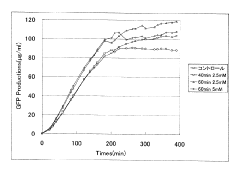
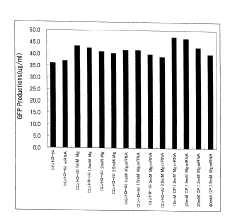
Integration of Artificial Metabolism in Cell-free Protein Synthesis
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Artificial Metabolism and CFPS Background
Cell-free protein synthesis (CFPS) systems have emerged as powerful platforms for the production of proteins outside living cells. These systems harness the cellular machinery necessary for transcription and translation while eliminating the constraints of cell viability and growth. The concept of CFPS dates back to the 1950s when Nirenberg and Matthaei used cell extracts to decipher the genetic code, but recent advances have transformed these systems into sophisticated biotechnological tools.
Artificial metabolism represents a critical advancement in CFPS technology. Traditional CFPS systems face limitations in energy supply and cofactor regeneration, which restricts their productivity and application scope. Artificial metabolism addresses these challenges by integrating metabolic pathways that can continuously regenerate ATP, NAD(P)H, and other essential cofactors required for protein synthesis.
The evolution of CFPS systems has progressed through several key phases. Early systems relied on high-energy phosphate compounds like phosphoenolpyruvate (PEP) for ATP regeneration, but these were expensive and produced inhibitory byproducts. The second generation incorporated substrate-level phosphorylation pathways using cheaper energy sources like glucose, but still faced limitations in energy efficiency.
Modern CFPS platforms now integrate more complex metabolic networks, including glycolysis, pentose phosphate pathway, and oxidative phosphorylation components. These artificial metabolic systems can utilize diverse carbon sources and maintain redox balance, significantly extending reaction durations from hours to days and improving protein yields by orders of magnitude.
The integration of artificial metabolism with CFPS has enabled numerous applications beyond simple protein production. These include the synthesis of complex proteins with post-translational modifications, the production of toxic proteins that would inhibit cell growth in vivo, and the rapid prototyping of metabolic pathways for synthetic biology applications.
Recent technological breakthroughs have further expanded CFPS capabilities through the incorporation of non-native metabolic pathways. These innovations allow for the incorporation of non-canonical amino acids, the synthesis of glycoproteins, and the production of membrane proteins in artificial lipid environments. The convergence of artificial metabolism with CFPS represents a paradigm shift in protein production technology, offering unprecedented control over the biochemical environment.
The field continues to evolve rapidly, with researchers developing increasingly sophisticated artificial metabolic networks that can be tailored to specific protein production requirements. These advances are positioning CFPS as a versatile platform for applications ranging from biopharmaceutical production to biosensing and synthetic cell construction.
Artificial metabolism represents a critical advancement in CFPS technology. Traditional CFPS systems face limitations in energy supply and cofactor regeneration, which restricts their productivity and application scope. Artificial metabolism addresses these challenges by integrating metabolic pathways that can continuously regenerate ATP, NAD(P)H, and other essential cofactors required for protein synthesis.
The evolution of CFPS systems has progressed through several key phases. Early systems relied on high-energy phosphate compounds like phosphoenolpyruvate (PEP) for ATP regeneration, but these were expensive and produced inhibitory byproducts. The second generation incorporated substrate-level phosphorylation pathways using cheaper energy sources like glucose, but still faced limitations in energy efficiency.
Modern CFPS platforms now integrate more complex metabolic networks, including glycolysis, pentose phosphate pathway, and oxidative phosphorylation components. These artificial metabolic systems can utilize diverse carbon sources and maintain redox balance, significantly extending reaction durations from hours to days and improving protein yields by orders of magnitude.
The integration of artificial metabolism with CFPS has enabled numerous applications beyond simple protein production. These include the synthesis of complex proteins with post-translational modifications, the production of toxic proteins that would inhibit cell growth in vivo, and the rapid prototyping of metabolic pathways for synthetic biology applications.
Recent technological breakthroughs have further expanded CFPS capabilities through the incorporation of non-native metabolic pathways. These innovations allow for the incorporation of non-canonical amino acids, the synthesis of glycoproteins, and the production of membrane proteins in artificial lipid environments. The convergence of artificial metabolism with CFPS represents a paradigm shift in protein production technology, offering unprecedented control over the biochemical environment.
