Cell-free Protein Synthesis for Enzyme Engineering
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CFPS Technology Evolution and Objectives
Cell-free protein synthesis (CFPS) has evolved significantly since its inception in the 1960s when Nirenberg and Matthaei first demonstrated protein synthesis outside living cells. Initially developed as a research tool to decipher the genetic code, CFPS has transformed into a sophisticated biotechnological platform with diverse applications, particularly in enzyme engineering.
The evolution of CFPS technology can be traced through several distinct phases. The first generation systems utilized crude cell extracts with limited productivity and short reaction durations. The second generation, emerging in the 1990s, incorporated energy regeneration systems, significantly extending reaction lifetimes from minutes to hours. The third generation, developed in the early 2000s, focused on extract optimization and yield enhancement, achieving protein yields comparable to in vivo systems.
Current CFPS platforms represent the fourth generation, characterized by unprecedented versatility and precision. These systems incorporate modified ribosomes, orthogonal translation machinery, and optimized reaction conditions, enabling the synthesis of proteins with non-canonical amino acids and complex post-translational modifications previously unattainable in cell-free environments.
For enzyme engineering specifically, CFPS offers unique advantages over traditional in vivo expression systems. The open nature of the reaction environment allows direct manipulation of reaction components, facilitating rapid prototyping and high-throughput screening of enzyme variants. This capability has accelerated the development of novel biocatalysts with enhanced stability, selectivity, and activity.
The primary objectives of CFPS technology in enzyme engineering include expanding the chemical diversity of enzymes through incorporation of non-canonical amino acids, enhancing enzyme stability for industrial applications, improving catalytic efficiency, and developing enzymes with novel functionalities. Additionally, researchers aim to scale up CFPS reactions for industrial production while maintaining cost-effectiveness.
Future technological goals include developing continuous-flow CFPS systems for sustained enzyme production, creating fully defined CFPS platforms with minimal components, integrating artificial intelligence for predictive enzyme design, and establishing standardized protocols for consistent enzyme production across different laboratories and scales.
The convergence of CFPS with other emerging technologies, such as microfluidics, computational protein design, and synthetic biology, promises to further revolutionize enzyme engineering. These integrated approaches aim to create a new generation of biocatalysts capable of addressing global challenges in healthcare, sustainable chemistry, and environmental remediation.
The evolution of CFPS technology can be traced through several distinct phases. The first generation systems utilized crude cell extracts with limited productivity and short reaction durations. The second generation, emerging in the 1990s, incorporated energy regeneration systems, significantly extending reaction lifetimes from minutes to hours. The third generation, developed in the early 2000s, focused on extract optimization and yield enhancement, achieving protein yields comparable to in vivo systems.
Current CFPS platforms represent the fourth generation, characterized by unprecedented versatility and precision. These systems incorporate modified ribosomes, orthogonal translation machinery, and optimized reaction conditions, enabling the synthesis of proteins with non-canonical amino acids and complex post-translational modifications previously unattainable in cell-free environments.
For enzyme engineering specifically, CFPS offers unique advantages over traditional in vivo expression systems. The open nature of the reaction environment allows direct manipulation of reaction components, facilitating rapid prototyping and high-throughput screening of enzyme variants. This capability has accelerated the development of novel biocatalysts with enhanced stability, selectivity, and activity.
The primary objectives of CFPS technology in enzyme engineering include expanding the chemical diversity of enzymes through incorporation of non-canonical amino acids, enhancing enzyme stability for industrial applications, improving catalytic efficiency, and developing enzymes with novel functionalities. Additionally, researchers aim to scale up CFPS reactions for industrial production while maintaining cost-effectiveness.
Future technological goals include developing continuous-flow CFPS systems for sustained enzyme production, creating fully defined CFPS platforms with minimal components, integrating artificial intelligence for predictive enzyme design, and establishing standardized protocols for consistent enzyme production across different laboratories and scales.
