Modeling of Reaction Kinetics in Cell-free Protein Synthesis
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis Background and Objectives
Cell-free protein synthesis (CFPS) has emerged as a powerful biotechnological platform that enables the production of proteins without the use of living cells. This technology originated in the 1950s when Nirenberg and Matthaei utilized cell extracts to decipher the genetic code. Since then, CFPS has evolved from a research tool into a versatile technology with applications spanning from fundamental research to industrial biomanufacturing.
The evolution of CFPS systems has been marked by significant improvements in protein yields, reaction durations, and cost-effectiveness. Early systems derived from E. coli extracts have been complemented by eukaryotic alternatives, including wheat germ, rabbit reticulocyte, and insect cell extracts, each offering distinct advantages for specific applications. Recent advancements have focused on developing CFPS platforms from non-model organisms and engineering systems for specialized functions.
Understanding the reaction kinetics in CFPS represents a critical frontier in optimizing these systems. The complex interplay between transcription, translation, energy metabolism, and substrate utilization determines the efficiency and productivity of protein synthesis. Mathematical modeling of these kinetic processes provides a framework for rational design and optimization of CFPS reactions.
The primary objectives of modeling reaction kinetics in CFPS include: (1) elucidating the rate-limiting steps in protein production, (2) predicting system behavior under various conditions, (3) optimizing reaction components and conditions for maximum yield, and (4) designing novel CFPS systems with enhanced performance characteristics.
Current modeling approaches range from empirical black-box models to detailed mechanistic representations of biochemical reactions. These models aim to capture the dynamic behavior of CFPS systems, including resource consumption, intermediate formation, and protein production rates. The integration of experimental data with computational models has accelerated our understanding of the underlying kinetic principles.
The technological trajectory indicates a growing interest in developing predictive models that can guide the design of CFPS reactions for specific applications. This includes tailoring systems for the production of difficult-to-express proteins, incorporation of non-canonical amino acids, and continuous-exchange cell-free systems for prolonged protein synthesis.
As CFPS technology continues to mature, the development of robust kinetic models becomes increasingly important for advancing the field toward industrial relevance. These models serve not only as tools for optimization but also as frameworks for understanding the fundamental principles governing cell-free protein synthesis.
The evolution of CFPS systems has been marked by significant improvements in protein yields, reaction durations, and cost-effectiveness. Early systems derived from E. coli extracts have been complemented by eukaryotic alternatives, including wheat germ, rabbit reticulocyte, and insect cell extracts, each offering distinct advantages for specific applications. Recent advancements have focused on developing CFPS platforms from non-model organisms and engineering systems for specialized functions.
Understanding the reaction kinetics in CFPS represents a critical frontier in optimizing these systems. The complex interplay between transcription, translation, energy metabolism, and substrate utilization determines the efficiency and productivity of protein synthesis. Mathematical modeling of these kinetic processes provides a framework for rational design and optimization of CFPS reactions.
The primary objectives of modeling reaction kinetics in CFPS include: (1) elucidating the rate-limiting steps in protein production, (2) predicting system behavior under various conditions, (3) optimizing reaction components and conditions for maximum yield, and (4) designing novel CFPS systems with enhanced performance characteristics.
Current modeling approaches range from empirical black-box models to detailed mechanistic representations of biochemical reactions. These models aim to capture the dynamic behavior of CFPS systems, including resource consumption, intermediate formation, and protein production rates. The integration of experimental data with computational models has accelerated our understanding of the underlying kinetic principles.
The technological trajectory indicates a growing interest in developing predictive models that can guide the design of CFPS reactions for specific applications. This includes tailoring systems for the production of difficult-to-express proteins, incorporation of non-canonical amino acids, and continuous-exchange cell-free systems for prolonged protein synthesis.
As CFPS technology continues to mature, the development of robust kinetic models becomes increasingly important for advancing the field toward industrial relevance. These models serve not only as tools for optimization but also as frameworks for understanding the fundamental principles governing cell-free protein synthesis.
Market Analysis for Cell-free Protein Expression Systems
The cell-free protein expression systems market has been experiencing significant growth, driven by increasing applications in synthetic biology, personalized medicine, and pharmaceutical research. The global market for cell-free protein expression systems was valued at approximately $208 million in 2020 and is projected to reach $271 million by 2025, growing at a CAGR of 5.5% during the forecast period.
