Comparative Study of Cell-free Protein Synthesis Kits and Platforms
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis Background and Objectives
Cell-free protein synthesis (CFPS) has evolved significantly since its inception in the 1960s, transforming from a fundamental research tool into a versatile biotechnological platform. This technology emerged from the pioneering work of Nirenberg and Matthaei, who utilized cell extracts to decipher the genetic code. Over subsequent decades, CFPS has undergone substantial refinements in extract preparation, reaction conditions, and supplementation strategies, leading to dramatic improvements in protein yields and functionality.
The evolution of CFPS systems reflects broader trends in biotechnology, moving from crude lysates toward increasingly defined and specialized systems. Early systems derived from E. coli have been complemented by eukaryotic alternatives based on wheat germ, rabbit reticulocytes, insect cells, and human cell lines, each offering distinct advantages for specific applications. Recent innovations have focused on enhancing system performance through genetic engineering of source organisms, optimization of energy regeneration pathways, and development of continuous-exchange platforms.
The primary objective of this comparative study is to systematically evaluate commercially available CFPS kits and platforms across multiple performance parameters. We aim to establish a comprehensive framework for assessing these systems based on protein yield, functionality, post-translational modification capabilities, scalability, and cost-effectiveness. This evaluation will provide valuable insights for researchers and industry professionals seeking to select optimal CFPS solutions for specific applications.
Additionally, this study seeks to identify technological gaps in current CFPS offerings and highlight emerging trends that may shape future developments. By analyzing the strengths and limitations of existing platforms, we can forecast potential innovation pathways and anticipate future market needs. This forward-looking perspective will help guide strategic research and development initiatives in the CFPS field.
The broader context of this analysis encompasses the growing importance of CFPS in various applications, including rapid protein production for structural studies, incorporation of non-canonical amino acids, vaccine development, biosensing, and on-demand biomanufacturing. As synthetic biology and bioengineering continue to advance, CFPS systems are positioned to play an increasingly central role in both research and industrial settings, particularly for applications requiring rapid prototyping, toxic protein expression, or point-of-care production.
Through this comprehensive technical assessment, we aim to provide a valuable resource that bridges the gap between academic research and practical implementation of CFPS technology across diverse sectors of the biotechnology industry.
The evolution of CFPS systems reflects broader trends in biotechnology, moving from crude lysates toward increasingly defined and specialized systems. Early systems derived from E. coli have been complemented by eukaryotic alternatives based on wheat germ, rabbit reticulocytes, insect cells, and human cell lines, each offering distinct advantages for specific applications. Recent innovations have focused on enhancing system performance through genetic engineering of source organisms, optimization of energy regeneration pathways, and development of continuous-exchange platforms.
The primary objective of this comparative study is to systematically evaluate commercially available CFPS kits and platforms across multiple performance parameters. We aim to establish a comprehensive framework for assessing these systems based on protein yield, functionality, post-translational modification capabilities, scalability, and cost-effectiveness. This evaluation will provide valuable insights for researchers and industry professionals seeking to select optimal CFPS solutions for specific applications.
Additionally, this study seeks to identify technological gaps in current CFPS offerings and highlight emerging trends that may shape future developments. By analyzing the strengths and limitations of existing platforms, we can forecast potential innovation pathways and anticipate future market needs. This forward-looking perspective will help guide strategic research and development initiatives in the CFPS field.
The broader context of this analysis encompasses the growing importance of CFPS in various applications, including rapid protein production for structural studies, incorporation of non-canonical amino acids, vaccine development, biosensing, and on-demand biomanufacturing. As synthetic biology and bioengineering continue to advance, CFPS systems are positioned to play an increasingly central role in both research and industrial settings, particularly for applications requiring rapid prototyping, toxic protein expression, or point-of-care production.
Through this comprehensive technical assessment, we aim to provide a valuable resource that bridges the gap between academic research and practical implementation of CFPS technology across diverse sectors of the biotechnology industry.
Market Analysis of Cell-free Expression Systems
The global market for cell-free expression systems has experienced significant growth in recent years, driven by increasing applications in synthetic biology, protein engineering, and diagnostics. As of 2023, the market size was valued at approximately 208 million USD, with projections indicating a compound annual growth rate (CAGR) of 8.5% through 2030, potentially reaching 370 million USD by the end of the forecast period.