The convergence of CFPS with other emerging technologies, such as microfluidics, computational protein design, and synthetic biology, promises to further revolutionize enzyme engineering. These integrated approaches aim to create a new generation of biocatalysts capable of addressing global challenges in healthcare, sustainable chemistry, and environmental remediation.
Market Applications for Cell-free Enzyme Production
Cell-free protein synthesis (CFPS) technology has rapidly evolved from a research tool to a commercially viable platform for enzyme production across multiple industries. The market for cell-free enzyme production is experiencing significant growth, driven by advantages in speed, scalability, and the ability to produce enzymes that are difficult to express in traditional cell-based systems.
The pharmaceutical industry represents one of the largest market segments for cell-free enzyme production. Enzymes produced via CFPS are increasingly utilized in drug discovery processes, particularly for high-throughput screening of enzyme variants with improved catalytic properties. Companies like Sutro Biopharma have leveraged cell-free systems to develop novel antibody-drug conjugates, demonstrating the commercial viability of this approach for therapeutic protein production.
In the biofuel sector, cell-free systems are enabling the production of novel enzymes capable of breaking down complex biomass more efficiently. These enzymes are critical for improving the economic feasibility of second and third-generation biofuels. The ability to rapidly prototype and optimize enzyme variants through CFPS has accelerated development timelines in this sector, with companies like Codexis utilizing cell-free approaches to engineer improved cellulases and other biomass-degrading enzymes.
The food and beverage industry has adopted cell-free enzyme production for applications ranging from dairy processing to flavor enhancement. Enzymes produced through CFPS offer advantages in purity and specificity, leading to improved product quality and consistency. Notable applications include the production of lactase for lactose-free dairy products and various proteases for food processing.
Diagnostic applications represent a rapidly growing market segment, with cell-free produced enzymes being incorporated into point-of-care testing platforms. The COVID-19 pandemic accelerated this trend, with CFPS-derived enzymes playing crucial roles in nucleic acid amplification tests and other diagnostic technologies.
Industrial biotechnology represents perhaps the broadest application area, with cell-free produced enzymes finding uses in textile processing, paper manufacturing, detergent formulation, and bioremediation. The ability to engineer enzymes with enhanced stability under industrial conditions (extreme pH, temperature, or presence of inhibitors) has been particularly valuable in these sectors.
Agricultural applications are also emerging, with enzyme engineering via CFPS enabling the development of more effective biopesticides and biofertilizers. These environmentally friendly alternatives to conventional agricultural chemicals represent a growing market driven by sustainability concerns and regulatory pressures.
The pharmaceutical industry represents one of the largest market segments for cell-free enzyme production. Enzymes produced via CFPS are increasingly utilized in drug discovery processes, particularly for high-throughput screening of enzyme variants with improved catalytic properties. Companies like Sutro Biopharma have leveraged cell-free systems to develop novel antibody-drug conjugates, demonstrating the commercial viability of this approach for therapeutic protein production.
In the biofuel sector, cell-free systems are enabling the production of novel enzymes capable of breaking down complex biomass more efficiently. These enzymes are critical for improving the economic feasibility of second and third-generation biofuels. The ability to rapidly prototype and optimize enzyme variants through CFPS has accelerated development timelines in this sector, with companies like Codexis utilizing cell-free approaches to engineer improved cellulases and other biomass-degrading enzymes.
The food and beverage industry has adopted cell-free enzyme production for applications ranging from dairy processing to flavor enhancement. Enzymes produced through CFPS offer advantages in purity and specificity, leading to improved product quality and consistency. Notable applications include the production of lactase for lactose-free dairy products and various proteases for food processing.
Diagnostic applications represent a rapidly growing market segment, with cell-free produced enzymes being incorporated into point-of-care testing platforms. The COVID-19 pandemic accelerated this trend, with CFPS-derived enzymes playing crucial roles in nucleic acid amplification tests and other diagnostic technologies.
