-lives under operational conditions, with many degrading within hours. This instability necessitates continuous replenishment strategies that add complexity and cost to system design, particularly in microreactor environments where precise control is essential.
The accumulation of inhibitory byproducts, such as inorganic phosphate and AMP, progressively impairs reaction efficiency in continuous systems. These byproducts interfere with translation machinery and energy regeneration pathways, creating feedback inhibition that becomes increasingly problematic in extended operations. Current byproduct removal techniques often introduce additional complexity without fully resolving the issue.
Microfluidic implementation challenges further complicate continuous CFPS systems. Surface adsorption of critical proteins and enzymes to microreactor walls reduces available reaction components and creates inconsistent reaction conditions across the flow path. Additionally, maintaining uniform temperature distribution and preventing bubble formation within microchannels remains technically demanding, particularly at the microscale where surface tension effects dominate.
Scale-up limitations represent perhaps the most significant barrier to industrial adoption. While microreactors excel at precise control and rapid prototyping, transitioning to production-scale volumes introduces challenges in maintaining consistent flow dynamics, mixing efficiency, and heat transfer characteristics. The parallelization approach often employed introduces system complexity and potential for variability between channels.
Monitoring and control systems for continuous CFPS also remain underdeveloped. Real-time analysis of reaction parameters and protein production rates is technically challenging in microfluidic environments, limiting feedback control capabilities. The integration of sensors without disrupting flow dynamics or introducing contamination presents ongoing engineering challenges.
Addressing these interconnected challenges requires interdisciplinary approaches combining protein engineering, microfluidic design innovations, and advanced control systems. Recent research has begun exploring enzyme immobilization strategies, novel energy regeneration systems, and integrated sensing technologies as potential solutions to overcome these limitations.
Current Continuous Flow CFPS Implementation Strategies
01 Microfluidic systems for cell-free protein synthesis
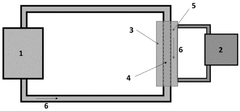
Microfluidic devices provide controlled environments for cell-free protein synthesis by enabling precise manipulation of reagents in small volumes. These systems incorporate channels, chambers, and flow control mechanisms to facilitate continuous protein production. The miniaturized format allows for efficient heat and mass transfer, reduced reagent consumption, and improved reaction kinetics compared to conventional batch methods.- Microfluidic systems for cell-free protein synthesis: Microfluidic devices provide controlled environments for cell-free protein synthesis by enabling precise manipulation of reagents in continuous flow. These systems typically incorporate channels, chambers, and flow control mechanisms to facilitate the mixing of cell lysates, DNA templates, and other necessary components. The miniaturized format allows for reduced reaction volumes, faster diffusion rates, and improved heat transfer, resulting in more efficient protein production compared to conventional batch methods.
- Continuous flow reactor designs for enhanced protein expression: Specialized reactor designs for continuous flow cell-free protein synthesis focus on optimizing reaction conditions throughout the process. These reactors incorporate features such as gradient generators, mixing zones, and residence time controllers to maintain optimal conditions for protein expression. Some designs include compartmentalization strategies to separate transcription and translation processes or to enable the continuous supply of energy sources and removal of inhibitory byproducts, thereby extending reaction lifetimes and increasing protein yields.
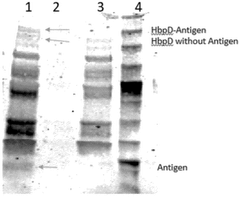
- Integration of purification and analysis in continuous flow systems: Advanced cell-free protein synthesis platforms integrate downstream processing steps directly into the continuous flow system. These integrated approaches incorporate on-chip purification modules using affinity chromatography, size exclusion, or other separation techniques to isolate the synthesized proteins. Additionally, real-time monitoring and analysis components such as fluorescence detection, mass spectrometry interfaces, or electrochemical sensors allow for immediate quality assessment of the produced proteins, enabling process optimization and quality control in a single unified system.