The field continues to evolve rapidly, with researchers developing increasingly sophisticated artificial metabolic networks that can be tailored to specific protein production requirements. These advances are positioning CFPS as a versatile platform for applications ranging from biopharmaceutical production to biosensing and synthetic cell construction.
Market Applications for Cell-free Metabolic Systems
Cell-free metabolic systems represent a transformative platform with diverse market applications across multiple industries. The pharmaceutical sector stands as a primary beneficiary, utilizing these systems for rapid prototyping and production of therapeutic proteins, vaccines, and personalized medicines. This technology enables pharmaceutical companies to accelerate drug discovery processes by quickly synthesizing candidate proteins for testing, significantly reducing development timelines from years to months.
The diagnostics industry has embraced cell-free systems for developing point-of-care testing solutions. These portable biosensors can detect pathogens, biomarkers, and environmental contaminants with high sensitivity and specificity. Companies like Sherlock Biosciences have leveraged CRISPR-based cell-free diagnostic platforms for rapid detection of infectious diseases, demonstrating the commercial viability of this approach.
In the biomanufacturing sector, cell-free metabolic systems offer sustainable alternatives for producing high-value biochemicals, biofuels, and biomaterials. The ability to engineer metabolic pathways without cellular constraints allows for the synthesis of compounds that would be toxic to living cells. This capability has attracted significant interest from chemical manufacturers seeking greener production methods and novel compounds.
The agricultural industry has begun exploring cell-free systems for crop protection and enhancement. These platforms can produce biopesticides, growth promoters, and other agricultural biologics with greater precision and environmental safety compared to traditional chemical approaches. Additionally, they enable rapid screening of plant-beneficial compounds before field testing.
Research tools represent another substantial market, with cell-free systems being adopted by academic and industrial laboratories for protein characterization, enzyme engineering, and synthetic biology applications. Companies like New England Biolabs and Promega have developed commercial kits based on cell-free protein synthesis, indicating growing market demand.
Emerging applications include environmental remediation, where engineered cell-free metabolic pathways can degrade pollutants, and cosmetics, where they can produce specialized ingredients with enhanced purity profiles. The food industry has also shown interest in using these systems to develop novel flavors, preservatives, and nutritional supplements.
The global market for cell-free protein synthesis alone was valued at approximately $250 million in 2021 and is projected to grow at a compound annual growth rate of 10-12% through 2028. When considering broader applications of cell-free metabolic systems, the potential market size expands significantly, potentially reaching several billion dollars within the next decade as adoption increases across industries.
The diagnostics industry has embraced cell-free systems for developing point-of-care testing solutions. These portable biosensors can detect pathogens, biomarkers, and environmental contaminants with high sensitivity and specificity. Companies like Sherlock Biosciences have leveraged CRISPR-based cell-free diagnostic platforms for rapid detection of infectious diseases, demonstrating the commercial viability of this approach.
In the biomanufacturing sector, cell-free metabolic systems offer sustainable alternatives for producing high-value biochemicals, biofuels, and biomaterials. The ability to engineer metabolic pathways without cellular constraints allows for the synthesis of compounds that would be toxic to living cells. This capability has attracted significant interest from chemical manufacturers seeking greener production methods and novel compounds.
The agricultural industry has begun exploring cell-free systems for crop protection and enhancement. These platforms can produce biopesticides, growth promoters, and other agricultural biologics with greater precision and environmental safety compared to traditional chemical approaches. Additionally, they enable rapid screening of plant-beneficial compounds before field testing.
Research tools represent another substantial market, with cell-free systems being adopted by academic and industrial laboratories for protein characterization, enzyme engineering, and synthetic biology applications. Companies like New England Biolabs and Promega have developed commercial kits based on cell-free protein synthesis, indicating growing market demand.
Emerging applications include environmental remediation, where engineered cell-free metabolic pathways can degrade pollutants, and cosmetics, where they can produce specialized ingredients with enhanced purity profiles. The food industry has also shown interest in using these systems to develop novel flavors, preservatives, and nutritional supplements.
The global market for cell-free protein synthesis alone was valued at approximately $250 million in 2021 and is projected to grow at a compound annual growth rate of 10-12% through 2028. When considering broader applications of cell-free metabolic systems, the potential market size expands significantly, potentially reaching several billion dollars within the next decade as adoption increases across industries.