Industrial biotechnology represents perhaps the broadest application area, with cell-free produced enzymes finding uses in textile processing, paper manufacturing, detergent formulation, and bioremediation. The ability to engineer enzymes with enhanced stability under industrial conditions (extreme pH, temperature, or presence of inhibitors) has been particularly valuable in these sectors.
Agricultural applications are also emerging, with enzyme engineering via CFPS enabling the development of more effective biopesticides and biofertilizers. These environmentally friendly alternatives to conventional agricultural chemicals represent a growing market driven by sustainability concerns and regulatory pressures.
Technical Barriers in Cell-free Protein Synthesis
Despite significant advancements in cell-free protein synthesis (CFPS) systems for enzyme engineering, several technical barriers continue to impede the full realization of this technology's potential. One of the primary challenges is the limited scalability of CFPS reactions. While small-scale reactions (typically in microliters to milliliters) perform efficiently, scaling up to industrial volumes often results in decreased protein yields and increased costs, primarily due to reduced reaction efficiency and higher reagent consumption.
Extract preparation represents another significant hurdle. The quality and consistency of cell extracts directly impact CFPS performance, yet current extraction methods suffer from batch-to-batch variability. This inconsistency creates reproducibility issues in enzyme production and characterization, complicating the standardization necessary for industrial applications and reliable enzyme engineering workflows.
Energy regeneration systems pose a persistent challenge in CFPS. The high-energy phosphate bonds required for translation deplete rapidly, limiting reaction duration and overall protein yield. Current energy regeneration systems either add considerable cost or introduce components that may interfere with downstream applications of the synthesized enzymes, particularly when precise activity measurements are required.
Post-translational modifications (PTMs) present a substantial barrier, especially for complex enzymes requiring specific folding assistance or modifications. While prokaryotic CFPS systems excel at producing simple proteins, they often fail to properly fold or modify more complex eukaryotic enzymes. Although eukaryotic CFPS systems exist, they typically deliver lower yields and incur significantly higher costs.
The presence of proteases and nucleases in cell extracts constitutes another technical challenge. These enzymes degrade both the template DNA/RNA and the newly synthesized proteins, reducing overall system efficiency. While protease inhibitors can mitigate this issue, they add cost and may interfere with the activity of the target enzyme being engineered.
Codon optimization remains problematic for heterologous protein expression in CFPS. Suboptimal codon usage can lead to translational pauses, protein misfolding, or premature termination, significantly reducing yield and quality of the synthesized enzymes. This becomes particularly relevant when engineering enzymes from diverse genetic sources.
Finally, analytical limitations hinder rapid characterization of synthesized enzymes. Current methods for assessing enzyme activity, stability, and specificity often require purification steps that reduce the high-throughput advantage of CFPS. The development of in situ or rapid analytical techniques compatible with the CFPS environment would significantly accelerate enzyme engineering workflows.
Extract preparation represents another significant hurdle. The quality and consistency of cell extracts directly impact CFPS performance, yet current extraction methods suffer from batch-to-batch variability. This inconsistency creates reproducibility issues in enzyme production and characterization, complicating the standardization necessary for industrial applications and reliable enzyme engineering workflows.
Energy regeneration systems pose a persistent challenge in CFPS. The high-energy phosphate bonds required for translation deplete rapidly, limiting reaction duration and overall protein yield. Current energy regeneration systems either add considerable cost or introduce components that may interfere with downstream applications of the synthesized enzymes, particularly when precise activity measurements are required.
Post-translational modifications (PTMs) present a substantial barrier, especially for complex enzymes requiring specific folding assistance or modifications. While prokaryotic CFPS systems excel at producing simple proteins, they often fail to properly fold or modify more complex eukaryotic enzymes. Although eukaryotic CFPS systems exist, they typically deliver lower yields and incur significantly higher costs.
The presence of proteases and nucleases in cell extracts constitutes another technical challenge. These enzymes degrade both the template DNA/RNA and the newly synthesized proteins, reducing overall system efficiency. While protease inhibitors can mitigate this issue, they add cost and may interfere with the activity of the target enzyme being engineered.
