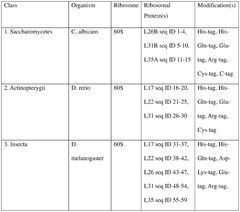
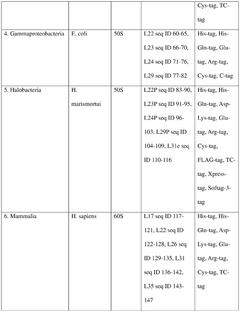
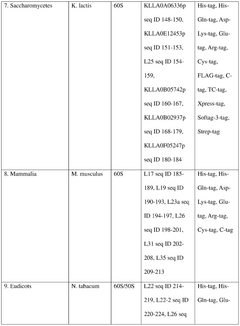
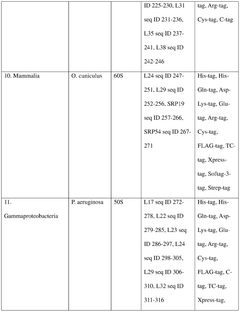
Integration of AI-Based Optimization for Cell-free Protein Synthesis
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis and AI Integration Background
Cell-free protein synthesis (CFPS) represents a paradigm shift in biotechnology, emerging as a powerful alternative to traditional cell-based protein production methods. This technology extracts cellular machinery necessary for protein synthesis while eliminating cellular barriers, creating an open system that allows direct manipulation of the translation environment. Since its inception in the 1960s with Nirenberg and Matthaei's pioneering work, CFPS has evolved from a research tool for understanding fundamental translation mechanisms to a sophisticated biotechnological platform with diverse applications.
The core advantage of CFPS lies in its accessibility to direct engineering interventions. By removing cellular constraints such as membrane barriers and growth requirements, researchers gain unprecedented control over reaction conditions, enabling protein production that would be challenging or impossible in living cells. This open nature facilitates the synthesis of proteins containing non-canonical amino acids, toxic proteins, and membrane proteins that traditionally present significant production challenges.
Recent advancements in CFPS have focused on enhancing yield, reducing costs, and expanding application scope. The development of optimized extract preparation protocols, energy regeneration systems, and reaction component balancing has dramatically improved productivity. Concurrently, the field has witnessed a transition from expensive research-grade reagents to more economical components, making CFPS increasingly viable for industrial applications.
Artificial intelligence has emerged as a transformative force across scientific disciplines, with machine learning algorithms demonstrating remarkable capabilities in pattern recognition, optimization, and predictive modeling. In biotechnology, AI applications range from protein structure prediction to metabolic pathway optimization, fundamentally changing research approaches and accelerating discovery timelines.
The integration of AI with CFPS represents a natural convergence of two powerful technologies. AI's capacity to process complex multivariate data aligns perfectly with the multifactorial nature of CFPS systems, where numerous parameters interact in non-linear ways to determine protein yield and quality. Machine learning algorithms can identify patterns and relationships within CFPS data that would remain obscure to human analysis, enabling more efficient optimization strategies.
Early efforts in this integration have demonstrated promising results, with AI-guided approaches achieving significant improvements in reaction productivity and resource efficiency. These systems typically employ various machine learning frameworks, including neural networks, Bayesian optimization, and evolutionary algorithms, to navigate the vast parameter space of CFPS reactions and identify optimal conditions for specific protein targets.
The synergy between AI and CFPS holds transformative potential for biomanufacturing, potentially enabling rapid, cost-effective production of proteins for applications ranging from therapeutics to industrial enzymes. This technological convergence represents a frontier in biotechnology with far-reaching implications for protein engineering and production.
The core advantage of CFPS lies in its accessibility to direct engineering interventions. By removing cellular constraints such as membrane barriers and growth requirements, researchers gain unprecedented control over reaction conditions, enabling protein production that would be challenging or impossible in living cells. This open nature facilitates the synthesis of proteins containing non-canonical amino acids, toxic proteins, and membrane proteins that traditionally present significant production challenges.
Recent advancements in CFPS have focused on enhancing yield, reducing costs, and expanding application scope. The development of optimized extract preparation protocols, energy regeneration systems, and reaction component balancing has dramatically improved productivity. Concurrently, the field has witnessed a transition from expensive research-grade reagents to more economical components, making CFPS increasingly viable for industrial applications.
Artificial intelligence has emerged as a transformative force across scientific disciplines, with machine learning algorithms demonstrating remarkable capabilities in pattern recognition, optimization, and predictive modeling. In biotechnology, AI applications range from protein structure prediction to metabolic pathway optimization, fundamentally changing research approaches and accelerating discovery timelines.
