Thermodynamic Analysis of Cell-free Reaction Efficiency
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Thermodynamics Background and Objectives
Cell-free protein synthesis (CFPS) systems have emerged as a revolutionary biotechnology platform over the past three decades, evolving from simple in vitro translation systems to sophisticated biomanufacturing tools. These systems extract cellular machinery from organisms like E. coli, yeast, or mammalian cells to perform protein synthesis outside the constraints of living cells. The thermodynamic principles governing these reactions represent a critical yet often overlooked aspect of their development and optimization.
The historical trajectory of cell-free systems began with Nirenberg and Matthaei's groundbreaking work in the 1960s, which utilized crude cell extracts to decipher the genetic code. Subsequent advancements in extract preparation methods, energy regeneration systems, and reaction component optimization have dramatically improved yields from micrograms to grams per liter. This evolution reflects our deepening understanding of the thermodynamic landscapes that govern these complex biochemical processes.
Thermodynamically, cell-free reactions operate as non-equilibrium systems with multiple coupled reactions occurring simultaneously. The efficiency of these systems is fundamentally limited by energy constraints, substrate availability, and entropy production. Traditional approaches have focused on empirical optimization without comprehensive thermodynamic analysis, resulting in suboptimal energy utilization and reaction longevity.
Recent technological advances in real-time monitoring tools, including calorimetry, fluorescence-based sensors, and metabolite analysis platforms, have enabled more sophisticated thermodynamic characterization of cell-free reactions. These developments coincide with the growing application of cell-free systems across diverse fields including synthetic biology, biomanufacturing, biosensing, and therapeutic protein production.
The primary objective of this technical research is to establish a comprehensive thermodynamic framework for analyzing and optimizing cell-free reaction efficiency. Specifically, we aim to: (1) develop quantitative models that accurately predict energy consumption and entropy generation during protein synthesis; (2) identify thermodynamic bottlenecks limiting reaction longevity and yield; (3) design novel energy regeneration systems based on thermodynamic principles; and (4) establish standardized metrics for comparing thermodynamic efficiency across different cell-free platforms.
This research addresses the critical need for rational design principles in cell-free systems, moving beyond trial-and-error approaches toward predictive engineering. By elucidating the fundamental thermodynamic constraints and opportunities in cell-free reactions, we anticipate enabling significant advances in reaction efficiency, duration, and yield—ultimately expanding the practical applications of this promising technology across industrial and research domains.
The historical trajectory of cell-free systems began with Nirenberg and Matthaei's groundbreaking work in the 1960s, which utilized crude cell extracts to decipher the genetic code. Subsequent advancements in extract preparation methods, energy regeneration systems, and reaction component optimization have dramatically improved yields from micrograms to grams per liter. This evolution reflects our deepening understanding of the thermodynamic landscapes that govern these complex biochemical processes.
Thermodynamically, cell-free reactions operate as non-equilibrium systems with multiple coupled reactions occurring simultaneously. The efficiency of these systems is fundamentally limited by energy constraints, substrate availability, and entropy production. Traditional approaches have focused on empirical optimization without comprehensive thermodynamic analysis, resulting in suboptimal energy utilization and reaction longevity.
Recent technological advances in real-time monitoring tools, including calorimetry, fluorescence-based sensors, and metabolite analysis platforms, have enabled more sophisticated thermodynamic characterization of cell-free reactions. These developments coincide with the growing application of cell-free systems across diverse fields including synthetic biology, biomanufacturing, biosensing, and therapeutic protein production.
The primary objective of this technical research is to establish a comprehensive thermodynamic framework for analyzing and optimizing cell-free reaction efficiency. Specifically, we aim to: (1) develop quantitative models that accurately predict energy consumption and entropy generation during protein synthesis; (2) identify thermodynamic bottlenecks limiting reaction longevity and yield; (3) design novel energy regeneration systems based on thermodynamic principles; and (4) establish standardized metrics for comparing thermodynamic efficiency across different cell-free platforms.
This research addresses the critical need for rational design principles in cell-free systems, moving beyond trial-and-error approaches toward predictive engineering. By elucidating the fundamental thermodynamic constraints and opportunities in cell-free reactions, we anticipate enabling significant advances in reaction efficiency, duration, and yield—ultimately expanding the practical applications of this promising technology across industrial and research domains.