The pharmaceutical and biotechnology sectors represent the largest end-user segments, accounting for over 60% of the market share. This dominance is attributed to the rising demand for therapeutic proteins and antibodies, where cell-free systems offer advantages in producing difficult-to-express proteins and those toxic to host cells. Academic and research institutions constitute another significant market segment, utilizing these systems for fundamental research in protein structure and function.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. The Asia-Pacific region, particularly China, Japan, and India, is expected to witness the highest growth rate due to increasing investments in life sciences research and biotechnology infrastructure development.
The market is segmented by product type into E. coli lysate systems, rabbit reticulocyte lysate systems, wheat germ extract systems, and insect cell lysate systems. E. coli-based systems dominate with nearly 45% market share due to their cost-effectiveness and high protein yield. However, eukaryotic systems are gaining traction for applications requiring post-translational modifications.
Key market drivers include the growing demand for rapid protein production methods, advancements in reaction kinetics modeling that improve yield predictability, and increasing adoption in vaccine development. The COVID-19 pandemic has further accelerated market growth, highlighting the value of cell-free systems for rapid vaccine candidate screening and production.
Challenges limiting market expansion include high costs associated with reagents, technical complexities in scaling up production, and competition from traditional cell-based expression systems. Additionally, the lack of standardized protocols for kinetic modeling across different cell-free systems presents obstacles to widespread industrial adoption.
Emerging trends include the development of continuous-exchange cell-free systems, integration with microfluidic technologies, and the rise of commercial kits optimized for specific applications. The market is also witnessing increased interest in on-demand, point-of-care protein synthesis applications, particularly for personalized medicine and remote healthcare settings.
The pharmaceutical and biotechnology sectors represent the largest end-user segments, accounting for over 60% of the market share. This dominance is attributed to the rising demand for therapeutic proteins and antibodies, where cell-free systems offer advantages in producing difficult-to-express proteins and those toxic to host cells. Academic and research institutions constitute another significant market segment, utilizing these systems for fundamental research in protein structure and function.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. The Asia-Pacific region, particularly China, Japan, and India, is expected to witness the highest growth rate due to increasing investments in life sciences research and biotechnology infrastructure development.
The market is segmented by product type into E. coli lysate systems, rabbit reticulocyte lysate systems, wheat germ extract systems, and insect cell lysate systems. E. coli-based systems dominate with nearly 45% market share due to their cost-effectiveness and high protein yield. However, eukaryotic systems are gaining traction for applications requiring post-translational modifications.
Key market drivers include the growing demand for rapid protein production methods, advancements in reaction kinetics modeling that improve yield predictability, and increasing adoption in vaccine development. The COVID-19 pandemic has further accelerated market growth, highlighting the value of cell-free systems for rapid vaccine candidate screening and production.
Challenges limiting market expansion include high costs associated with reagents, technical complexities in scaling up production, and competition from traditional cell-based expression systems. Additionally, the lack of standardized protocols for kinetic modeling across different cell-free systems presents obstacles to widespread industrial adoption.
Emerging trends include the development of continuous-exchange cell-free systems, integration with microfluidic technologies, and the rise of commercial kits optimized for specific applications. The market is also witnessing increased interest in on-demand, point-of-care protein synthesis applications, particularly for personalized medicine and remote healthcare settings.
Current Challenges in CFPS Reaction Kinetics Modeling
Despite significant advancements in Cell-free Protein Synthesis (CFPS) systems, modeling reaction kinetics remains a formidable challenge. Current models struggle to capture the complex, multi-component nature of CFPS reactions, which involve numerous enzymes, substrates, and cofactors interacting simultaneously. This complexity creates computational barriers that limit predictive accuracy and model utility.
A primary challenge is the incomplete mechanistic understanding of reaction pathways. While individual enzymatic reactions are well-characterized, their collective behavior in the CFPS environment differs significantly from in vivo conditions. The absence of cellular compartmentalization and regulatory mechanisms creates unique kinetic profiles that existing models fail to accurately represent.