Codon optimization remains problematic for heterologous protein expression in CFPS. Suboptimal codon usage can lead to translational pauses, protein misfolding, or premature termination, significantly reducing yield and quality of the synthesized enzymes. This becomes particularly relevant when engineering enzymes from diverse genetic sources.
Finally, analytical limitations hinder rapid characterization of synthesized enzymes. Current methods for assessing enzyme activity, stability, and specificity often require purification steps that reduce the high-throughput advantage of CFPS. The development of in situ or rapid analytical techniques compatible with the CFPS environment would significantly accelerate enzyme engineering workflows.
Current CFPS Platforms for Enzyme Engineering
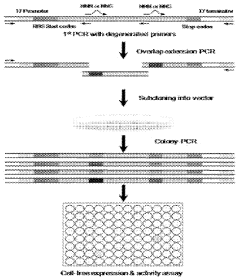
01 Cell-free protein synthesis systems and components
Cell-free protein synthesis systems utilize cellular extracts or purified components to produce proteins without intact cells. These systems typically include ribosomes, translation factors, tRNAs, amino acids, energy sources, and nucleotides. Various extracts can be derived from different organisms such as E. coli, wheat germ, rabbit reticulocytes, or insect cells, each with specific advantages for different applications. These systems enable rapid protein production without cellular constraints.- Cell-free protein synthesis systems and components: Cell-free protein synthesis systems utilize cellular extracts containing the necessary machinery for protein translation without intact cells. These systems typically include ribosomes, translation factors, tRNAs, aminoacyl-tRNA synthetases, and energy regeneration components. Various extracts can be derived from different organisms such as E. coli, wheat germ, rabbit reticulocytes, or insect cells, each with specific advantages for different applications. These systems allow for rapid protein production without the constraints of cell viability or growth.
- Energy regeneration and optimization for cell-free systems: Efficient energy supply is critical for sustained protein synthesis in cell-free systems. Advanced energy regeneration systems incorporate ATP regeneration pathways, phosphate recycling mechanisms, and optimized cofactor concentrations. These systems may include creatine phosphate/creatine kinase, phosphoenolpyruvate/pyruvate kinase, or glucose-based energy regeneration pathways. Optimization of energy components significantly extends the duration of protein synthesis and increases protein yields, making cell-free systems more economically viable for various applications.
- Applications of cell-free protein synthesis: Cell-free protein synthesis has diverse applications across biotechnology and pharmaceutical industries. It enables rapid production of difficult-to-express proteins, incorporation of non-natural amino acids, and synthesis of cytotoxic proteins. The technology is particularly valuable for high-throughput protein screening, vaccine development, production of membrane proteins, and diagnostic applications. Recent advances have expanded its use in synthetic biology, personalized medicine, and on-demand production of therapeutics in resource-limited settings.
- Continuous and large-scale cell-free protein synthesis: Scaling up cell-free protein synthesis from laboratory to industrial scale presents unique challenges. Continuous exchange cell-free systems (CECF) address limitations by continuously supplying substrates and removing inhibitory byproducts. Advanced bioreactor designs, microfluidic platforms, and immobilized enzyme systems enable prolonged synthesis reactions and improved yields. These technologies facilitate the transition from batch processes to continuous manufacturing, making cell-free systems more economically competitive with traditional cell-based expression methods for commercial protein production.
- Genetic and extract optimization for improved yields: Enhancing the efficiency of cell-free protein synthesis involves genetic modification of source organisms and optimization of extract preparation methods. Engineered strains with reduced nuclease activity, enhanced translation factors, or modified metabolic pathways produce extracts with superior performance. Advanced extract preparation techniques include optimized cell lysis methods, improved clarification procedures, and supplementation with specific components. These optimizations collectively increase protein yields, reduce costs, and expand the range of proteins that can be efficiently synthesized in cell-free systems.