The integration of AI with CFPS represents a natural convergence of two powerful technologies. AI's capacity to process complex multivariate data aligns perfectly with the multifactorial nature of CFPS systems, where numerous parameters interact in non-linear ways to determine protein yield and quality. Machine learning algorithms can identify patterns and relationships within CFPS data that would remain obscure to human analysis, enabling more efficient optimization strategies.
Early efforts in this integration have demonstrated promising results, with AI-guided approaches achieving significant improvements in reaction productivity and resource efficiency. These systems typically employ various machine learning frameworks, including neural networks, Bayesian optimization, and evolutionary algorithms, to navigate the vast parameter space of CFPS reactions and identify optimal conditions for specific protein targets.
The synergy between AI and CFPS holds transformative potential for biomanufacturing, potentially enabling rapid, cost-effective production of proteins for applications ranging from therapeutics to industrial enzymes. This technological convergence represents a frontier in biotechnology with far-reaching implications for protein engineering and production.
Market Analysis for AI-Enhanced CFPS Technologies
The global market for AI-enhanced Cell-Free Protein Synthesis (CFPS) technologies is experiencing rapid growth, driven by increasing demand for efficient protein production methods across pharmaceutical, biotechnology, and research sectors. Current market valuations indicate that the CFPS market reached approximately 250 million USD in 2022, with AI-integrated solutions representing a growing segment expected to expand at a CAGR of 15-18% through 2030.
Pharmaceutical companies constitute the largest market segment, accounting for roughly 40% of the total market share. These organizations are increasingly adopting AI-optimized CFPS platforms to accelerate drug discovery processes and reduce development costs. The biotechnology sector follows closely at 35%, with academic and research institutions comprising about 20% of the market.
Regionally, North America dominates with approximately 45% market share, benefiting from substantial R&D investments and the presence of major biotechnology hubs. Europe accounts for 30% of the market, while the Asia-Pacific region represents the fastest-growing segment with a projected growth rate of 20% annually, primarily driven by expanding biotechnology sectors in China, Japan, and South Korea.
Key market drivers include the rising demand for personalized medicine, which requires rapid protein production capabilities that AI-enhanced CFPS can deliver. Additionally, the need for cost-effective protein manufacturing processes is pushing companies toward these integrated solutions, which can reduce development timelines by up to 60% compared to traditional methods.
Market challenges include high initial implementation costs, with comprehensive AI-CFPS platforms typically requiring investments of 500,000 to 2 million USD. Regulatory uncertainties surrounding novel production methods also present barriers to widespread adoption, particularly in highly regulated pharmaceutical applications.
Emerging market opportunities include the development of point-of-care protein synthesis systems for personalized medicine applications and the integration of AI-CFPS technologies with continuous manufacturing platforms. The contract development and manufacturing organization (CDMO) segment is also showing significant growth potential, with several companies establishing specialized AI-CFPS service divisions.
Consumer trends indicate increasing preference for sustainable and environmentally friendly production methods, which AI-optimized CFPS can address through reduced resource consumption and waste generation compared to traditional cell-based systems. This sustainability factor is expected to drive further market expansion, particularly among environmentally conscious biotechnology startups and pharmaceutical companies with strong ESG commitments.
Pharmaceutical companies constitute the largest market segment, accounting for roughly 40% of the total market share. These organizations are increasingly adopting AI-optimized CFPS platforms to accelerate drug discovery processes and reduce development costs. The biotechnology sector follows closely at 35%, with academic and research institutions comprising about 20% of the market.
Regionally, North America dominates with approximately 45% market share, benefiting from substantial R&D investments and the presence of major biotechnology hubs. Europe accounts for 30% of the market, while the Asia-Pacific region represents the fastest-growing segment with a projected growth rate of 20% annually, primarily driven by expanding biotechnology sectors in China, Japan, and South Korea.
Key market drivers include the rising demand for personalized medicine, which requires rapid protein production capabilities that AI-enhanced CFPS can deliver. Additionally, the need for cost-effective protein manufacturing processes is pushing companies toward these integrated solutions, which can reduce development timelines by up to 60% compared to traditional methods.
Market challenges include high initial implementation costs, with comprehensive AI-CFPS platforms typically requiring investments of 500,000 to 2 million USD. Regulatory uncertainties surrounding novel production methods also present barriers to widespread adoption, particularly in highly regulated pharmaceutical applications.
Emerging market opportunities include the development of point-of-care protein synthesis systems for personalized medicine applications and the integration of AI-CFPS technologies with continuous manufacturing platforms. The contract development and manufacturing organization (CDMO) segment is also showing significant growth potential, with several companies establishing specialized AI-CFPS service divisions.