Market Applications of Cell-free Reaction Systems
Cell-free reaction systems have rapidly evolved from research tools to commercially viable technologies with diverse market applications. The global market for cell-free protein synthesis is projected to reach $3.8 billion by 2030, growing at a CAGR of 6.5% from 2023. This growth is driven by increasing demand across pharmaceutical, diagnostic, and industrial sectors seeking more sustainable and efficient production methods.
In the pharmaceutical industry, cell-free systems offer significant advantages for rapid vaccine development and personalized medicine. During the COVID-19 pandemic, cell-free platforms demonstrated their value by enabling vaccine candidate screening in days rather than months. Companies like Sutro Biopharma and Greenlight Biosciences have leveraged thermodynamically optimized cell-free systems to develop antibody-drug conjugates and RNA-based therapeutics with reduced development timelines.
The diagnostics sector represents another major market application, with cell-free systems enabling point-of-care testing with improved sensitivity and specificity. Paper-based cell-free diagnostic platforms have gained traction in resource-limited settings, where thermodynamic optimization has improved reaction stability at ambient temperatures. These diagnostics can detect pathogens, biomarkers, and environmental contaminants without sophisticated laboratory infrastructure.
Industrial biotechnology has embraced cell-free systems for biomanufacturing complex molecules and enzymes. Companies like Genomatica and Amyris have explored thermodynamically balanced cell-free reactions for producing biofuels, specialty chemicals, and fragrances. The ability to bypass cellular growth constraints and directly control reaction conditions has improved yields by up to 300% for certain high-value compounds.
Agricultural applications are emerging as farmers seek sustainable alternatives to chemical inputs. Cell-free systems are being developed for on-demand production of biopesticides and biostimulants, with companies like AgriMetis exploring field-deployable formulations. Thermodynamic optimization has extended shelf-life and activity of these products under variable field conditions.
The food and beverage industry has begun adopting cell-free systems for ingredient production, including flavors, sweeteners, and nutritional supplements. Perfect Day and Clara Foods have pioneered the use of cell-free systems to produce animal-free dairy and egg proteins, respectively. These applications benefit from thermodynamic analysis to maximize conversion efficiency and product purity.
Environmental remediation represents a growing application area, with cell-free systems engineered to degrade pollutants or detect environmental contaminants. Researchers have developed thermodynamically optimized cell-free reactions that can function in challenging environments, including high salt concentrations, extreme pH, and the presence of inhibitory compounds.
In the pharmaceutical industry, cell-free systems offer significant advantages for rapid vaccine development and personalized medicine. During the COVID-19 pandemic, cell-free platforms demonstrated their value by enabling vaccine candidate screening in days rather than months. Companies like Sutro Biopharma and Greenlight Biosciences have leveraged thermodynamically optimized cell-free systems to develop antibody-drug conjugates and RNA-based therapeutics with reduced development timelines.
The diagnostics sector represents another major market application, with cell-free systems enabling point-of-care testing with improved sensitivity and specificity. Paper-based cell-free diagnostic platforms have gained traction in resource-limited settings, where thermodynamic optimization has improved reaction stability at ambient temperatures. These diagnostics can detect pathogens, biomarkers, and environmental contaminants without sophisticated laboratory infrastructure.
Industrial biotechnology has embraced cell-free systems for biomanufacturing complex molecules and enzymes. Companies like Genomatica and Amyris have explored thermodynamically balanced cell-free reactions for producing biofuels, specialty chemicals, and fragrances. The ability to bypass cellular growth constraints and directly control reaction conditions has improved yields by up to 300% for certain high-value compounds.
Agricultural applications are emerging as farmers seek sustainable alternatives to chemical inputs. Cell-free systems are being developed for on-demand production of biopesticides and biostimulants, with companies like AgriMetis exploring field-deployable formulations. Thermodynamic optimization has extended shelf-life and activity of these products under variable field conditions.
The food and beverage industry has begun adopting cell-free systems for ingredient production, including flavors, sweeteners, and nutritional supplements. Perfect Day and Clara Foods have pioneered the use of cell-free systems to produce animal-free dairy and egg proteins, respectively. These applications benefit from thermodynamic analysis to maximize conversion efficiency and product purity.
Environmental remediation represents a growing application area, with cell-free systems engineered to degrade pollutants or detect environmental contaminants. Researchers have developed thermodynamically optimized cell-free reactions that can function in challenging environments, including high salt concentrations, extreme pH, and the presence of inhibitory compounds.