Technical Challenges in Metabolic Integration
The integration of artificial metabolism into cell-free protein synthesis (CFPS) systems faces several significant technical challenges that currently limit its widespread application and efficiency. One of the primary obstacles is the maintenance of metabolic pathway stability over extended reaction periods. Unlike living cells that continuously regenerate enzymes and cofactors, CFPS systems experience gradual degradation of these components, leading to diminished metabolic activity over time.
Energy regeneration represents another critical challenge. Traditional CFPS systems rely on high-energy phosphate compounds like phosphoenolpyruvate or creatine phosphate, which are expensive and can accumulate inhibitory byproducts. Implementing complete metabolic cycles for ATP regeneration requires precise balancing of multiple enzymatic reactions, with each enzyme having distinct optimal conditions for activity.
Cofactor regeneration and maintenance pose similar difficulties. Essential cofactors such as NAD(P)H, ATP, and coenzyme A are consumed during protein synthesis and metabolic reactions. Their continuous regeneration requires additional enzymatic pathways that must function efficiently within the artificial environment of CFPS systems, often competing with the primary protein synthesis reactions for resources.
The spatial organization of metabolic pathways presents a unique challenge in CFPS. In living cells, metabolism benefits from compartmentalization and enzyme clustering that enhance reaction efficiency through substrate channeling. Recreating these spatial arrangements in cell-free systems requires innovative approaches such as enzyme immobilization, synthetic scaffolds, or liposome encapsulation, each with its own technical complexities.
Redox balance maintenance is particularly problematic in artificial metabolic systems. The accumulation of reducing equivalents or oxidizing agents can rapidly disrupt metabolic processes. Without cellular regulatory mechanisms, CFPS systems require careful engineering to maintain appropriate redox conditions throughout the reaction period.
Scaling up CFPS with integrated metabolism introduces additional challenges related to oxygen transfer, heat dissipation, and mixing efficiency. These parameters, easily managed in small-scale reactions, become significant hurdles when transitioning to industrial production scales.
Furthermore, the integration of complex metabolic pathways often results in unpredictable interactions and competition for resources. Metabolic flux analysis and modeling of these systems remain difficult due to the absence of cellular regulatory mechanisms and the dynamic nature of the cell-free environment. This unpredictability hampers rational design approaches and necessitates extensive empirical optimization.
Finally, the cost-effectiveness of implementing artificial metabolism in CFPS systems remains a significant barrier to commercial adoption. The purification of multiple enzymes and cofactors substantially increases production costs compared to simpler CFPS approaches, limiting practical applications despite potential performance advantages.
Energy regeneration represents another critical challenge. Traditional CFPS systems rely on high-energy phosphate compounds like phosphoenolpyruvate or creatine phosphate, which are expensive and can accumulate inhibitory byproducts. Implementing complete metabolic cycles for ATP regeneration requires precise balancing of multiple enzymatic reactions, with each enzyme having distinct optimal conditions for activity.
Cofactor regeneration and maintenance pose similar difficulties. Essential cofactors such as NAD(P)H, ATP, and coenzyme A are consumed during protein synthesis and metabolic reactions. Their continuous regeneration requires additional enzymatic pathways that must function efficiently within the artificial environment of CFPS systems, often competing with the primary protein synthesis reactions for resources.
The spatial organization of metabolic pathways presents a unique challenge in CFPS. In living cells, metabolism benefits from compartmentalization and enzyme clustering that enhance reaction efficiency through substrate channeling. Recreating these spatial arrangements in cell-free systems requires innovative approaches such as enzyme immobilization, synthetic scaffolds, or liposome encapsulation, each with its own technical complexities.
Redox balance maintenance is particularly problematic in artificial metabolic systems. The accumulation of reducing equivalents or oxidizing agents can rapidly disrupt metabolic processes. Without cellular regulatory mechanisms, CFPS systems require careful engineering to maintain appropriate redox conditions throughout the reaction period.
Scaling up CFPS with integrated metabolism introduces additional challenges related to oxygen transfer, heat dissipation, and mixing efficiency. These parameters, easily managed in small-scale reactions, become significant hurdles when transitioning to industrial production scales.
Furthermore, the integration of complex metabolic pathways often results in unpredictable interactions and competition for resources. Metabolic flux analysis and modeling of these systems remain difficult due to the absence of cellular regulatory mechanisms and the dynamic nature of the cell-free environment. This unpredictability hampers rational design approaches and necessitates extensive empirical optimization.