North America currently dominates the market landscape, accounting for roughly 40% of the global share, followed by Europe at 30% and Asia-Pacific at 20%. This regional distribution reflects the concentration of research institutions, biotechnology companies, and pharmaceutical giants that are primary consumers of cell-free protein synthesis technologies.
The market segmentation reveals distinct categories based on product types, with commercial kits representing 45% of the market, followed by reagents (30%), expression systems (15%), and accessories (10%). Among commercial kits, E. coli-based systems maintain the largest market share at approximately 35%, while rabbit reticulocyte lysate systems account for 25%, wheat germ extract systems for 20%, and insect cell-based systems for 15%.
Key market drivers include the growing demand for rapid protein production methods, increasing research in structural biology, and the expanding applications in vaccine development and personalized medicine. The COVID-19 pandemic significantly accelerated market growth, as cell-free systems proved valuable for rapid diagnostic development and vaccine research.
Pricing analysis indicates considerable variation across platforms, with high-end commercial kits ranging from 500 to 2,000 USD per reaction set, while academic-focused systems may be available at lower price points. This price disparity creates distinct market segments catering to different user groups, from well-funded pharmaceutical research to budget-conscious academic laboratories.
Market challenges include the high cost of commercial kits, technical complexity requiring specialized expertise, and reproducibility issues across different batches. These factors have limited broader adoption, particularly in resource-constrained settings and developing regions.
Emerging trends suggest a shift toward continuous-exchange cell-free systems, miniaturization for high-throughput applications, and the development of freeze-dried cell-free systems for point-of-care diagnostics. Additionally, there is growing interest in cell-free systems derived from non-model organisms for specialized applications, potentially opening new market niches.
North America currently dominates the market landscape, accounting for roughly 40% of the global share, followed by Europe at 30% and Asia-Pacific at 20%. This regional distribution reflects the concentration of research institutions, biotechnology companies, and pharmaceutical giants that are primary consumers of cell-free protein synthesis technologies.
The market segmentation reveals distinct categories based on product types, with commercial kits representing 45% of the market, followed by reagents (30%), expression systems (15%), and accessories (10%). Among commercial kits, E. coli-based systems maintain the largest market share at approximately 35%, while rabbit reticulocyte lysate systems account for 25%, wheat germ extract systems for 20%, and insect cell-based systems for 15%.
Key market drivers include the growing demand for rapid protein production methods, increasing research in structural biology, and the expanding applications in vaccine development and personalized medicine. The COVID-19 pandemic significantly accelerated market growth, as cell-free systems proved valuable for rapid diagnostic development and vaccine research.
Pricing analysis indicates considerable variation across platforms, with high-end commercial kits ranging from 500 to 2,000 USD per reaction set, while academic-focused systems may be available at lower price points. This price disparity creates distinct market segments catering to different user groups, from well-funded pharmaceutical research to budget-conscious academic laboratories.
Market challenges include the high cost of commercial kits, technical complexity requiring specialized expertise, and reproducibility issues across different batches. These factors have limited broader adoption, particularly in resource-constrained settings and developing regions.
Emerging trends suggest a shift toward continuous-exchange cell-free systems, miniaturization for high-throughput applications, and the development of freeze-dried cell-free systems for point-of-care diagnostics. Additionally, there is growing interest in cell-free systems derived from non-model organisms for specialized applications, potentially opening new market niches.
Current Landscape and Technical Barriers
Cell-free protein synthesis (CFPS) technology has evolved significantly over the past decade, with numerous commercial kits and platforms now available in the market. The current landscape is characterized by a diverse range of offerings from established biotechnology companies and emerging startups. Major players include New England Biolabs, Thermo Fisher Scientific, Promega, and Takara Bio, who offer standardized CFPS kits based primarily on E. coli extracts. More specialized systems from companies like Sutro Biopharma and Arbor Biosciences provide eukaryotic-based systems utilizing wheat germ, rabbit reticulocyte, or insect cell extracts.