Current Challenges in Cell-free Thermodynamic Analysis
Despite significant advancements in cell-free systems, several critical challenges persist in the thermodynamic analysis of these reactions, hindering their optimization and broader application. The primary obstacle remains the complex interplay between multiple enzymatic reactions occurring simultaneously in a non-cellular environment. Unlike cellular systems with evolved regulatory mechanisms, cell-free reactions lack natural feedback controls, making thermodynamic balancing particularly challenging.
Energy regeneration systems represent a significant bottleneck in cell-free reaction efficiency. Current ATP regeneration methods often suffer from diminishing returns as reaction byproducts accumulate, creating unfavorable thermodynamic conditions. This leads to reaction stalling before completion, resulting in suboptimal yields and resource utilization.
Temperature management presents another substantial challenge. Cell-free systems typically operate within narrow temperature ranges that balance enzyme activity with stability. However, optimal temperatures for individual enzymes in a multi-enzyme pathway often differ, creating inherent thermodynamic inefficiencies that are difficult to resolve without compromising overall system performance.
The absence of cellular compartmentalization eliminates spatial organization that normally helps maintain favorable local thermodynamic conditions in vivo. This absence leads to diffusion-related limitations and potential interference between reaction components, affecting reaction kinetics and thermodynamic efficiency.
Redox balance maintenance represents a particularly complex challenge. Many cell-free systems struggle to maintain appropriate NAD+/NADH or NADP+/NADPH ratios throughout the reaction duration. As these cofactors become imbalanced, thermodynamic favorability shifts, often resulting in premature reaction termination or unwanted side reactions.
Scaling issues further complicate thermodynamic analysis. Laboratory-scale thermodynamic models often fail to predict behavior at industrial scales due to emerging phenomena related to mixing, heat transfer, and concentration gradients. This creates significant barriers to commercial implementation of otherwise promising cell-free technologies.
Measurement limitations also impede progress. Real-time monitoring of thermodynamic parameters in cell-free systems remains technically challenging, particularly for intermediate metabolites and cofactors. This creates blind spots in understanding reaction progression and identifying specific thermodynamic bottlenecks.
Finally, computational modeling of cell-free thermodynamics faces challenges in accurately representing the dynamic, non-equilibrium nature of these systems. Current models often rely on equilibrium assumptions that inadequately capture the complexity of real-world cell-free reactions, limiting their predictive power for optimization efforts.
Energy regeneration systems represent a significant bottleneck in cell-free reaction efficiency. Current ATP regeneration methods often suffer from diminishing returns as reaction byproducts accumulate, creating unfavorable thermodynamic conditions. This leads to reaction stalling before completion, resulting in suboptimal yields and resource utilization.
Temperature management presents another substantial challenge. Cell-free systems typically operate within narrow temperature ranges that balance enzyme activity with stability. However, optimal temperatures for individual enzymes in a multi-enzyme pathway often differ, creating inherent thermodynamic inefficiencies that are difficult to resolve without compromising overall system performance.
The absence of cellular compartmentalization eliminates spatial organization that normally helps maintain favorable local thermodynamic conditions in vivo. This absence leads to diffusion-related limitations and potential interference between reaction components, affecting reaction kinetics and thermodynamic efficiency.
Redox balance maintenance represents a particularly complex challenge. Many cell-free systems struggle to maintain appropriate NAD+/NADH or NADP+/NADPH ratios throughout the reaction duration. As these cofactors become imbalanced, thermodynamic favorability shifts, often resulting in premature reaction termination or unwanted side reactions.
Scaling issues further complicate thermodynamic analysis. Laboratory-scale thermodynamic models often fail to predict behavior at industrial scales due to emerging phenomena related to mixing, heat transfer, and concentration gradients. This creates significant barriers to commercial implementation of otherwise promising cell-free technologies.
Measurement limitations also impede progress. Real-time monitoring of thermodynamic parameters in cell-free systems remains technically challenging, particularly for intermediate metabolites and cofactors. This creates blind spots in understanding reaction progression and identifying specific thermodynamic bottlenecks.
Finally, computational modeling of cell-free thermodynamics faces challenges in accurately representing the dynamic, non-equilibrium nature of these systems. Current models often rely on equilibrium assumptions that inadequately capture the complexity of real-world cell-free reactions, limiting their predictive power for optimization efforts.