Finally, the cost-effectiveness of implementing artificial metabolism in CFPS systems remains a significant barrier to commercial adoption. The purification of multiple enzymes and cofactors substantially increases production costs compared to simpler CFPS approaches, limiting practical applications despite potential performance advantages.
Current Metabolic Integration Approaches
01 Energy regeneration systems for CFPS
Cell-free protein synthesis (CFPS) systems can be enhanced by incorporating energy regeneration mechanisms that mimic cellular metabolism. These systems typically include ATP regeneration pathways, cofactor recycling components, and energy-rich substrates to sustain protein production over extended periods. By integrating artificial metabolic pathways that continuously replenish ATP and other high-energy molecules, the efficiency and duration of cell-free protein synthesis can be significantly improved compared to conventional batch reactions.- Energy regeneration systems for CFPS: Energy regeneration systems are crucial for enhancing the efficiency of cell-free protein synthesis (CFPS). These systems involve the integration of metabolic pathways that continuously regenerate ATP and other energy-rich compounds needed for protein synthesis. By incorporating enzymes that catalyze the regeneration of ATP from secondary energy sources, the duration and yield of CFPS reactions can be significantly improved. These systems often utilize glycolysis, oxidative phosphorylation mimics, or other metabolic pathways to maintain energy supply during protein production.
- Cofactor regeneration for sustained CFPS: Efficient cofactor regeneration is essential for maintaining the productivity of cell-free protein synthesis systems. This approach involves the integration of artificial metabolic cycles that continuously regenerate critical cofactors such as NAD+/NADH, NADP+/NADPH, and other redox molecules required for protein synthesis. By incorporating specific enzymes or enzyme cascades that facilitate cofactor recycling, the system can operate continuously without the need for external supplementation. This regeneration strategy significantly improves the cost-effectiveness and sustainability of cell-free protein production.
- Artificial metabolic modules for CFPS optimization: Artificial metabolic modules can be designed and integrated into cell-free protein synthesis systems to optimize specific aspects of protein production. These engineered metabolic pathways can be tailored to provide precursors for amino acid synthesis, manage byproduct accumulation, or maintain optimal redox conditions. By incorporating synthetic metabolic networks that complement the protein synthesis machinery, researchers can enhance production efficiency, reduce inhibitory effects, and extend reaction lifetimes. These modules often involve rationally designed enzyme cascades that work synergistically with the translation apparatus.
- Microfluidic and compartmentalized CFPS systems: Microfluidic and compartmentalized approaches enhance cell-free protein synthesis by creating controlled microenvironments that mimic cellular compartmentalization. These systems utilize microfluidic devices, liposomes, or other artificial compartments to spatially organize the protein synthesis machinery and associated metabolic pathways. By controlling the diffusion of substrates, products, and inhibitors, these systems can achieve higher efficiency and longer-lasting protein production. The physical separation of different metabolic functions allows for better regulation of reaction conditions and improved integration of artificial metabolism with protein synthesis.
- Genetic circuit integration with CFPS: Genetic circuits can be integrated with cell-free protein synthesis systems to create sophisticated control mechanisms for protein production. These circuits involve engineered DNA constructs that respond to specific signals or conditions, allowing for dynamic regulation of protein synthesis and metabolic activities. By incorporating genetic switches, feedback loops, or cascades into CFPS systems, researchers can achieve programmable protein expression that adapts to changing conditions. This integration enables the development of smart CFPS systems with artificial metabolism that can self-regulate based on substrate availability, product accumulation, or other environmental factors.