The market segmentation is primarily divided between prokaryotic and eukaryotic expression systems, with further differentiation based on application specificity, throughput capacity, and level of automation. Recent technological advancements have led to the development of microfluidic platforms and continuous-exchange cell-free (CECF) systems that significantly enhance protein yield and reduce reaction volumes.
Despite these advancements, several technical barriers persist in the CFPS field. Extract preparation remains highly variable between batches, affecting reproducibility and scalability. This variability stems from differences in cell growth conditions, lysis methods, and extract processing procedures. Standardization efforts are underway but have not yet reached industry-wide consensus.
Energy regeneration systems represent another significant challenge. Current ATP regeneration methods often become limiting factors in extended reactions, with phosphate accumulation inhibiting protein synthesis over time. Alternative energy sources and regeneration pathways are being explored but have not been widely implemented in commercial kits.
Post-translational modifications (PTMs) present a substantial hurdle, particularly for therapeutic protein production. While eukaryotic CFPS systems can perform some PTMs, they often lack the complete cellular machinery necessary for complex modifications like glycosylation. This limitation restricts the application of CFPS for certain classes of proteins, particularly monoclonal antibodies and other biologics.
Scale-up challenges also persist across platforms. Most commercial kits are optimized for microliter to milliliter reaction volumes, with significant performance drops observed at larger scales. This limitation hampers industrial adoption for biomanufacturing applications where gram to kilogram quantities are required.
Cost remains a significant barrier to widespread adoption. Current commercial kits range from $2-20 per microliter of reaction, making them prohibitively expensive for many applications. The high cost of specialized components, particularly nucleotides and energy regeneration systems, contributes significantly to this economic barrier.
The market segmentation is primarily divided between prokaryotic and eukaryotic expression systems, with further differentiation based on application specificity, throughput capacity, and level of automation. Recent technological advancements have led to the development of microfluidic platforms and continuous-exchange cell-free (CECF) systems that significantly enhance protein yield and reduce reaction volumes.
Despite these advancements, several technical barriers persist in the CFPS field. Extract preparation remains highly variable between batches, affecting reproducibility and scalability. This variability stems from differences in cell growth conditions, lysis methods, and extract processing procedures. Standardization efforts are underway but have not yet reached industry-wide consensus.
Energy regeneration systems represent another significant challenge. Current ATP regeneration methods often become limiting factors in extended reactions, with phosphate accumulation inhibiting protein synthesis over time. Alternative energy sources and regeneration pathways are being explored but have not been widely implemented in commercial kits.
Post-translational modifications (PTMs) present a substantial hurdle, particularly for therapeutic protein production. While eukaryotic CFPS systems can perform some PTMs, they often lack the complete cellular machinery necessary for complex modifications like glycosylation. This limitation restricts the application of CFPS for certain classes of proteins, particularly monoclonal antibodies and other biologics.
Scale-up challenges also persist across platforms. Most commercial kits are optimized for microliter to milliliter reaction volumes, with significant performance drops observed at larger scales. This limitation hampers industrial adoption for biomanufacturing applications where gram to kilogram quantities are required.
Cost remains a significant barrier to widespread adoption. Current commercial kits range from $2-20 per microliter of reaction, making them prohibitively expensive for many applications. The high cost of specialized components, particularly nucleotides and energy regeneration systems, contributes significantly to this economic barrier.
Comparative Analysis of Commercial CFPS Kits
01 Cell-free protein synthesis systems based on cell extracts
Cell-free protein synthesis systems utilizing extracts from various cell types (bacterial, mammalian, plant) as the foundation for in vitro protein production. These systems contain the necessary cellular machinery including ribosomes, translation factors, and enzymes required for transcription and translation. The extracts are optimized to provide efficient protein synthesis outside the living cell environment, allowing for rapid protein production without cellular constraints.- Cell-free protein synthesis systems based on cell extracts: Cell-free protein synthesis systems utilizing extracts from various cell types (bacterial, mammalian, plant) as the foundation for in vitro translation. These systems contain the necessary components for protein synthesis including ribosomes, tRNAs, aminoacyl-tRNA synthetases, translation factors, and energy regeneration systems. The extracts provide the cellular machinery required for efficient protein production outside of living cells.