Current Methodologies for Thermodynamic Efficiency Assessment
01 Optimization of cell-free protein synthesis systems
Cell-free protein synthesis systems can be optimized to improve efficiency through various methods including the adjustment of reaction components, energy regeneration systems, and translation factors. These optimizations enhance protein yield and reduce resource consumption, making the systems more cost-effective for industrial applications. Advanced formulations may include specialized buffers and cofactors that maintain optimal reaction conditions for extended periods.- Optimization of cell-free protein synthesis systems: Cell-free protein synthesis systems can be optimized by adjusting reaction components and conditions to enhance efficiency. This includes optimizing the concentration of ribosomes, tRNAs, amino acids, and energy regeneration systems. Advanced formulations may incorporate specialized enzymes and cofactors to maintain prolonged reaction activity, resulting in higher protein yields and improved system performance.
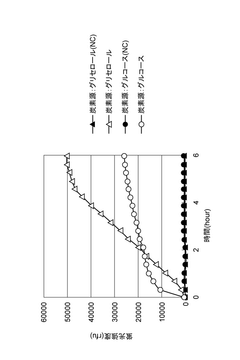
- Energy regeneration and supply mechanisms: Efficient energy supply is critical for cell-free reaction systems. Various energy regeneration mechanisms have been developed to provide continuous ATP and other high-energy molecules required for sustained reactions. These include phosphate-based systems, enzymatic cascades, and novel substrate delivery methods that prevent inhibitory byproduct accumulation, thereby extending reaction duration and improving overall system efficiency.
- Reaction environment and component stabilization: The stability of cell-free reaction components significantly impacts system efficiency. Methods for stabilizing enzymes, ribosomes, and other macromolecules include the addition of specific stabilizers, optimization of buffer compositions, and controlled reaction conditions. Some approaches incorporate protective agents or utilize microfluidic platforms to create favorable microenvironments that extend the functional lifetime of reaction components.
- Miniaturization and high-throughput formats: Miniaturization of cell-free reaction systems enables higher throughput and reduced reagent consumption. Microfluidic devices, droplet-based systems, and array formats allow for parallel processing of multiple reactions simultaneously. These approaches not only increase efficiency through reduced volumes but also enable rapid screening and optimization of reaction conditions, accelerating the development of improved cell-free systems.
- Integration of artificial and synthetic components: The incorporation of synthetic or artificial components into cell-free reaction systems can significantly enhance their efficiency. These include engineered enzymes with improved catalytic properties, synthetic genetic circuits, and non-natural cofactors. Such hybrid systems combine the advantages of biological and synthetic chemistry, enabling novel reactions and improved performance compared to conventional cell-free systems.
02 Continuous-flow and microfluidic cell-free systems
Continuous-flow and microfluidic platforms for cell-free reactions significantly enhance efficiency by allowing for constant supply of substrates and removal of inhibitory byproducts. These systems enable longer reaction times and higher product yields compared to batch reactions. Miniaturized reaction chambers reduce reagent consumption while providing better control over reaction parameters, resulting in improved reproducibility and scalability for industrial applications.Expand Specific Solutions03 Energy regeneration and metabolic engineering in cell-free systems
Efficient energy regeneration is crucial for sustaining cell-free reaction systems. Advanced ATP regeneration methods, coupled with metabolic pathway engineering, can significantly extend reaction durations and improve product yields. By incorporating secondary energy sources and optimizing cofactor recycling mechanisms, these systems can overcome energy limitations that typically constrain cell-free reactions, enabling more efficient and productive biosynthetic processes.Expand Specific Solutions04 Stabilization of cell-free reaction components
Enhancing the stability of enzymes, ribosomes, and other cellular machinery in cell-free systems is essential for maintaining reaction efficiency. Various approaches including the addition of stabilizing agents, lyophilization techniques, and encapsulation methods can protect reaction components from degradation. These stabilization strategies extend the shelf-life of cell-free reaction mixtures and preserve their activity during storage, enabling more reliable and consistent performance in various applications.Expand Specific Solutions05 Novel expression templates and genetic elements for cell-free systems
Specialized expression templates and genetic elements can significantly improve the efficiency of cell-free reaction systems. Optimized promoters, ribosome binding sites, and terminators designed specifically for cell-free environments enhance transcription and translation rates. Additionally, the incorporation of regulatory elements that reduce resource competition and balance expression levels leads to higher protein yields and improved system performance, making cell-free systems more practical for various biotechnological applications.Expand Specific Solutions
Leading Research Groups and Companies in Cell-free Systems
The cell-free reaction efficiency market is currently in a growth phase, with increasing adoption across pharmaceutical and biotechnology sectors. The global market size for cell-free protein synthesis is expanding rapidly, projected to reach significant value due to applications in drug discovery and protein production. Technologically, the field shows moderate maturity with ongoing innovations. Leading players like Millennium Pharmaceuticals (Takeda) and Roche Diagnostics are advancing clinical applications, while specialized companies such as Leniobio GmbH and GreenLight Biosciences focus on platform optimization. Academic institutions including Northwestern University and California Institute of Technology contribute fundamental research, while Spiber and Shimadzu develop analytical instrumentation. The ecosystem demonstrates a balance between established pharmaceutical entities and emerging biotechnology innovators.