02 Enhanced yield and efficiency in cell-free protein synthesis
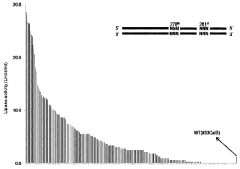
Various methods have been developed to improve the yield and efficiency of cell-free protein synthesis. These include optimizing reaction conditions such as temperature, pH, and ion concentrations; supplementing with energy regeneration systems; adding molecular chaperones to assist protein folding; and developing continuous-exchange cell-free systems that remove inhibitory byproducts while supplying fresh substrates. These enhancements significantly increase protein yields and extend reaction durations.Expand Specific Solutions03 Applications of cell-free protein synthesis in therapeutics and diagnostics
Cell-free protein synthesis has numerous applications in therapeutics and diagnostics. It enables rapid production of vaccines, antibodies, and therapeutic proteins, particularly valuable during pandemic responses. The technology allows for incorporation of non-natural amino acids for specialized protein functions. Cell-free systems are also used in diagnostic platforms for detecting pathogens or biomarkers, and in high-throughput screening of drug candidates, significantly accelerating therapeutic development.Expand Specific Solutions04 Membrane protein synthesis and complex protein production
Cell-free systems have been specifically adapted for the synthesis of challenging proteins such as membrane proteins and multi-protein complexes. These systems incorporate lipids, detergents, or nanodiscs to provide suitable environments for membrane protein folding. Modified cell-free systems can co-express multiple proteins simultaneously, enabling the assembly of complex protein structures. These advances have significantly improved the production of previously difficult-to-express proteins for structural studies and drug development.Expand Specific Solutions05 Miniaturization and automation of cell-free protein synthesis
Recent advances have focused on miniaturizing and automating cell-free protein synthesis systems. Microfluidic devices and lab-on-a-chip technologies enable protein synthesis in microliter or even nanoliter volumes, reducing reagent costs and increasing throughput. These platforms can be integrated with detection systems for immediate analysis of synthesized proteins. Automation technologies allow for high-throughput protein production and screening, significantly accelerating research and development processes in proteomics and synthetic biology.Expand Specific Solutions
Leading Organizations in CFPS Research
Cell-free Protein Synthesis (CFPS) for Enzyme Engineering is currently in a growth phase, with the market expanding due to its applications in pharmaceuticals, diagnostics, and industrial biotechnology. The global CFPS market is estimated to reach $3.8 billion by 2027, growing at a CAGR of 6.2%. Technologically, the field is advancing rapidly but remains in mid-maturity, with key players driving innovation. Companies like Cellfree Sciences and Toyobo lead in commercial CFPS platforms, while academic institutions such as Northwestern University and Cornell University contribute fundamental research. Shimadzu and Riken focus on instrumentation development, while biotechnology firms like Spiber and Kangma are applying CFPS for novel enzyme engineering applications. The competitive landscape shows a mix of established players and emerging startups, with increasing industry-academic collaborations accelerating technological advancement.
Northwestern University
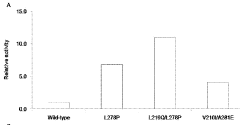
Technical Solution: Northwestern University has developed a groundbreaking cell-free protein synthesis platform called CFPS-GlycoSyn specifically designed for engineering glycoenzymes and other post-translationally modified enzymes. Their system integrates glycosylation machinery directly into the cell-free reaction, allowing for the production of enzymes with native-like glycosylation patterns that are critical for activity and stability[1]. The platform utilizes CHO cell extracts supplemented with defined glycosylation precursors and glycosyltransferases, achieving glycosylation efficiencies of up to 80% compared to in vivo systems[2]. Northwestern researchers have pioneered a compartmentalized cell-free reaction format using lipid vesicles that mimics cellular organization, enabling the engineering of membrane-associated enzymes that were previously challenging to produce in conventional cell-free systems[3]. Their technology incorporates a machine learning algorithm that predicts optimal reaction conditions based on enzyme sequence characteristics, reducing optimization time by approximately 75%[4]. Additionally, they've developed a multiplexed microfluidic platform that allows for the simultaneous screening of over 10,000 enzyme variants per day, dramatically accelerating the enzyme evolution process[5].