- Continuous exchange cell-free protein synthesis platforms: Advanced cell-free protein synthesis platforms that employ continuous exchange of reagents to enhance protein yield and quality. These systems feature specialized reaction chambers that allow for the continuous supply of energy sources and amino acids while removing inhibitory byproducts. This approach significantly extends reaction lifetimes and increases protein production efficiency compared to batch-mode systems.
- Lyophilized cell-free protein synthesis kits: Ready-to-use freeze-dried cell-free protein synthesis kits that offer improved stability and shelf life. These kits contain pre-measured, lyophilized components that maintain activity during storage and can be quickly reconstituted with minimal preparation. The lyophilization process preserves the functionality of the translation machinery while allowing for room temperature storage and transportation.
- Cell-free protein synthesis with post-translational modifications: Specialized cell-free protein synthesis systems designed to incorporate post-translational modifications such as glycosylation, phosphorylation, and disulfide bond formation. These advanced platforms include additional enzymatic components and cofactors that enable the production of proteins with proper folding and modifications similar to those produced in living cells, resulting in improved functionality and bioactivity.
- Microfluidic and miniaturized cell-free protein synthesis platforms: Innovative miniaturized cell-free protein synthesis platforms that utilize microfluidic technology for high-throughput protein production and screening. These systems enable parallel synthesis of multiple proteins in small volumes, reducing reagent consumption and increasing experimental efficiency. The microfluidic design allows for precise control of reaction conditions and integration with downstream analysis methods.
02 Commercial kit formulations for cell-free protein synthesis
Ready-to-use commercial kits containing pre-optimized components for cell-free protein synthesis. These kits typically include cell extracts, energy regeneration systems, nucleotides, amino acids, and reaction buffers. The formulations are designed for ease of use, reproducibility, and high protein yields. Some kits are specialized for specific applications such as membrane protein production, isotope labeling, or high-throughput screening.Expand Specific Solutions03 Energy regeneration systems for sustained protein synthesis
Specialized energy regeneration systems that maintain ATP and GTP levels during cell-free protein synthesis reactions. These systems typically include energy sources (such as phosphoenolpyruvate, creatine phosphate, or glucose), along with the enzymes required for their metabolism. By continuously regenerating energy molecules, these systems extend reaction lifetimes and increase protein yields compared to batch reactions with limited energy sources.Expand Specific Solutions04 Continuous-exchange cell-free protein synthesis platforms
Advanced platforms that enable continuous-exchange or flow-based cell-free protein synthesis. These systems feature specialized reaction chambers that allow for the continuous supply of substrates and removal of inhibitory byproducts during the synthesis reaction. By maintaining optimal reaction conditions over extended periods, these platforms achieve significantly higher protein yields than conventional batch reactions and can be scaled for larger production volumes.Expand Specific Solutions05 Modified cell-free systems for specialized protein production
Customized cell-free protein synthesis systems designed for specialized applications such as production of complex proteins, incorporation of non-canonical amino acids, or post-translational modifications. These systems may include supplementary components like chaperones for proper protein folding, disulfide bond formation enzymes, glycosylation machinery, or orthogonal translation systems. The modifications enable the production of proteins that would be difficult to express in conventional cell-based or cell-free systems.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The cell-free protein synthesis (CFPS) market is currently in a growth phase, with an estimated global market size of $250-300 million and projected annual growth of 8-10%. The competitive landscape features established academic institutions (MIT, Northwestern University, Cornell, Tsinghua) driving fundamental research alongside commercial players developing proprietary platforms. Leading companies like Shimadzu, RIKEN, and Spiber have commercialized advanced CFPS kits, while emerging players such as Swiftscale Biologics and Kangma Biological Technology are introducing innovative technologies focused on scalability and yield optimization. Technical maturity varies significantly across platforms, with recent advancements in extract preparation, energy regeneration systems, and reaction optimization improving protein yields from micrograms to grams per liter. Chinese institutions and companies are rapidly gaining ground with significant R&D investments in this previously Western-dominated field.