02 Continuous-exchange CFPS platforms
Continuous-exchange cell-free protein synthesis platforms represent an advanced approach to improving synthesis efficiency. These systems utilize semipermeable membranes or microfluidic devices to continuously supply fresh substrates while removing inhibitory byproducts. By maintaining optimal reaction conditions through the constant exchange of materials, these platforms can achieve higher protein yields and longer reaction durations than traditional batch systems. The integration of artificial metabolism in these continuous systems further enhances their performance by providing sustained energy supply.Expand Specific Solutions03 Cofactor recycling and stabilization
Efficient cofactor recycling and stabilization are crucial for maintaining artificial metabolism in cell-free protein synthesis systems. This approach involves the addition of enzymes and substrates that regenerate essential cofactors such as NAD+/NADH, NADP+/NADPH, and flavin nucleotides. By incorporating these recycling pathways, the system can maintain redox balance and support continuous protein production. Additionally, stabilizing agents can be added to prevent cofactor degradation, further enhancing the overall efficiency of the integrated metabolic network.Expand Specific Solutions04 Multi-enzyme cascade integration
The integration of multi-enzyme cascades into cell-free protein synthesis systems creates sophisticated artificial metabolic networks that can perform complex biochemical transformations. These cascades typically involve sequential enzymatic reactions that convert primary substrates into the necessary precursors for protein synthesis. By carefully designing these enzyme cascades to work in concert with the translation machinery, researchers can create highly efficient systems that minimize resource consumption and maximize protein yield. This approach often incorporates both native and engineered enzymes to optimize pathway performance.Expand Specific Solutions05 Synthetic cell mimics for CFPS
Synthetic cell mimics represent an advanced approach to cell-free protein synthesis that aims to recreate cellular compartmentalization and organization. These systems utilize liposomes, polymersomes, or other artificial structures to encapsulate the protein synthesis machinery along with artificial metabolic components. By mimicking the spatial organization of cellular processes, these systems can achieve higher efficiency through increased local concentrations of reactants and improved coupling between metabolic and synthetic processes. The compartmentalization also provides protection against inhibitory factors and allows for the creation of concentration gradients that can drive reactions forward.Expand Specific Solutions
Leading Research Groups and Biotech Companies
The integration of Artificial Metabolism in Cell-free Protein Synthesis represents an emerging field at the intersection of synthetic biology and protein engineering. Currently in its early growth phase, this technology is gaining momentum with a projected market size reaching several billion dollars by 2030. The competitive landscape features academic institutions (Tsinghua University, Northwestern University, Cornell) collaborating with specialized biotechnology companies. Leading commercial players include Cellfree Sciences, Leniobio GmbH, and Kangma Biological Technology, who are advancing cell-free systems with metabolic capabilities. Established corporations like Shimadzu and Toyobo are entering the space through strategic partnerships, while research organizations such as RIKEN and The Babraham Institute contribute fundamental scientific advancements, creating a dynamic ecosystem poised for significant technological breakthroughs.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed a proprietary WEPRO® system for cell-free protein synthesis (CFPS) that integrates artificial metabolism components to enhance energy regeneration. Their technology utilizes wheat germ extract as the primary platform, which offers advantages in expressing complex eukaryotic proteins. The company has engineered metabolic pathways that incorporate ATP regeneration systems based on creatine phosphate and creatine kinase, allowing for sustained energy supply during protein synthesis reactions. Additionally, they've implemented secondary energy recycling mechanisms using pyruvate oxidase and acetate kinase to convert byproducts back into usable energy sources. This integrated metabolic network significantly extends reaction duration from typical 2-4 hours to over 24 hours, increasing protein yield by 3-5 fold compared to conventional CFPS systems.
Strengths: Superior expression of complex eukaryotic proteins with proper folding and post-translational modifications; extended reaction longevity through efficient energy recycling; lower inhibition by reaction byproducts. Weaknesses: Higher cost compared to E. coli-based systems; more complex setup requirements; potential scalability limitations for industrial production.
Toyobo Co., Ltd.
Technical Solution: Toyobo has pioneered the PURE (Protein synthesis Using Recombinant Elements) system approach to cell-free protein synthesis with integrated artificial metabolism. Their technology reconstructs the translation machinery using purified components rather than crude cell extracts, allowing precise control over the reaction environment. Toyobo's PURE system incorporates engineered metabolic modules including an enhanced ATP regeneration system based on phosphoenolpyruvate (PEP) and pyruvate kinase, coupled with secondary pathways for recycling nucleotides. They've further developed a continuous-exchange cell-free system that removes inhibitory byproducts while supplying fresh substrates, enabling reaction times exceeding 48 hours. The company has also integrated artificial chaperone systems to improve protein folding efficiency, particularly for membrane proteins and disulfide-bonded proteins that are traditionally challenging to produce.
Strengths: Highly defined system with minimal background reactions; excellent for producing toxic proteins; superior control over reaction conditions allowing optimization for specific proteins. Weaknesses: Higher production costs due to purified components; lower protein yields compared to extract-based systems; requires more specialized expertise to operate effectively.