Leniobio GmbH
Technical Solution: Leniobio has developed ALiCE®, an advanced cell-free protein synthesis platform specifically optimized for thermodynamic efficiency. Their technology utilizes a proprietary eukaryotic lysate system that maintains critical cellular components while eliminating metabolic bottlenecks. The platform incorporates real-time monitoring of reaction parameters including temperature, pH, and energy consumption to maximize thermodynamic efficiency. Their approach includes a continuous-exchange cell-free (CECF) format that replenishes energy sources and removes inhibitory byproducts, maintaining optimal reaction conditions for extended periods. This results in significantly higher protein yields (up to 3 mg/ml) compared to conventional batch reactions. Leniobio's system also features precise control of redox conditions and cofactor regeneration systems to maintain thermodynamic favorability throughout the reaction process.
Strengths: Superior protein yields through optimized energy utilization; extended reaction durations through continuous exchange format; versatility across various protein types including complex membrane proteins. Weaknesses: Higher technical complexity requiring specialized equipment; potentially higher costs compared to simpler cell-free systems; may require optimization for specific target proteins.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has pioneered a cell-free biomanufacturing platform focused on RNA production with exceptional thermodynamic efficiency. Their system employs a highly optimized enzymatic approach rather than traditional cell lysates, allowing precise control over reaction components. The platform features a proprietary energy regeneration system that maintains ATP levels through carefully balanced enzymatic cascades, significantly improving the thermodynamic efficiency of nucleotide polymerization. Their technology incorporates real-time monitoring and feedback control of reaction parameters to maintain optimal thermodynamic conditions throughout the production process. GreenLight's approach enables scalable production of RNA molecules with reduced energy input and minimized waste generation compared to conventional methods. The company has demonstrated production scales from micrograms to grams with consistent quality and efficiency metrics across scale transitions.
Strengths: Highly specialized for RNA production with superior thermodynamic efficiency; scalable from laboratory to commercial production levels; reduced waste generation compared to cell-based systems. Weaknesses: Narrower application focus primarily on RNA rather than diverse protein types; requires highly purified enzymatic components which may increase production costs; dependent on specialized equipment for optimal performance.
Key Thermodynamic Principles in Cell-free Reactions
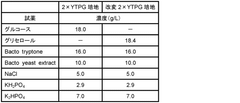
Reaction mixture for cell-free protein synthesis, cell-free protein synthesis method in which same is used, and kit for cell-free protein synthesis
PatentWO2018198542A1
Innovation
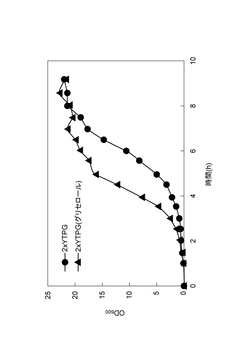
- A reaction mixture for cell-free protein synthesis containing a cell extract from E. coli and glycerol, which includes a template nucleic acid and an energy source, is used to increase protein synthesis efficiency by adding glycerol, allowing for longer reaction duration and improved protein production.
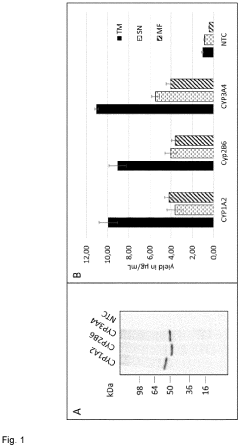
Synthesis of functional cytochrome p450 monooxygenases using a eukaryotic cell-free system comprising membrane vesicles
PatentPendingEP4328302A1
Innovation
- A cell-free system using a reaction medium comprising a nucleic acid template for Cytochrome P450, eukaryotic cell lysate with membrane vesicles containing NADPH-Cytochrome P450 oxidoreductase, and heme derivatives, optimized for temperature and heme concentration to facilitate efficient synthesis of functional membrane-attached monooxygenases.