- Energy regeneration systems for sustained protein synthesis: Continuous flow cell-free protein synthesis requires sustained energy supply to maintain production rates. Energy regeneration systems incorporate secondary enzymatic pathways to recycle key metabolites like ATP and GTP or provide alternative energy sources. These systems may include immobilized enzymes on microreactor surfaces, co-flowing energy substrate channels, or timed release of energy components. Such approaches significantly extend the productive lifetime of cell-free reactions from hours to days, enabling higher protein yields and more efficient use of costly reagents.
- Encapsulation and compartmentalization strategies: Encapsulation techniques create isolated microenvironments for cell-free protein synthesis within continuous flow systems. These approaches use droplet microfluidics, liposomes, polymersomes, or hydrogel particles to compartmentalize reaction components. Each microcompartment functions as an individual bioreactor, allowing for high-throughput screening of different conditions or templates. Some systems enable the exchange of small molecules across compartment boundaries while retaining larger components, mimicking cellular organization and potentially improving protein folding and activity in the synthesized products.
02 Continuous flow bioreactors for protein expression
Continuous flow bioreactors enable sustained cell-free protein synthesis by constantly supplying fresh reagents while removing inhibitory byproducts. These systems maintain optimal reaction conditions over extended periods, resulting in higher protein yields compared to batch processes. The continuous exchange of materials helps overcome resource limitations and product inhibition that typically restrict conventional cell-free protein synthesis methods.Expand Specific Solutions03 Immobilization techniques for cell-free components
Immobilization of cell-free protein synthesis components on solid supports or within matrices enhances stability and enables reusability in continuous flow systems. Techniques include attachment of ribosomes, enzymes, and transcription/translation machinery to surfaces or encapsulation within protective structures. These approaches extend the functional lifetime of the biological components and facilitate their integration into microreactor designs for sustained protein production.Expand Specific Solutions04 Energy regeneration systems for extended synthesis
Energy regeneration systems maintain ATP and other high-energy molecules required for continuous cell-free protein synthesis. These systems incorporate enzymatic cascades that recycle spent energy carriers and provide a constant supply of metabolic resources. By integrating energy regeneration with continuous flow microreactors, the duration and efficiency of protein synthesis can be significantly extended beyond what is possible in conventional batch reactions.Expand Specific Solutions05 Integrated sensing and control for optimized production
Integration of sensors and control systems in cell-free protein synthesis microreactors enables real-time monitoring and adjustment of reaction conditions. These systems can detect parameters such as pH, temperature, substrate concentrations, and protein production rates, allowing for automated optimization of the synthesis process. Advanced control algorithms can respond to changing conditions, maintaining optimal performance throughout extended continuous flow operations.Expand Specific Solutions
Leading Organizations in CFPS Microreactor Development
Continuous Flow Cell-free Protein Synthesis in Microreactors is emerging as a transformative technology in the early growth phase of its industry development. The market is expanding rapidly, with an estimated size reaching several hundred million dollars, driven by applications in pharmaceuticals, diagnostics, and synthetic biology. Technologically, the field shows varying maturity levels across players. Leading institutions like Northwestern University and Tsinghua University are pioneering academic research, while companies such as Cellfree Sciences, Shimadzu Corp., and QIAGEN GmbH demonstrate advanced commercial implementations. Fraunhofer-Gesellschaft and Riken Corp. are developing scalable industrial applications, while startups like Invitris GmbH and Ionovation GmbH focus on specialized niche applications, indicating a diversifying competitive landscape with significant room for innovation and market expansion.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed a proprietary PURESYSTEM® technology for continuous flow cell-free protein synthesis (CFPS) in microreactors. Their approach utilizes a wheat germ extract-based system that allows for extended reaction times and higher protein yields compared to traditional batch methods. The company has engineered microfluidic devices with specialized compartments that enable continuous supply of energy sources and amino acids while removing inhibitory byproducts. Their system incorporates real-time monitoring capabilities through integrated sensors that track reaction parameters such as pH, temperature, and protein concentration. This allows for dynamic adjustment of reaction conditions to optimize protein production. The technology also features modular microreactor designs that can be scaled or connected in series for multi-step protein processing, including folding and post-translational modifications.