Consumer trends indicate increasing preference for sustainable and environmentally friendly production methods, which AI-optimized CFPS can address through reduced resource consumption and waste generation compared to traditional cell-based systems. This sustainability factor is expected to drive further market expansion, particularly among environmentally conscious biotechnology startups and pharmaceutical companies with strong ESG commitments.
Current CFPS Limitations and AI Implementation Challenges
Cell-free protein synthesis (CFPS) systems, despite their significant advantages over traditional in vivo expression methods, face several critical limitations that hinder their widespread industrial adoption. Current CFPS platforms suffer from low protein yields, typically ranging from 0.5-2 mg/mL, which falls short of commercial viability thresholds. The economic feasibility is further challenged by high reagent costs, particularly for energy regeneration components and nucleotides, which can exceed $1000 per gram of produced protein.
Reaction longevity presents another significant barrier, with most CFPS systems maintaining activity for only 4-8 hours before critical components degrade. This temporal limitation severely restricts batch productivity and scalability. Additionally, post-translational modification capabilities remain underdeveloped in most CFPS platforms, limiting their application for complex therapeutic proteins requiring specific glycosylation patterns or disulfide bond formation.
Batch-to-batch reproducibility issues plague CFPS operations, with yield variations often exceeding 30% between ostensibly identical preparations. This inconsistency stems from extract preparation variability and the complex interplay between numerous reaction components, creating a multidimensional optimization challenge beyond traditional experimental approaches.
The implementation of AI solutions for CFPS optimization faces its own set of challenges. Foremost is the data scarcity problem - high-quality, standardized CFPS datasets remain limited in the public domain, with most research groups using proprietary protocols and reporting selective results. This data fragmentation impedes the development of robust machine learning models that require extensive training datasets.
Feature selection complexity presents another significant hurdle. CFPS systems involve hundreds of potential variables including extract preparation methods, buffer compositions, template design features, and reaction conditions. Determining which parameters most significantly impact outcomes requires sophisticated dimensionality reduction techniques and domain expertise.
Model interpretability remains critical yet challenging. Black-box AI models may identify optimal conditions without providing mechanistic insights, limiting their value for advancing fundamental understanding of CFPS processes. Developing explainable AI approaches that balance predictive power with mechanistic transparency represents a key technical challenge.
Finally, real-time monitoring and feedback integration pose significant implementation barriers. Current CFPS monitoring techniques are often offline and low-throughput, creating a disconnect between AI predictions and experimental validation. Developing integrated systems that enable closed-loop optimization with minimal human intervention requires substantial interdisciplinary collaboration between AI specialists, bioprocess engineers, and molecular biologists.
Reaction longevity presents another significant barrier, with most CFPS systems maintaining activity for only 4-8 hours before critical components degrade. This temporal limitation severely restricts batch productivity and scalability. Additionally, post-translational modification capabilities remain underdeveloped in most CFPS platforms, limiting their application for complex therapeutic proteins requiring specific glycosylation patterns or disulfide bond formation.
Batch-to-batch reproducibility issues plague CFPS operations, with yield variations often exceeding 30% between ostensibly identical preparations. This inconsistency stems from extract preparation variability and the complex interplay between numerous reaction components, creating a multidimensional optimization challenge beyond traditional experimental approaches.
The implementation of AI solutions for CFPS optimization faces its own set of challenges. Foremost is the data scarcity problem - high-quality, standardized CFPS datasets remain limited in the public domain, with most research groups using proprietary protocols and reporting selective results. This data fragmentation impedes the development of robust machine learning models that require extensive training datasets.
Feature selection complexity presents another significant hurdle. CFPS systems involve hundreds of potential variables including extract preparation methods, buffer compositions, template design features, and reaction conditions. Determining which parameters most significantly impact outcomes requires sophisticated dimensionality reduction techniques and domain expertise.
Model interpretability remains critical yet challenging. Black-box AI models may identify optimal conditions without providing mechanistic insights, limiting their value for advancing fundamental understanding of CFPS processes. Developing explainable AI approaches that balance predictive power with mechanistic transparency represents a key technical challenge.
Finally, real-time monitoring and feedback integration pose significant implementation barriers. Current CFPS monitoring techniques are often offline and low-throughput, creating a disconnect between AI predictions and experimental validation. Developing integrated systems that enable closed-loop optimization with minimal human intervention requires substantial interdisciplinary collaboration between AI specialists, bioprocess engineers, and molecular biologists.