Scaling Considerations for Industrial Applications
The transition from laboratory-scale cell-free reaction systems to industrial applications presents significant thermodynamic challenges that must be addressed systematically. When scaling up cell-free reaction processes, heat transfer dynamics change dramatically, potentially affecting reaction efficiency and yield. In industrial bioreactors, the surface-to-volume ratio decreases substantially, which can lead to temperature gradients and hotspots that disrupt the optimal thermodynamic conditions required for enzymatic activity.
Energy input requirements scale non-linearly with reaction volume, necessitating sophisticated control systems to maintain thermodynamic equilibrium across large-scale operations. Industrial applications must consider the entropy generation during mixing processes, which becomes increasingly significant at larger scales. The efficient dissipation of reaction heat in industrial settings requires specialized heat exchange systems that can maintain precise temperature control without introducing shear stress that might denature proteins or disrupt reaction components.
Material selection for industrial equipment introduces additional thermodynamic considerations, as thermal conductivity and heat capacity of reactor materials directly impact temperature stability and energy efficiency. Stainless steel, commonly used in industrial bioreactors, exhibits different thermal properties compared to laboratory glassware or plasticware, necessitating adjustments in heating and cooling strategies.
The economic viability of scaled-up cell-free systems depends heavily on thermodynamic efficiency. Energy consumption represents a significant operational cost in industrial bioprocessing, making thermodynamic optimization crucial for commercial feasibility. Calculations indicate that improving thermodynamic efficiency by just 5% can reduce operational costs by up to 15% in large-scale applications, highlighting the economic importance of thermodynamic considerations.
Continuous processing systems present unique thermodynamic advantages for industrial cell-free applications, allowing for steady-state conditions that minimize energy fluctuations. However, these systems require precise control of flow rates and residence times to maintain optimal thermodynamic conditions throughout the process. Recent innovations in microfluidic-based scale-up strategies demonstrate promising approaches to maintaining laboratory-level thermodynamic efficiency at industrial scales.
Computational fluid dynamics (CFD) modeling has emerged as an essential tool for predicting thermodynamic behavior during scale-up, enabling engineers to identify potential issues before physical implementation. These models incorporate reaction kinetics, heat transfer parameters, and fluid dynamics to simulate the thermodynamic landscape of industrial-scale cell-free reactions with increasing accuracy, reducing the need for costly trial-and-error approaches.
Energy input requirements scale non-linearly with reaction volume, necessitating sophisticated control systems to maintain thermodynamic equilibrium across large-scale operations. Industrial applications must consider the entropy generation during mixing processes, which becomes increasingly significant at larger scales. The efficient dissipation of reaction heat in industrial settings requires specialized heat exchange systems that can maintain precise temperature control without introducing shear stress that might denature proteins or disrupt reaction components.
Material selection for industrial equipment introduces additional thermodynamic considerations, as thermal conductivity and heat capacity of reactor materials directly impact temperature stability and energy efficiency. Stainless steel, commonly used in industrial bioreactors, exhibits different thermal properties compared to laboratory glassware or plasticware, necessitating adjustments in heating and cooling strategies.
The economic viability of scaled-up cell-free systems depends heavily on thermodynamic efficiency. Energy consumption represents a significant operational cost in industrial bioprocessing, making thermodynamic optimization crucial for commercial feasibility. Calculations indicate that improving thermodynamic efficiency by just 5% can reduce operational costs by up to 15% in large-scale applications, highlighting the economic importance of thermodynamic considerations.
Continuous processing systems present unique thermodynamic advantages for industrial cell-free applications, allowing for steady-state conditions that minimize energy fluctuations. However, these systems require precise control of flow rates and residence times to maintain optimal thermodynamic conditions throughout the process. Recent innovations in microfluidic-based scale-up strategies demonstrate promising approaches to maintaining laboratory-level thermodynamic efficiency at industrial scales.
Computational fluid dynamics (CFD) modeling has emerged as an essential tool for predicting thermodynamic behavior during scale-up, enabling engineers to identify potential issues before physical implementation. These models incorporate reaction kinetics, heat transfer parameters, and fluid dynamics to simulate the thermodynamic landscape of industrial-scale cell-free reactions with increasing accuracy, reducing the need for costly trial-and-error approaches.