Northwestern University
Technical Solution: Northwestern University has pioneered the development of cell-free protein synthesis platforms optimized for point-of-care diagnostic applications. Their "CFPS-Dx" system utilizes specially prepared E. coli extracts with enhanced tolerance to clinical sample matrices like blood, saliva, and urine[9]. The platform incorporates freeze-dried technology that stabilizes all components for room-temperature storage while maintaining activity upon rehydration. Northwestern's system features a colorimetric output module that couples protein synthesis to visible color changes, enabling equipment-free detection of target analytes. Their technology achieves detection limits in the picomolar range for nucleic acid targets and nanomolar range for protein targets within 30-60 minutes[10]. The university has also developed specialized formulations for on-demand production of therapeutic proteins, including antimicrobial peptides and vaccine antigens, with demonstrated activity in animal models. Recent innovations include paper-based formats that further simplify deployment in resource-limited settings.
Strengths: Exceptional stability in lyophilized format; robust performance in complex biological matrices; rapid time-to-result; minimal equipment requirements for field deployment. Weaknesses: Lower protein yields compared to research-grade systems; limited post-translational modifications; higher cost per reaction than conventional laboratory systems; narrower operating temperature range.
Jiangsu Fulcrum Biotechnology Co., Ltd.
Technical Solution: Jiangsu Fulcrum Biotechnology has developed a hybrid cell-free protein synthesis platform called "FulCFPS" that combines elements of both E. coli and wheat germ extracts to achieve high yields while maintaining complex protein folding capabilities. Their system incorporates a proprietary pre-treatment process that removes inhibitory components from crude extracts while preserving essential translation machinery[7]. The platform features a dual-phase reaction format with an initial rapid expression phase followed by a extended maturation phase that enhances proper protein folding. Fulcrum's technology includes specialized chaperone supplements that facilitate the production of complex eukaryotic proteins with multiple disulfide bonds. Their system achieves consistent yields of 1-1.5 mg/mL for standard proteins and maintains activity for up to 12 hours through an optimized energy regeneration system[8]. The company offers customized formulations for different protein classes, including membrane proteins, antibodies, and enzymes.
Strengths: Balanced performance across yield, cost, and protein complexity; good scalability from microliter to liter scale; effective for both prokaryotic and eukaryotic proteins. Weaknesses: Less specialized than competitor systems focused on specific applications; moderate cost-performance ratio; requires optimization for maximum yield with complex proteins.
Key Patents and Scientific Breakthroughs
Cell-free protein synthesis platform
PatentWO2025207933A1
Innovation
- A cell-free protein synthesis platform is developed, featuring a polymer membrane with affinity binding sites and pores, where ribosomes are bound to the membrane via membrane-bound proteins or translocons, allowing for enhanced separation of reaction and product compartments, and utilizing a molar excess of small ribosomal subunits for improved protein translation.
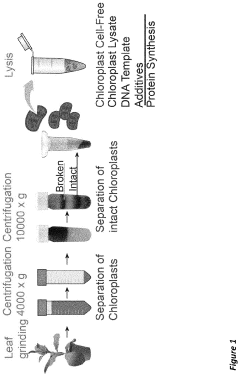
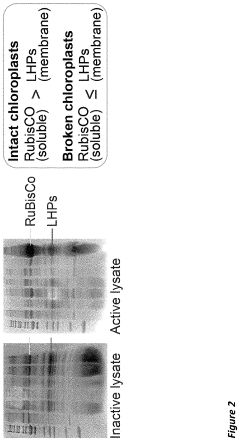
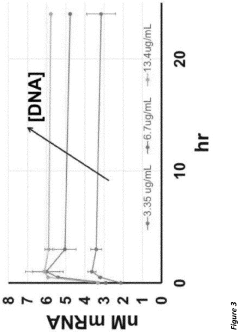
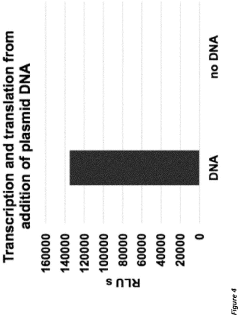
Combined transcription and translation platform derived from plant plastids and methods for in vitro protein synthesis and prototyping of genetic expression in plants
PatentPendingUS20210163969A1
Innovation
- A cell-free protein synthesis system using components prepared from isolated plant plastids and extracts, particularly from Nicotiana tabacum and Zea mays, enabling high-throughput genetic analysis and protein production for prototyping genetic parts and establishing part libraries.