Parameter estimation presents another significant obstacle. CFPS systems contain hundreds of parameters that must be determined experimentally, many of which are difficult to measure directly. This leads to underdetermined models with high uncertainty. Current approaches often rely on simplified assumptions or lumped parameters that sacrifice mechanistic insight for computational tractability.
Scale-bridging between molecular-level events and system-level outcomes remains problematic. Molecular dynamics simulations provide detailed insights into specific interactions but are computationally prohibitive for system-scale modeling. Conversely, phenomenological models may capture overall system behavior but lack mechanistic explanations needed for rational system optimization.
Resource depletion and byproduct accumulation dynamics are inadequately represented in current models. As CFPS reactions progress, essential components are consumed while inhibitory byproducts accumulate, creating time-dependent changes in reaction environments that most models fail to capture accurately. This limitation severely restricts the ability to predict extended reaction performance.
Batch-to-batch variability presents a significant modeling challenge. Extract preparation methods introduce inherent variability that affects reaction kinetics in ways that deterministic models cannot predict. This variability necessitates stochastic modeling approaches that are computationally intensive and difficult to parameterize.
Integration of multi-omics data remains underdeveloped. While proteomics, metabolomics, and transcriptomics generate vast datasets on CFPS systems, computational frameworks to leverage this information for improved kinetic models are lacking. Current models typically utilize only a fraction of available experimental data.
The absence of standardized benchmarking datasets and model comparison frameworks hinders progress. Without consensus validation methods, comparing model performance across different research groups becomes problematic, slowing the identification and adoption of superior modeling approaches.
A primary challenge is the incomplete mechanistic understanding of reaction pathways. While individual enzymatic reactions are well-characterized, their collective behavior in the CFPS environment differs significantly from in vivo conditions. The absence of cellular compartmentalization and regulatory mechanisms creates unique kinetic profiles that existing models fail to accurately represent.
Parameter estimation presents another significant obstacle. CFPS systems contain hundreds of parameters that must be determined experimentally, many of which are difficult to measure directly. This leads to underdetermined models with high uncertainty. Current approaches often rely on simplified assumptions or lumped parameters that sacrifice mechanistic insight for computational tractability.
Scale-bridging between molecular-level events and system-level outcomes remains problematic. Molecular dynamics simulations provide detailed insights into specific interactions but are computationally prohibitive for system-scale modeling. Conversely, phenomenological models may capture overall system behavior but lack mechanistic explanations needed for rational system optimization.
Resource depletion and byproduct accumulation dynamics are inadequately represented in current models. As CFPS reactions progress, essential components are consumed while inhibitory byproducts accumulate, creating time-dependent changes in reaction environments that most models fail to capture accurately. This limitation severely restricts the ability to predict extended reaction performance.
Batch-to-batch variability presents a significant modeling challenge. Extract preparation methods introduce inherent variability that affects reaction kinetics in ways that deterministic models cannot predict. This variability necessitates stochastic modeling approaches that are computationally intensive and difficult to parameterize.
Integration of multi-omics data remains underdeveloped. While proteomics, metabolomics, and transcriptomics generate vast datasets on CFPS systems, computational frameworks to leverage this information for improved kinetic models are lacking. Current models typically utilize only a fraction of available experimental data.
The absence of standardized benchmarking datasets and model comparison frameworks hinders progress. Without consensus validation methods, comparing model performance across different research groups becomes problematic, slowing the identification and adoption of superior modeling approaches.
Established Methodologies for CFPS Kinetic Modeling
01 Mathematical modeling of cell-free protein synthesis kinetics
Mathematical models are developed to simulate and predict the kinetics of cell-free protein synthesis systems. These models incorporate various reaction parameters such as transcription rates, translation efficiency, resource consumption, and product formation. By accurately modeling these kinetic processes, researchers can optimize reaction conditions, predict protein yields, and understand the limiting factors in cell-free protein synthesis systems.- Kinetic modeling of cell-free protein synthesis systems: Mathematical models are developed to describe the reaction kinetics in cell-free protein synthesis (CFPS) systems. These models incorporate various parameters such as transcription rates, translation rates, and resource consumption to predict protein yield and system behavior. By understanding the kinetic relationships between different components, researchers can optimize reaction conditions and improve the efficiency of protein production in cell-free environments.