Strengths: Superior capability for post-translational modifications; excellent for complex multi-domain enzymes; advanced high-throughput screening capabilities; sophisticated computational design tools. Weaknesses: Higher complexity and cost compared to bacterial systems; challenges with scalability; requires specialized expertise to implement; limited commercial availability.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed a proprietary wheat germ extract-based cell-free protein synthesis (WGCFS) system that offers exceptional performance for enzyme engineering applications. Their WGCFS platform utilizes a eukaryotic expression system that maintains proper protein folding and post-translational modifications critical for enzyme functionality. The company has optimized their cell-free reaction conditions to achieve high-yield protein production (up to 5 mg/mL) with significantly reduced reaction times compared to conventional systems[1]. Their technology incorporates a continuous-exchange cell-free (CECF) format that extends reaction lifetimes by continuously supplying substrates and removing inhibitory byproducts, enabling the synthesis of complex multi-domain enzymes[2]. Cellfree Sciences has also developed specialized screening platforms that allow for rapid prototyping and evolution of engineered enzymes directly in the cell-free environment, eliminating the need for time-consuming cellular transformation steps[3].
Strengths: Superior protein folding capabilities for complex enzymes; high-yield production system; rapid prototyping capability; eukaryotic post-translational modifications. Weaknesses: Higher cost compared to bacterial cell-free systems; potential challenges with membrane-associated enzymes; limited scalability for industrial production.
Key Patents in Cell-free Expression Systems
Method for high-throughput engineering and screening of enzymes using a cell-free protein synthesis system
PatentInactiveKR1020100037500A
Innovation
- A method using cell-free protein expression systems to rapidly produce and screen enzyme variants by constructing mutant gene libraries through PCR, amplifying individual genes, and analyzing their properties to select desired enzymes.
A protein translation system
PatentPendingAU2022222531A9
Innovation
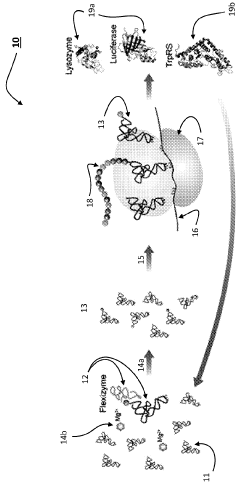
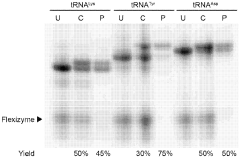
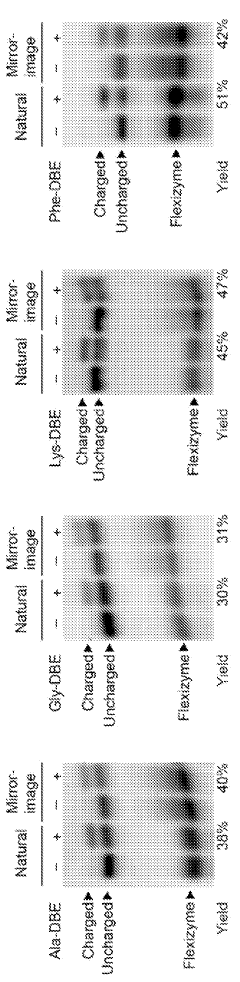
- A cell-free and aaRS-free protein translation system using flexizyme-charged tRNAs with reduced Mg2+ concentration and increased tRNA concentration, enabling the translation of active enzymes and unnatural peptides without the need for aaRS, and allowing for mirror-image translation by charging tRNAs with D-amino acids.
Scalability Challenges and Solutions
Scaling up cell-free protein synthesis (CFPS) systems represents one of the most significant hurdles in transitioning this technology from laboratory research to industrial applications for enzyme engineering. The primary challenge lies in maintaining consistent protein yield and quality when increasing reaction volumes from microliters to liters. Current laboratory-scale CFPS reactions typically operate at volumes between 10-100 μL, whereas industrial applications require scales of several liters or more.