Scalability and Industrial Applications
The scalability of cell-free protein synthesis (CFPS) systems represents a critical frontier for transitioning this technology from laboratory research to industrial applications. Current commercial CFPS kits demonstrate varying degrees of scalability, with systems like the Promega S30 T7 High-Yield and New England Biolabs PURExpress showing promising results in scaled-up reactions from microliter to milliliter volumes. However, significant challenges emerge when attempting further scale-up to industrial levels of liters or beyond.
Key factors affecting scalability include oxygen transfer limitations, heat dissipation issues, and the maintenance of homogeneous reaction conditions across larger volumes. Commercial platforms have addressed these challenges through different approaches. For instance, continuous-flow CFPS systems developed by Sutro Biopharma have demonstrated production capabilities at the 100-liter scale, while Arbor Biosciences has focused on optimizing their myTXTL kit for batch processing at intermediate scales.
Industrial applications of CFPS technology span multiple sectors, with biopharmaceuticals representing the most mature market. Companies like GreenLight Biosciences and Sutro Biopharma have leveraged CFPS platforms for rapid production of vaccine candidates and therapeutic proteins, respectively. The ability to bypass cell viability requirements enables production of proteins that would be toxic to living cells, expanding the range of potential biopharmaceutical products.
Beyond pharmaceuticals, industrial enzymes represent another significant application area. Synthetic Genomics and Synvitrobio have developed CFPS platforms specifically optimized for enzyme screening and production. These systems allow rapid prototyping and optimization of industrial enzymes for applications in detergents, food processing, and biofuel production, significantly reducing development timelines compared to traditional cell-based methods.
Agricultural applications are emerging as a promising frontier, with companies like AgriMetis utilizing CFPS for the development of novel crop protection compounds. The rapid prototyping capabilities of CFPS systems enable efficient screening of bioactive peptides and proteins that could serve as environmentally friendly alternatives to conventional pesticides.
Cost considerations remain paramount for industrial adoption. Current commercial kits range from $0.1-1 per microliter of reaction, making large-scale applications prohibitively expensive. However, recent advances in extract preparation methods and energy regeneration systems have demonstrated potential cost reductions of up to 100-fold, potentially enabling economically viable industrial applications in the near future.
Key factors affecting scalability include oxygen transfer limitations, heat dissipation issues, and the maintenance of homogeneous reaction conditions across larger volumes. Commercial platforms have addressed these challenges through different approaches. For instance, continuous-flow CFPS systems developed by Sutro Biopharma have demonstrated production capabilities at the 100-liter scale, while Arbor Biosciences has focused on optimizing their myTXTL kit for batch processing at intermediate scales.
Industrial applications of CFPS technology span multiple sectors, with biopharmaceuticals representing the most mature market. Companies like GreenLight Biosciences and Sutro Biopharma have leveraged CFPS platforms for rapid production of vaccine candidates and therapeutic proteins, respectively. The ability to bypass cell viability requirements enables production of proteins that would be toxic to living cells, expanding the range of potential biopharmaceutical products.
Beyond pharmaceuticals, industrial enzymes represent another significant application area. Synthetic Genomics and Synvitrobio have developed CFPS platforms specifically optimized for enzyme screening and production. These systems allow rapid prototyping and optimization of industrial enzymes for applications in detergents, food processing, and biofuel production, significantly reducing development timelines compared to traditional cell-based methods.
Agricultural applications are emerging as a promising frontier, with companies like AgriMetis utilizing CFPS for the development of novel crop protection compounds. The rapid prototyping capabilities of CFPS systems enable efficient screening of bioactive peptides and proteins that could serve as environmentally friendly alternatives to conventional pesticides.
Cost considerations remain paramount for industrial adoption. Current commercial kits range from $0.1-1 per microliter of reaction, making large-scale applications prohibitively expensive. However, recent advances in extract preparation methods and energy regeneration systems have demonstrated potential cost reductions of up to 100-fold, potentially enabling economically viable industrial applications in the near future.