- Resource allocation and metabolic modeling in CFPS: Cell-free protein synthesis systems require careful consideration of resource allocation and metabolic pathways. Models that account for energy regeneration, substrate utilization, and byproduct formation help predict how resources are distributed during protein synthesis. These models enable the design of more efficient reaction mixtures by balancing the supply of amino acids, nucleotides, and energy sources to maintain sustained protein production and avoid premature reaction termination.
- Real-time monitoring and control of CFPS reaction kinetics: Technologies for real-time monitoring of cell-free protein synthesis reactions allow for dynamic tracking of reaction kinetics. These approaches use various detection methods to measure parameters such as protein production rates, substrate consumption, and byproduct formation during the synthesis process. The real-time data can be fed into control systems that adjust reaction conditions to optimize protein yield and quality based on kinetic models, enabling more precise and efficient protein production processes.
- Enzyme kinetics and stability in cell-free systems: The activity and stability of enzymes involved in transcription, translation, and energy regeneration significantly impact the overall kinetics of cell-free protein synthesis. Models that account for enzyme degradation, inhibition, and temperature dependence help predict system performance over time. By understanding these enzyme kinetics, researchers can develop strategies to extend reaction lifetimes, such as adding stabilizing agents or implementing continuous-exchange systems that remove inhibitory byproducts.
- Multi-scale modeling approaches for CFPS optimization: Multi-scale modeling approaches combine molecular-level simulations with system-level kinetic models to provide comprehensive understanding of cell-free protein synthesis. These integrated models account for interactions between different reaction components across various time and spatial scales. By connecting molecular mechanisms to observable reaction kinetics, researchers can identify rate-limiting steps and bottlenecks in the synthesis process, leading to rational design strategies for improving protein yield, reducing resource consumption, and enhancing the overall efficiency of cell-free systems.
02 Optimization of reaction components for enhanced protein synthesis
The optimization of reaction components is crucial for improving the efficiency of cell-free protein synthesis. This includes adjusting concentrations of enzymes, ribosomes, amino acids, nucleotides, and energy sources to achieve optimal reaction kinetics. By systematically analyzing the impact of each component on the overall synthesis process, researchers can develop more productive and cost-effective cell-free protein production systems.Expand Specific Solutions03 Energy regeneration systems in cell-free protein synthesis
Energy regeneration systems play a critical role in sustaining cell-free protein synthesis reactions. These systems maintain adequate ATP levels throughout the reaction by continuously regenerating energy from secondary substrates. The kinetics of these energy regeneration pathways significantly impact the duration and yield of protein synthesis. Various approaches include phosphate-based systems, glucose-based systems, and hybrid energy regeneration mechanisms.Expand Specific Solutions04 Continuous-flow and microfluidic systems for reaction kinetics analysis
Continuous-flow and microfluidic systems enable real-time monitoring and control of cell-free protein synthesis reactions. These platforms allow for precise manipulation of reaction conditions and kinetic parameters, facilitating the study of reaction dynamics at microscale levels. By integrating sensors and analytical tools, researchers can observe protein synthesis kinetics with high temporal resolution, leading to improved understanding of the process and better predictive models.Expand Specific Solutions05 Inhibition factors and reaction longevity in cell-free systems
Understanding inhibition factors that affect reaction kinetics is essential for extending the longevity of cell-free protein synthesis systems. These inhibitory factors include byproduct accumulation, resource depletion, and enzyme degradation. By identifying and mitigating these factors through buffer optimization, component replenishment strategies, or continuous exchange systems, researchers can significantly extend reaction durations and improve overall protein yields.Expand Specific Solutions
Leading Research Groups and Companies in CFPS Technology
Cell-free protein synthesis (CFPS) technology is currently in a growth phase, with the market expanding rapidly due to increasing applications in synthetic biology, pharmaceuticals, and diagnostics. The global CFPS market is estimated to reach approximately $3 billion by 2027, growing at a CAGR of 8-10%. Technologically, the field is advancing from early commercial applications toward greater standardization and scalability. Leading players include established research institutions like Cornell University, Tsinghua University, and RIKEN, alongside commercial entities such as Shimadzu Corp. and Spiber Inc. These organizations are developing proprietary CFPS platforms with improved yields, reduced costs, and enhanced reaction kinetics modeling. Emerging companies like Nature's Toolbox and Kangma Biological Technology are introducing innovative approaches to reaction optimization and real-time monitoring systems, while academic-industry partnerships are accelerating the translation of fundamental kinetic models into practical applications.