Strengths: Superior protein yield (up to 5mg/ml) compared to other CFPS systems; exceptional capability for producing complex eukaryotic proteins with proper folding; longer reaction sustainability (up to 72 hours) in continuous mode. Weaknesses: Higher cost compared to E. coli-based systems; requires specialized equipment and expertise; wheat germ extract preparation can introduce batch-to-batch variability.
Northwestern University
Technical Solution: Northwestern University has pioneered an innovative approach to continuous flow cell-free protein synthesis through their Jewett Lab, which has developed the Continuous Exchange Cell-Free (CECF) system integrated with microfluidic platforms. Their technology utilizes a semi-permeable membrane-based reactor design that allows for continuous diffusion of small molecules while retaining larger macromolecules and enzymes within the reaction chamber. The system incorporates precisely controlled fluid dynamics to maintain optimal concentrations of ATP, amino acids, and other substrates while continuously removing inhibitory byproducts. Northwestern's approach also features genetically engineered cell extracts optimized for extended protein production lifetimes, achieving reaction durations exceeding 24 hours compared to conventional batch reactions that typically terminate within 4 hours. Their microreactor designs include specialized surface treatments to minimize protein adsorption and aggregation, significantly improving yield and quality of synthesized proteins. The university has also developed computational models that predict and optimize reaction parameters in real-time, allowing for adaptive control of synthesis conditions.
Strengths: Exceptional reaction longevity (>24 hours) with sustained productivity; highly optimized extract preparation protocols that enhance energy efficiency; sophisticated integration with downstream purification processes. Weaknesses: Complex setup requirements limiting accessibility to specialized laboratories; higher initial investment costs compared to traditional batch systems; challenges in scaling to industrial production volumes.
Key Innovations in Microreactor Design for CFPS
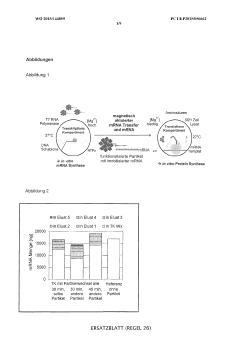
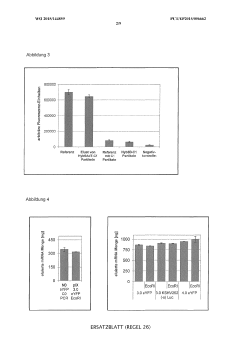
Cell-free protein synthesis with semi-continuous or continuous supply of RNA template
PatentWO2015144859A9
Innovation
- The method involves immobilizing RNA during continuous in vitro transcription and repeatedly transferring it to the translation compartment without interrupting transcription, allowing for continuous or semi-continuous mRNA replenishment, which can be automated to maintain optimal mRNA levels.
Continuous protein expression
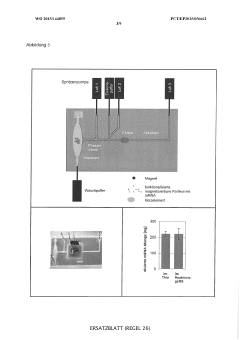
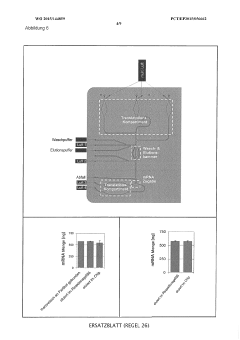
PatentWO2025176840A1
Innovation
- A continuous cell-free expression method using a partitioned reaction space with an inner and outer compartment allows for the production and purification of proteins, including bacteriophages, by supplying components as needed during the expression process, enabling the assembly and modification of multi-protein complexes.
Scale-up Considerations for Industrial CFPS Applications
Scaling up Continuous Flow Cell-free Protein Synthesis (CFPS) systems from microreactors to industrial applications presents significant engineering challenges that must be addressed systematically. The transition from laboratory-scale microfluidic devices to production-scale bioreactors requires careful consideration of several critical factors to maintain process efficiency and product quality.