Key Innovations in Artificial Metabolism
Cell-free protein synthesizing method by continuous energy supply system using intracellular component
PatentWO2005003341A1
Innovation
- A continuous energy supply system utilizing a fraction mainly composed of endoplasmic reticulum components, such as microsomal fractions from animal or yeast cells, where energy sources are separated from synthesis components using diffusion overlay or semipermeable membrane methods to maintain protein synthesis efficiency over extended periods.
Cell-free synthesizing method for protein
PatentInactiveJP2005312436A
Innovation
- A cell-free protein synthesis system using a reaction solution containing ribosomes, mRNA, substrate amino acids, ATP, GTP, magnesium ions, creatine phosphate, and creatine phosphate kinase, with periodic addition of creatine phosphate or its salt to sustain the synthesis reaction.
Biosafety and Containment Considerations
The integration of artificial metabolism in cell-free protein synthesis (CFPS) systems presents unique biosafety challenges that require careful consideration. Unlike traditional contained cellular systems, CFPS operates without intact cell membranes, potentially increasing exposure risks to biological components. This fundamental difference necessitates specialized containment strategies beyond conventional biosafety protocols designed for whole-cell systems.
Primary biosafety concerns include the potential for reconstitution of infectious agents, horizontal gene transfer of engineered genetic elements, and unintended environmental release of synthetic biological components. The cell-free nature of these systems means that metabolic pathways and protein products exist in solution rather than within cellular compartments, creating novel exposure pathways that must be addressed through comprehensive risk assessment frameworks.
Physical containment measures for CFPS systems should incorporate multiple redundant barriers. These typically include sealed reaction vessels, negative pressure environments, HEPA filtration systems, and dedicated equipment that remains within controlled laboratory spaces. For industrial applications, closed-loop processing systems with integrated decontamination capabilities represent the gold standard for preventing environmental release.
Chemical inactivation protocols specific to cell-free systems require optimization beyond standard approaches. Traditional decontamination methods may be insufficient for certain synthetic components with enhanced stability. Research indicates that combinations of detergents, nucleases, and proteases are more effective for complete inactivation of CFPS reaction components than single-agent approaches.
Regulatory frameworks for CFPS technologies remain underdeveloped in many jurisdictions, creating compliance challenges. Current regulations primarily address contained use of genetically modified organisms rather than cell-free systems. Industry stakeholders should engage proactively with regulatory bodies to develop appropriate guidelines that balance innovation with responsible development.
Emerging biosecurity considerations include the potential dual-use applications of CFPS technology. The portability and simplified infrastructure requirements of cell-free systems lower technical barriers for both beneficial and potentially harmful applications. Implementation of secure laboratory practices, comprehensive personnel training, and careful documentation of materials transfer becomes particularly important in this context.
Risk assessment methodologies for artificial metabolism in CFPS should incorporate both component-level and system-level analyses. This includes evaluation of individual metabolic pathways, genetic elements, and protein products, as well as emergent properties that may arise from their integration. Quantitative risk models specifically calibrated for cell-free systems represent an important area for future development.
Primary biosafety concerns include the potential for reconstitution of infectious agents, horizontal gene transfer of engineered genetic elements, and unintended environmental release of synthetic biological components. The cell-free nature of these systems means that metabolic pathways and protein products exist in solution rather than within cellular compartments, creating novel exposure pathways that must be addressed through comprehensive risk assessment frameworks.
Physical containment measures for CFPS systems should incorporate multiple redundant barriers. These typically include sealed reaction vessels, negative pressure environments, HEPA filtration systems, and dedicated equipment that remains within controlled laboratory spaces. For industrial applications, closed-loop processing systems with integrated decontamination capabilities represent the gold standard for preventing environmental release.
Chemical inactivation protocols specific to cell-free systems require optimization beyond standard approaches. Traditional decontamination methods may be insufficient for certain synthetic components with enhanced stability. Research indicates that combinations of detergents, nucleases, and proteases are more effective for complete inactivation of CFPS reaction components than single-agent approaches.
Regulatory frameworks for CFPS technologies remain underdeveloped in many jurisdictions, creating compliance challenges. Current regulations primarily address contained use of genetically modified organisms rather than cell-free systems. Industry stakeholders should engage proactively with regulatory bodies to develop appropriate guidelines that balance innovation with responsible development.