Shimadzu Corp.
Technical Solution: Shimadzu Corporation has developed an integrated analytical and modeling platform for cell-free protein synthesis that combines high-precision measurement technologies with kinetic modeling. Their approach utilizes mass spectrometry-based proteomics and metabolomics to quantify reaction components with exceptional accuracy, providing high-quality data for model parameterization. Shimadzu's models incorporate detailed enzyme kinetics for translation factors and aminoacyl-tRNA synthetases, accounting for their concentration-dependent effects on overall synthesis rates. Their technology includes specialized microfluidic devices that enable real-time monitoring of reaction progress while maintaining precise environmental control. Shimadzu has pioneered models that account for molecular crowding effects on reaction kinetics, incorporating the impact of macromolecular concentration on diffusion rates and enzyme activities. Their recent work has focused on developing predictive models for scale-up, addressing challenges in maintaining consistent kinetics between microliter research scales and larger production volumes. Shimadzu's platform includes specialized software tools that integrate experimental data acquisition with model fitting and prediction, creating a seamless workflow from experiment to optimization.
Strengths: Exceptional analytical precision provides high-quality data for model development and validation. Their integrated hardware-software approach creates a comprehensive solution from measurement to modeling. Weaknesses: Heavy reliance on expensive analytical equipment increases the cost of implementation. Their models sometimes prioritize measurement precision over biological mechanism, potentially limiting insights into fundamental processes.
Riken Corp.
Technical Solution: Riken has developed a comprehensive cell-free protein synthesis (CFPS) modeling framework that integrates reaction kinetics with metabolic networks. Their approach uses differential equation systems to describe transcription, translation, and metabolite consumption rates in CFPS reactions. Notably, their PURE (Protein synthesis Using Recombinant Elements) system provides a defined environment where all components are purified recombinant proteins, allowing precise control and modeling of reaction parameters. Riken's models incorporate mRNA degradation kinetics, ribosome binding site strength, and aminoacyl-tRNA synthetase activities to predict protein yield under various conditions. Their recent advancements include incorporating machine learning algorithms to optimize reaction conditions based on kinetic models, achieving up to 5-fold improvements in protein yield compared to standard conditions. Riken has also developed computational tools that simulate the effect of crowding agents and ionic strength on reaction kinetics, providing insights into how these factors affect protein folding during synthesis.
Strengths: Highly controlled and defined system components allow for precise parameter estimation and model validation. Their integrated approach connecting transcription, translation and metabolism provides comprehensive understanding of reaction limitations. Weaknesses: Models require extensive experimental data for parameterization, making them resource-intensive to develop. The PURE system's high cost limits industrial scalability despite its excellent modeling potential.
Critical Patents and Publications in CFPS Reaction Kinetics
Methods of synthesizing cell-free protein
PatentWO2002024939A1
Innovation
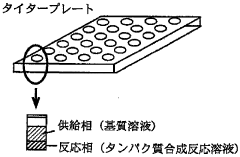
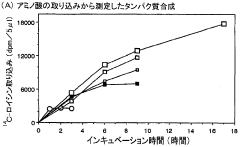
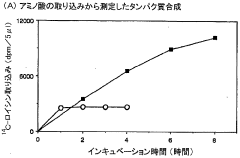
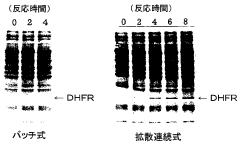
- A diffusion continuous batch system where a biological extract is in direct contact with a substrate and energy source supply solution, allowing free diffusion and continuous supply of substrates and energy while eliminating by-products, enhancing synthesis efficiency and duration, using wheat germ or E. coli extracts and employing techniques like gel filtration or dialysis for resupplying reactants and removing by-products.