Reactor design represents the primary consideration, as industrial CFPS applications demand specialized bioreactors that can maintain the advantages of microreactors while operating at larger volumes. Continuous stirred-tank reactors (CSTRs) modified with enhanced mixing capabilities and plug-flow reactors (PFRs) with optimized residence time distributions have shown promise for maintaining reaction homogeneity at scale.
Process control complexity increases exponentially with scale. Industrial CFPS systems require sophisticated monitoring and control systems for temperature, pH, oxygen levels, and substrate concentrations. Real-time analytics using spectroscopic methods and integrated sensors become essential for maintaining optimal reaction conditions throughout extended production runs.
Economic viability presents another crucial consideration. The cost of cell extract production, energy requirements for continuous operation, and raw material expenses must be carefully balanced against productivity. Recent advances in extract preparation methods have reduced costs by up to 70%, making industrial CFPS more economically feasible, though further optimization remains necessary.
Regulatory compliance introduces additional complexity for pharmaceutical applications. Establishing robust quality control protocols, validation procedures, and documentation systems that meet GMP requirements is essential. The continuous nature of the process requires implementation of Process Analytical Technology (PAT) frameworks to ensure consistent product quality.
Supply chain management becomes increasingly important at industrial scale. Securing reliable sources of high-quality reagents, particularly nucleotides and amino acids, and establishing cold chain logistics for enzyme components are critical for uninterrupted production. Some companies have begun developing specialized supply chains specifically for CFPS applications.
Finally, integration with downstream processing represents a significant challenge. Continuous product recovery systems must be designed to handle the specific characteristics of CFPS output streams without disrupting the upstream synthesis process. Membrane-based separation technologies and continuous chromatography systems have shown particular promise for seamless integration with CFPS bioreactors.
Reactor design represents the primary consideration, as industrial CFPS applications demand specialized bioreactors that can maintain the advantages of microreactors while operating at larger volumes. Continuous stirred-tank reactors (CSTRs) modified with enhanced mixing capabilities and plug-flow reactors (PFRs) with optimized residence time distributions have shown promise for maintaining reaction homogeneity at scale.
Process control complexity increases exponentially with scale. Industrial CFPS systems require sophisticated monitoring and control systems for temperature, pH, oxygen levels, and substrate concentrations. Real-time analytics using spectroscopic methods and integrated sensors become essential for maintaining optimal reaction conditions throughout extended production runs.
Economic viability presents another crucial consideration. The cost of cell extract production, energy requirements for continuous operation, and raw material expenses must be carefully balanced against productivity. Recent advances in extract preparation methods have reduced costs by up to 70%, making industrial CFPS more economically feasible, though further optimization remains necessary.
Regulatory compliance introduces additional complexity for pharmaceutical applications. Establishing robust quality control protocols, validation procedures, and documentation systems that meet GMP requirements is essential. The continuous nature of the process requires implementation of Process Analytical Technology (PAT) frameworks to ensure consistent product quality.
Supply chain management becomes increasingly important at industrial scale. Securing reliable sources of high-quality reagents, particularly nucleotides and amino acids, and establishing cold chain logistics for enzyme components are critical for uninterrupted production. Some companies have begun developing specialized supply chains specifically for CFPS applications.
Finally, integration with downstream processing represents a significant challenge. Continuous product recovery systems must be designed to handle the specific characteristics of CFPS output streams without disrupting the upstream synthesis process. Membrane-based separation technologies and continuous chromatography systems have shown particular promise for seamless integration with CFPS bioreactors.
Sustainability and Economic Viability of Continuous CFPS
The economic sustainability of Continuous Flow Cell-free Protein Synthesis (CFPS) systems represents a critical factor in their commercial viability and widespread adoption. Current analyses indicate that while batch CFPS processes face significant cost barriers, continuous flow systems offer promising economic advantages through improved resource utilization and extended reaction lifetimes.
Material costs constitute a substantial portion of CFPS expenses, with energy substrates and nucleotides representing up to 30-40% of total operational costs. Continuous flow systems demonstrate superior economics by enabling the constant replenishment of these components while removing inhibitory byproducts, effectively extending reaction durations from hours to days. This extension translates to higher volumetric productivity and reduced cost per unit protein produced.