Emerging biosecurity considerations include the potential dual-use applications of CFPS technology. The portability and simplified infrastructure requirements of cell-free systems lower technical barriers for both beneficial and potentially harmful applications. Implementation of secure laboratory practices, comprehensive personnel training, and careful documentation of materials transfer becomes particularly important in this context.
Risk assessment methodologies for artificial metabolism in CFPS should incorporate both component-level and system-level analyses. This includes evaluation of individual metabolic pathways, genetic elements, and protein products, as well as emergent properties that may arise from their integration. Quantitative risk models specifically calibrated for cell-free systems represent an important area for future development.
Scalability and Industrial Implementation
The scalability of cell-free protein synthesis (CFPS) systems with integrated artificial metabolism represents a critical challenge for industrial implementation. Current laboratory-scale CFPS reactions typically range from microliters to milliliters, which is insufficient for commercial production demands. The transition to industrial scales requires addressing several key engineering challenges, particularly maintaining reaction homogeneity and efficient energy regeneration across larger volumes.
Recent advancements in bioreactor design have shown promising results for scaling CFPS systems. Continuous-flow reactors have demonstrated the ability to maintain protein synthesis for extended periods (up to 24 hours) compared to batch reactions that typically plateau after 4-6 hours. These systems allow for the continuous addition of substrates and removal of inhibitory byproducts, significantly enhancing productivity and cost-effectiveness.
The integration of artificial metabolic pathways introduces additional complexity to scaling efforts. Maintaining optimal concentrations of cofactors and intermediates becomes increasingly difficult at larger scales. Research indicates that immobilization techniques, where enzymes are attached to solid supports or encapsulated in microcompartments, can improve stability and reusability of the metabolic components, making larger-scale operations more feasible.
Economic analysis reveals that raw material costs, particularly for energy substrates and nucleotides, remain the primary barrier to industrial implementation. The development of more efficient artificial metabolic pathways that can utilize cheaper, renewable substrates could significantly reduce production costs. Companies like Sutro Biopharma and GreenLight Biosciences have made substantial progress in this direction, developing proprietary CFPS platforms with integrated metabolism that operate at 100-liter scales.
Regulatory considerations also impact industrial implementation. The regulatory pathway for products manufactured using CFPS with artificial metabolism is still evolving. However, the closed nature of these systems, with defined components, potentially offers advantages in terms of product consistency and quality control compared to traditional cell-based manufacturing.
Energy efficiency remains a critical factor for industrial viability. Current artificial metabolic systems in CFPS typically achieve 20-30% conversion efficiency from primary energy substrate to protein product. Computational modeling suggests that optimized pathways could theoretically reach 50-60% efficiency, which would dramatically improve economic feasibility at industrial scales.
Recent advancements in bioreactor design have shown promising results for scaling CFPS systems. Continuous-flow reactors have demonstrated the ability to maintain protein synthesis for extended periods (up to 24 hours) compared to batch reactions that typically plateau after 4-6 hours. These systems allow for the continuous addition of substrates and removal of inhibitory byproducts, significantly enhancing productivity and cost-effectiveness.
The integration of artificial metabolic pathways introduces additional complexity to scaling efforts. Maintaining optimal concentrations of cofactors and intermediates becomes increasingly difficult at larger scales. Research indicates that immobilization techniques, where enzymes are attached to solid supports or encapsulated in microcompartments, can improve stability and reusability of the metabolic components, making larger-scale operations more feasible.
Economic analysis reveals that raw material costs, particularly for energy substrates and nucleotides, remain the primary barrier to industrial implementation. The development of more efficient artificial metabolic pathways that can utilize cheaper, renewable substrates could significantly reduce production costs. Companies like Sutro Biopharma and GreenLight Biosciences have made substantial progress in this direction, developing proprietary CFPS platforms with integrated metabolism that operate at 100-liter scales.
Regulatory considerations also impact industrial implementation. The regulatory pathway for products manufactured using CFPS with artificial metabolism is still evolving. However, the closed nature of these systems, with defined components, potentially offers advantages in terms of product consistency and quality control compared to traditional cell-based manufacturing.
Energy efficiency remains a critical factor for industrial viability. Current artificial metabolic systems in CFPS typically achieve 20-30% conversion efficiency from primary energy substrate to protein product. Computational modeling suggests that optimized pathways could theoretically reach 50-60% efficiency, which would dramatically improve economic feasibility at industrial scales.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!