Method for synthesizing cell-free protein using adenosine 3',5'-biphosphate, and synthetic reaction solution for cell-free protein
PatentInactiveJP2009142193A
Innovation
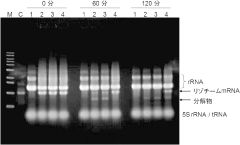
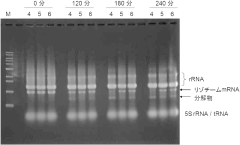
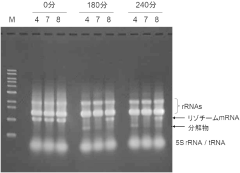
- Incorporating adenosine 3',5'-bisphosphate (pAp) into the cell-free protein synthesis reaction solution, optionally with lithium ions, to suppress mRNA degradation by inhibiting exonucleases, thereby maintaining mRNA integrity during the synthesis process.
Regulatory Considerations for CFPS-derived Products
Cell-free protein synthesis (CFPS) products are increasingly entering commercial and clinical applications, necessitating careful consideration of regulatory frameworks. These products exist in a unique regulatory space that spans pharmaceutical, biological, and industrial domains, requiring specialized approaches to ensure compliance and safety.
The regulatory landscape for CFPS-derived products varies significantly across different regions. In the United States, the FDA categorizes these products based on their intended use, with therapeutic proteins falling under the Center for Biologics Evaluation and Research (CBER) or Center for Drug Evaluation and Research (CDER). Industrial enzymes and non-therapeutic proteins typically face less stringent oversight from agencies such as the EPA or USDA.
European regulatory frameworks, governed by the European Medicines Agency (EMA), generally apply more precautionary principles to novel biotechnology products. This includes comprehensive risk assessments for CFPS systems, particularly those utilizing components from genetically modified organisms. The absence of cellular containment in CFPS raises unique regulatory considerations regarding product purity and potential contaminants.
Key regulatory challenges specific to CFPS-derived products include the characterization of reaction components, demonstration of batch-to-batch consistency, and validation of kinetic models that predict product quality. Regulatory bodies increasingly require manufacturers to implement Quality by Design (QbD) approaches, where understanding reaction kinetics becomes essential for defining critical quality attributes and process parameters.
The absence of cellular membranes in CFPS systems eliminates certain regulatory concerns related to living cells but introduces new considerations regarding the purity of input components and potential immunogenic responses to residual reaction components. Manufacturers must develop robust purification strategies and analytical methods to address these concerns.
Emerging regulatory trends indicate increasing acceptance of computational models for reaction kinetics as supporting evidence in regulatory submissions. These models can demonstrate process understanding and control, potentially streamlining approval processes. However, regulatory agencies typically require experimental validation of model predictions, particularly for critical quality attributes that impact safety and efficacy.
For companies developing CFPS-derived products, early engagement with regulatory authorities through pre-submission consultations is highly recommended. This approach allows for alignment on modeling approaches, validation requirements, and quality control strategies before significant resources are committed to development pathways.
The regulatory landscape for CFPS-derived products varies significantly across different regions. In the United States, the FDA categorizes these products based on their intended use, with therapeutic proteins falling under the Center for Biologics Evaluation and Research (CBER) or Center for Drug Evaluation and Research (CDER). Industrial enzymes and non-therapeutic proteins typically face less stringent oversight from agencies such as the EPA or USDA.
European regulatory frameworks, governed by the European Medicines Agency (EMA), generally apply more precautionary principles to novel biotechnology products. This includes comprehensive risk assessments for CFPS systems, particularly those utilizing components from genetically modified organisms. The absence of cellular containment in CFPS raises unique regulatory considerations regarding product purity and potential contaminants.
Key regulatory challenges specific to CFPS-derived products include the characterization of reaction components, demonstration of batch-to-batch consistency, and validation of kinetic models that predict product quality. Regulatory bodies increasingly require manufacturers to implement Quality by Design (QbD) approaches, where understanding reaction kinetics becomes essential for defining critical quality attributes and process parameters.
The absence of cellular membranes in CFPS systems eliminates certain regulatory concerns related to living cells but introduces new considerations regarding the purity of input components and potential immunogenic responses to residual reaction components. Manufacturers must develop robust purification strategies and analytical methods to address these concerns.