Equipment investment presents another economic consideration. Microfluidic reactors for continuous CFPS require specialized fabrication techniques and materials, initially resulting in higher capital expenditure compared to conventional batch reactors. However, the scalability of these systems through parallelization rather than volume increase offers advantages in terms of process control and product consistency, potentially reducing downstream processing costs.
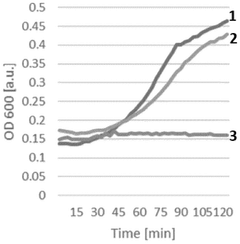
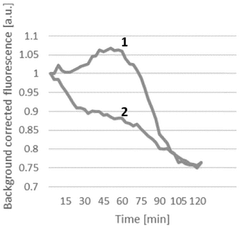
Energy efficiency metrics reveal that continuous CFPS systems achieve 2-5 fold improvements in ATP utilization compared to batch processes. This efficiency stems from the maintenance of optimal reaction conditions and the prevention of feedback inhibition. Studies demonstrate that continuous systems can achieve protein yields of 1.0-1.5 g/L/day compared to 0.5-0.7 g/L/day in batch operations, significantly enhancing economic performance.
Life cycle assessment (LCA) of continuous CFPS systems indicates reduced environmental impact compared to traditional cell-based manufacturing. The elimination of cell culture steps decreases water consumption by approximately 65% and energy requirements by 40%. Additionally, the absence of complex media formulations and sterilization procedures contributes to a more sustainable production platform.
Market analysis suggests that continuous CFPS is particularly economically viable for high-value biopharmaceuticals, personalized medicines, and rapid-response applications. The technology enables decentralized manufacturing models that can reduce transportation costs and cold chain requirements, further enhancing economic sustainability in global health applications.
Regulatory considerations also impact economic viability. The simplified process flow of continuous CFPS potentially streamlines regulatory approval pathways, reducing time-to-market and associated costs. However, novel quality control strategies must be developed to ensure consistent product quality throughout extended production runs.
Material costs constitute a substantial portion of CFPS expenses, with energy substrates and nucleotides representing up to 30-40% of total operational costs. Continuous flow systems demonstrate superior economics by enabling the constant replenishment of these components while removing inhibitory byproducts, effectively extending reaction durations from hours to days. This extension translates to higher volumetric productivity and reduced cost per unit protein produced.
Equipment investment presents another economic consideration. Microfluidic reactors for continuous CFPS require specialized fabrication techniques and materials, initially resulting in higher capital expenditure compared to conventional batch reactors. However, the scalability of these systems through parallelization rather than volume increase offers advantages in terms of process control and product consistency, potentially reducing downstream processing costs.
Energy efficiency metrics reveal that continuous CFPS systems achieve 2-5 fold improvements in ATP utilization compared to batch processes. This efficiency stems from the maintenance of optimal reaction conditions and the prevention of feedback inhibition. Studies demonstrate that continuous systems can achieve protein yields of 1.0-1.5 g/L/day compared to 0.5-0.7 g/L/day in batch operations, significantly enhancing economic performance.
Life cycle assessment (LCA) of continuous CFPS systems indicates reduced environmental impact compared to traditional cell-based manufacturing. The elimination of cell culture steps decreases water consumption by approximately 65% and energy requirements by 40%. Additionally, the absence of complex media formulations and sterilization procedures contributes to a more sustainable production platform.
Market analysis suggests that continuous CFPS is particularly economically viable for high-value biopharmaceuticals, personalized medicines, and rapid-response applications. The technology enables decentralized manufacturing models that can reduce transportation costs and cold chain requirements, further enhancing economic sustainability in global health applications.
Regulatory considerations also impact economic viability. The simplified process flow of continuous CFPS potentially streamlines regulatory approval pathways, reducing time-to-market and associated costs. However, novel quality control strategies must be developed to ensure consistent product quality throughout extended production runs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!