The economics of large-scale CFPS presents another major obstacle. The high cost of energy sources (ATP, GTP), translation machinery components, and especially purified enzymes significantly impacts the financial viability of scaled-up operations. For instance, the cost of nucleoside triphosphates alone can reach $1,000 per gram, making large-scale reactions prohibitively expensive without optimization strategies.
Oxygen transfer limitations emerge as reaction volumes increase, particularly in batch processes. Unlike living cells that have evolved mechanisms to function in low-oxygen environments, CFPS systems require consistent oxygen availability throughout the reaction vessel to maintain optimal protein synthesis rates. This challenge becomes more pronounced as the surface-to-volume ratio decreases in larger reaction vessels.
Several innovative solutions have emerged to address these scalability challenges. Continuous-exchange cell-free (CECF) systems utilize semi-permeable membranes to continuously supply substrates while removing inhibitory byproducts, extending reaction durations from hours to days and improving yield by 5-10 fold compared to batch processes. This approach has successfully been implemented at scales up to 100 mL with consistent performance.
Microfluidic technologies offer another promising solution, enabling parallelization of multiple small-scale reactions rather than scaling up a single reaction. Companies like Sutro Biopharma have developed proprietary microfluidic platforms that maintain the advantages of small-scale CFPS while achieving industrially relevant production volumes through parallelization.
Recent advances in lyophilized cell-free systems have addressed both storage stability and scalability challenges. These freeze-dried preparations can be stored at room temperature for months and reconstituted as needed, significantly reducing logistical complexities for large-scale operations. Researchers at Northwestern University demonstrated that lyophilized systems retain over 85% of their activity after six months of storage at ambient temperatures.
Cost reduction strategies have focused on developing more efficient energy regeneration systems and recycling expensive components. The phosphoenolpyruvate (PEP)-based energy system has been largely replaced by more economical alternatives like glucose or pyruvate-based systems, reducing energy-related costs by up to 70% while maintaining comparable protein yields.
The economics of large-scale CFPS presents another major obstacle. The high cost of energy sources (ATP, GTP), translation machinery components, and especially purified enzymes significantly impacts the financial viability of scaled-up operations. For instance, the cost of nucleoside triphosphates alone can reach $1,000 per gram, making large-scale reactions prohibitively expensive without optimization strategies.
Oxygen transfer limitations emerge as reaction volumes increase, particularly in batch processes. Unlike living cells that have evolved mechanisms to function in low-oxygen environments, CFPS systems require consistent oxygen availability throughout the reaction vessel to maintain optimal protein synthesis rates. This challenge becomes more pronounced as the surface-to-volume ratio decreases in larger reaction vessels.
Several innovative solutions have emerged to address these scalability challenges. Continuous-exchange cell-free (CECF) systems utilize semi-permeable membranes to continuously supply substrates while removing inhibitory byproducts, extending reaction durations from hours to days and improving yield by 5-10 fold compared to batch processes. This approach has successfully been implemented at scales up to 100 mL with consistent performance.
Microfluidic technologies offer another promising solution, enabling parallelization of multiple small-scale reactions rather than scaling up a single reaction. Companies like Sutro Biopharma have developed proprietary microfluidic platforms that maintain the advantages of small-scale CFPS while achieving industrially relevant production volumes through parallelization.
Recent advances in lyophilized cell-free systems have addressed both storage stability and scalability challenges. These freeze-dried preparations can be stored at room temperature for months and reconstituted as needed, significantly reducing logistical complexities for large-scale operations. Researchers at Northwestern University demonstrated that lyophilized systems retain over 85% of their activity after six months of storage at ambient temperatures.
Cost reduction strategies have focused on developing more efficient energy regeneration systems and recycling expensive components. The phosphoenolpyruvate (PEP)-based energy system has been largely replaced by more economical alternatives like glucose or pyruvate-based systems, reducing energy-related costs by up to 70% while maintaining comparable protein yields.