Emerging regulatory trends indicate increasing acceptance of computational models for reaction kinetics as supporting evidence in regulatory submissions. These models can demonstrate process understanding and control, potentially streamlining approval processes. However, regulatory agencies typically require experimental validation of model predictions, particularly for critical quality attributes that impact safety and efficacy.
For companies developing CFPS-derived products, early engagement with regulatory authorities through pre-submission consultations is highly recommended. This approach allows for alignment on modeling approaches, validation requirements, and quality control strategies before significant resources are committed to development pathways.
Scale-up Challenges and Industrial Applications
The transition from laboratory-scale cell-free protein synthesis (CFPS) to industrial production presents significant challenges that must be addressed for commercial viability. Primary among these is the issue of reaction volume scaling, where parameters optimized at microliter scales often fail to translate directly to liter-scale production. This discrepancy stems from altered mixing dynamics, temperature gradients, and oxygen transfer limitations that emerge in larger reaction vessels. Mathematical models that accurately capture these scale-dependent phenomena are essential but remain underdeveloped in current literature.
Cost considerations represent another critical barrier to industrial adoption. The high cost of energy-rich compounds like ATP and GTP, along with expensive enzymes and translation factors, makes large-scale CFPS economically prohibitive for many applications. Researchers are exploring alternative energy regeneration systems and continuous-exchange cell-free (CECF) formats to address these economic constraints, requiring corresponding adjustments to kinetic models.
Batch-to-batch reproducibility poses a substantial challenge for industrial implementation. Variations in extract quality, component degradation rates, and reaction environment stability can lead to inconsistent protein yields. Robust predictive models incorporating stochastic elements and sensitivity analyses are being developed to anticipate and compensate for these variations, though significant refinement is still needed.
Despite these challenges, several promising industrial applications have emerged. Pharmaceutical companies are utilizing CFPS for rapid production of personalized therapeutics and vaccines, where the ability to quickly synthesize proteins in response to emerging health threats outweighs cost considerations. The on-demand nature of CFPS aligns well with the just-in-time manufacturing paradigm increasingly adopted in biopharmaceutical production.
Diagnostic applications represent another growth area, with companies developing portable CFPS systems for point-of-care protein-based diagnostics. These applications benefit from the reduced scale requirements and the ability to lyophilize CFPS components for extended shelf life. Kinetic models optimized for these specialized applications are being developed to enhance sensitivity and specificity.
Enzyme production for industrial catalysis is gaining traction as a CFPS application, particularly for enzymes that are difficult to express in conventional systems due to toxicity or inclusion body formation. Companies are developing continuous-flow CFPS reactors with integrated purification systems, supported by sophisticated reaction kinetics models that optimize throughput and yield.
Cost considerations represent another critical barrier to industrial adoption. The high cost of energy-rich compounds like ATP and GTP, along with expensive enzymes and translation factors, makes large-scale CFPS economically prohibitive for many applications. Researchers are exploring alternative energy regeneration systems and continuous-exchange cell-free (CECF) formats to address these economic constraints, requiring corresponding adjustments to kinetic models.
Batch-to-batch reproducibility poses a substantial challenge for industrial implementation. Variations in extract quality, component degradation rates, and reaction environment stability can lead to inconsistent protein yields. Robust predictive models incorporating stochastic elements and sensitivity analyses are being developed to anticipate and compensate for these variations, though significant refinement is still needed.
Despite these challenges, several promising industrial applications have emerged. Pharmaceutical companies are utilizing CFPS for rapid production of personalized therapeutics and vaccines, where the ability to quickly synthesize proteins in response to emerging health threats outweighs cost considerations. The on-demand nature of CFPS aligns well with the just-in-time manufacturing paradigm increasingly adopted in biopharmaceutical production.
Diagnostic applications represent another growth area, with companies developing portable CFPS systems for point-of-care protein-based diagnostics. These applications benefit from the reduced scale requirements and the ability to lyophilize CFPS components for extended shelf life. Kinetic models optimized for these specialized applications are being developed to enhance sensitivity and specificity.
Enzyme production for industrial catalysis is gaining traction as a CFPS application, particularly for enzymes that are difficult to express in conventional systems due to toxicity or inclusion body formation. Companies are developing continuous-flow CFPS reactors with integrated purification systems, supported by sophisticated reaction kinetics models that optimize throughput and yield.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!