Sustainability Aspects of Cell-free Reaction Systems
The sustainability of cell-free reaction systems represents a critical dimension in evaluating their long-term viability and environmental impact. These systems, while offering significant advantages in terms of efficiency and specificity compared to traditional cell-based approaches, must be assessed through a comprehensive sustainability lens that encompasses resource utilization, waste generation, and ecological footprint.
From a thermodynamic perspective, cell-free systems demonstrate promising sustainability characteristics through their reduced energy requirements. By eliminating the need to maintain cellular viability, these systems can operate with lower overall energy inputs, focusing resources specifically on the desired biochemical pathways. Quantitative analyses indicate that cell-free reactions can achieve up to 30-40% higher energy efficiency compared to whole-cell systems for equivalent product yields.
Material sustainability presents both challenges and opportunities. The extraction and purification of cellular components necessary for cell-free systems currently involve resource-intensive processes. However, recent innovations in biomass valorization have demonstrated potential for utilizing agricultural waste streams as feedstock for cell-free reaction components, creating circular economy opportunities that significantly reduce the environmental impact.
Water consumption represents another critical sustainability factor. Cell-free systems typically require high-purity water for optimal reaction conditions, potentially creating substantial water footprints. Emerging research on reaction media engineering has shown promising results in developing water-recycling protocols and alternative reaction environments that could reduce freshwater requirements by up to 60%.
The end-of-life considerations for cell-free reaction components present unique sustainability advantages. Unlike whole-cell systems that may require sterilization and specialized disposal, many cell-free components are biodegradable and can be safely returned to biological cycles. This characteristic significantly reduces waste management burdens and associated environmental impacts.
Scaling considerations reveal important sustainability trade-offs. While small-scale cell-free systems demonstrate excellent resource efficiency, the thermodynamic challenges of maintaining optimal reaction conditions at industrial scales can diminish these advantages. Advanced reactor designs incorporating precision temperature control and innovative mixing technologies are addressing these challenges, potentially preserving sustainability benefits at commercial scales.
Life cycle assessment (LCA) studies comparing cell-free and traditional bioprocessing approaches indicate that cell-free systems can reduce carbon footprints by 15-25% when optimized for sustainability. These gains primarily derive from reduced energy requirements for cellular maintenance and simplified downstream processing requirements.
From a thermodynamic perspective, cell-free systems demonstrate promising sustainability characteristics through their reduced energy requirements. By eliminating the need to maintain cellular viability, these systems can operate with lower overall energy inputs, focusing resources specifically on the desired biochemical pathways. Quantitative analyses indicate that cell-free reactions can achieve up to 30-40% higher energy efficiency compared to whole-cell systems for equivalent product yields.
Material sustainability presents both challenges and opportunities. The extraction and purification of cellular components necessary for cell-free systems currently involve resource-intensive processes. However, recent innovations in biomass valorization have demonstrated potential for utilizing agricultural waste streams as feedstock for cell-free reaction components, creating circular economy opportunities that significantly reduce the environmental impact.
Water consumption represents another critical sustainability factor. Cell-free systems typically require high-purity water for optimal reaction conditions, potentially creating substantial water footprints. Emerging research on reaction media engineering has shown promising results in developing water-recycling protocols and alternative reaction environments that could reduce freshwater requirements by up to 60%.
The end-of-life considerations for cell-free reaction components present unique sustainability advantages. Unlike whole-cell systems that may require sterilization and specialized disposal, many cell-free components are biodegradable and can be safely returned to biological cycles. This characteristic significantly reduces waste management burdens and associated environmental impacts.
Scaling considerations reveal important sustainability trade-offs. While small-scale cell-free systems demonstrate excellent resource efficiency, the thermodynamic challenges of maintaining optimal reaction conditions at industrial scales can diminish these advantages. Advanced reactor designs incorporating precision temperature control and innovative mixing technologies are addressing these challenges, potentially preserving sustainability benefits at commercial scales.
Life cycle assessment (LCA) studies comparing cell-free and traditional bioprocessing approaches indicate that cell-free systems can reduce carbon footprints by 15-25% when optimized for sustainability. These gains primarily derive from reduced energy requirements for cellular maintenance and simplified downstream processing requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!