Regulatory Considerations for CFPS Products
Cell-free protein synthesis (CFPS) products are subject to a complex regulatory landscape that varies significantly across different regions and applications. The regulatory framework for CFPS technologies primarily depends on their intended use, with distinctions made between research tools, diagnostic applications, and therapeutic products. Each category faces distinct regulatory scrutiny and compliance requirements.
For research-grade CFPS kits, regulatory oversight is relatively minimal, focusing mainly on biosafety considerations and proper handling of biological materials. However, when CFPS platforms are utilized for diagnostic purposes, they must adhere to clinical laboratory standards such as CLIA (Clinical Laboratory Improvement Amendments) in the United States or equivalent regulations in other jurisdictions.
CFPS products intended for therapeutic applications face the most stringent regulatory requirements. These products must navigate the full pharmaceutical regulatory pathway, including extensive preclinical testing, clinical trials, and manufacturing process validation. The FDA, EMA, and other regulatory bodies require comprehensive documentation of raw material sourcing, quality control measures, and batch-to-batch consistency for CFPS-derived therapeutics.
A critical regulatory consideration for CFPS products is the classification of the cell extracts used in these systems. Depending on the source organism and preparation method, these extracts may be subject to different regulatory frameworks. For instance, extracts derived from human cells face additional scrutiny compared to those from bacterial or plant sources due to potential contamination risks and ethical considerations.
Intellectual property protection represents another significant regulatory dimension for CFPS technologies. Many commercial CFPS platforms incorporate patented components or processes, necessitating careful navigation of licensing agreements and potential infringement issues. Companies developing novel CFPS products must conduct thorough freedom-to-operate analyses to mitigate legal risks.
Emerging regulatory trends indicate increasing attention to the environmental impact of CFPS technologies, particularly regarding waste management and biosecurity concerns. Regulatory bodies are developing frameworks to address potential misuse of CFPS systems for bioweapon production or unauthorized synthesis of controlled substances, reflecting the dual-use nature of these powerful biotechnological tools.
International harmonization efforts are underway to standardize regulatory approaches to CFPS products, though significant regional variations persist. Companies operating in this space must develop comprehensive regulatory strategies that account for these differences while maintaining compliance with evolving requirements across global markets.
For research-grade CFPS kits, regulatory oversight is relatively minimal, focusing mainly on biosafety considerations and proper handling of biological materials. However, when CFPS platforms are utilized for diagnostic purposes, they must adhere to clinical laboratory standards such as CLIA (Clinical Laboratory Improvement Amendments) in the United States or equivalent regulations in other jurisdictions.
CFPS products intended for therapeutic applications face the most stringent regulatory requirements. These products must navigate the full pharmaceutical regulatory pathway, including extensive preclinical testing, clinical trials, and manufacturing process validation. The FDA, EMA, and other regulatory bodies require comprehensive documentation of raw material sourcing, quality control measures, and batch-to-batch consistency for CFPS-derived therapeutics.
A critical regulatory consideration for CFPS products is the classification of the cell extracts used in these systems. Depending on the source organism and preparation method, these extracts may be subject to different regulatory frameworks. For instance, extracts derived from human cells face additional scrutiny compared to those from bacterial or plant sources due to potential contamination risks and ethical considerations.
Intellectual property protection represents another significant regulatory dimension for CFPS technologies. Many commercial CFPS platforms incorporate patented components or processes, necessitating careful navigation of licensing agreements and potential infringement issues. Companies developing novel CFPS products must conduct thorough freedom-to-operate analyses to mitigate legal risks.
Emerging regulatory trends indicate increasing attention to the environmental impact of CFPS technologies, particularly regarding waste management and biosecurity concerns. Regulatory bodies are developing frameworks to address potential misuse of CFPS systems for bioweapon production or unauthorized synthesis of controlled substances, reflecting the dual-use nature of these powerful biotechnological tools.
International harmonization efforts are underway to standardize regulatory approaches to CFPS products, though significant regional variations persist. Companies operating in this space must develop comprehensive regulatory strategies that account for these differences while maintaining compliance with evolving requirements across global markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!