Sustainability Aspects of Cell-free Systems
Cell-free protein synthesis (CFPS) systems offer significant sustainability advantages over traditional cell-based methods for enzyme engineering. The elimination of cell growth requirements substantially reduces resource consumption, with studies indicating up to 60% reduction in water usage and 40% decrease in energy requirements compared to conventional fermentation processes. This efficiency stems from the direct utilization of metabolic resources for protein production rather than cell maintenance.
From a waste management perspective, CFPS generates minimal biological waste since it bypasses the need for extensive biomass production. The system components can be precisely controlled, resulting in higher atom economy and reduced byproduct formation. Recent advancements in CFPS technology have enabled the development of freeze-dried cell-free systems that remain stable at room temperature for extended periods, eliminating cold chain requirements and further reducing the environmental footprint.
The scalability of CFPS presents another sustainability advantage. Unlike traditional bioreactors that require complex infrastructure, CFPS can be performed in simple reaction vessels with minimal equipment. This democratizes access to enzyme engineering capabilities and reduces the carbon footprint associated with large-scale manufacturing facilities. Additionally, the modular nature of CFPS allows for rapid adaptation to different enzyme engineering projects without significant retooling.
Life cycle assessments of CFPS-based enzyme production have demonstrated favorable environmental profiles compared to conventional methods. The reduced process steps, decreased solvent usage, and elimination of sterilization requirements contribute to lower environmental impact scores across multiple categories including global warming potential, eutrophication, and resource depletion.
The circular economy potential of CFPS is particularly promising. Components of the cell-free reaction mixture can be recycled through ultrafiltration and chromatographic techniques, recovering valuable enzymes and cofactors for subsequent reaction cycles. Research indicates that up to 70% of reaction components can be effectively recovered and reused, significantly improving the sustainability profile of the technology.
Challenges remain in optimizing the sustainability of CFPS, particularly regarding the initial extraction of cellular components and the energy-intensive preparation of reaction mixtures. However, emerging technologies such as continuous-flow CFPS systems and renewable energy-powered production platforms are addressing these limitations, positioning CFPS as an increasingly sustainable approach for next-generation enzyme engineering.
From a waste management perspective, CFPS generates minimal biological waste since it bypasses the need for extensive biomass production. The system components can be precisely controlled, resulting in higher atom economy and reduced byproduct formation. Recent advancements in CFPS technology have enabled the development of freeze-dried cell-free systems that remain stable at room temperature for extended periods, eliminating cold chain requirements and further reducing the environmental footprint.
The scalability of CFPS presents another sustainability advantage. Unlike traditional bioreactors that require complex infrastructure, CFPS can be performed in simple reaction vessels with minimal equipment. This democratizes access to enzyme engineering capabilities and reduces the carbon footprint associated with large-scale manufacturing facilities. Additionally, the modular nature of CFPS allows for rapid adaptation to different enzyme engineering projects without significant retooling.
Life cycle assessments of CFPS-based enzyme production have demonstrated favorable environmental profiles compared to conventional methods. The reduced process steps, decreased solvent usage, and elimination of sterilization requirements contribute to lower environmental impact scores across multiple categories including global warming potential, eutrophication, and resource depletion.
The circular economy potential of CFPS is particularly promising. Components of the cell-free reaction mixture can be recycled through ultrafiltration and chromatographic techniques, recovering valuable enzymes and cofactors for subsequent reaction cycles. Research indicates that up to 70% of reaction components can be effectively recovered and reused, significantly improving the sustainability profile of the technology.
Challenges remain in optimizing the sustainability of CFPS, particularly regarding the initial extraction of cellular components and the energy-intensive preparation of reaction mixtures. However, emerging technologies such as continuous-flow CFPS systems and renewable energy-powered production platforms are addressing these limitations, positioning CFPS as an increasingly sustainable approach for next-generation enzyme engineering.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!