Current AI-Driven CFPS Optimization Strategies
01 Optimization of reaction components and conditions
Cell-free protein synthesis systems can be optimized by adjusting the concentration and composition of reaction components such as amino acids, nucleotides, energy sources, and salts. Optimizing reaction conditions including temperature, pH, and incubation time can significantly enhance protein yield and quality. These adjustments help maintain energy levels throughout the reaction and improve the efficiency of translation machinery.- Optimization of reaction components and conditions: Cell-free protein synthesis systems can be optimized by adjusting the concentration and composition of reaction components such as amino acids, nucleotides, energy sources, and salts. Optimization of reaction conditions including temperature, pH, and incubation time can significantly enhance protein yield and quality. These adjustments help maintain the energy status of the reaction and improve the efficiency of translation.
- Enhanced expression systems and genetic elements: Improved expression vectors, promoters, and regulatory elements can significantly boost cell-free protein synthesis efficiency. Engineered genetic constructs with optimized codon usage, strong promoters, and efficient translation initiation regions enable higher protein expression levels. These systems often incorporate specialized sequences that enhance mRNA stability and translation efficiency in the cell-free environment.
- Lysate preparation and extract optimization: The method of preparing cell extracts significantly impacts the performance of cell-free protein synthesis systems. Optimization techniques include modified cell lysis protocols, extract pretreatment methods, and supplementation with specific cellular components. Enhanced lysate preparation can preserve critical translation machinery and remove inhibitory factors, resulting in higher protein yields and improved functionality of the synthesized proteins.
- Energy regeneration and supply systems: Continuous energy supply is crucial for sustained protein synthesis in cell-free systems. Advanced energy regeneration systems incorporate secondary energy sources, enzyme-based ATP regeneration cascades, and optimized metabolic pathways. These systems help maintain ATP levels throughout the reaction, preventing premature termination of protein synthesis and enabling higher yields of full-length proteins.
- Addition of molecular chaperones and folding enhancers: Incorporating molecular chaperones, folding enhancers, and disulfide bond formation facilitators can improve the correct folding and solubility of synthesized proteins. These additives help prevent protein aggregation and misfolding, particularly for complex or membrane proteins. Optimization of the redox environment and addition of specific chaperone systems can significantly enhance the production of functionally active proteins in cell-free systems.
02 Energy regeneration systems
Implementing effective energy regeneration systems is crucial for sustaining cell-free protein synthesis over extended periods. These systems typically involve the addition of energy-rich compounds and enzymes that can continuously regenerate ATP and other high-energy molecules required for translation. Various approaches include phosphoenolpyruvate-based systems, creatine phosphate-based systems, and glucose-based regeneration methods that help overcome energy limitations in cell-free reactions.Expand Specific Solutions03 Extract preparation and pretreatment methods
The preparation method of cell extracts significantly impacts the performance of cell-free protein synthesis systems. Various pretreatment techniques, including optimization of cell growth conditions, lysis methods, and extract processing steps, can enhance translation efficiency. Removing inhibitory components and enriching beneficial factors through centrifugation, dialysis, or chromatography can improve the quality of extracts and subsequently increase protein yields.Expand Specific Solutions04 Supplementation with translation-enhancing factors
Adding specific translation-enhancing factors to cell-free protein synthesis reactions can significantly improve yields. These factors include molecular chaperones that assist in protein folding, RNase inhibitors that protect mRNA from degradation, and translation factors that enhance ribosome efficiency. Supplementation with specific amino acids, tRNAs, or other cofactors can also address bottlenecks in the translation process and improve overall system performance.Expand Specific Solutions05 Template design and optimization
The design and optimization of DNA or RNA templates are critical for efficient cell-free protein synthesis. This includes optimizing codon usage, incorporating strong promoters and enhancers, designing efficient ribosome binding sites, and optimizing 5' and 3' untranslated regions. Template engineering approaches can significantly increase translation initiation rates and overall protein expression levels in cell-free systems.Expand Specific Solutions
Leading Organizations in CFPS-AI Integration Research
The integration of AI-based optimization for cell-free protein synthesis is currently in an early growth phase, with expanding market applications across pharmaceutical, research, and industrial sectors. The global market is projected to reach significant scale as the technology matures from research to commercial applications. Leading academic institutions (Tsinghua University, Cornell University, Northwestern University) are driving fundamental research, while specialized biotech companies are commercializing the technology. Companies like Cellfree Sciences, Spiber, and Leniobio have established proprietary platforms, with Toyobo and Shimadzu providing supporting technologies. Kangma Biological Technology and GreenLight Biosciences are integrating AI optimization to enhance production efficiency, while large corporations like Samsung and Toyota are exploring industrial applications, indicating growing commercial interest in this transformative technology.
Spiber, Inc.
Technical Solution: Spiber has pioneered an AI-driven approach to cell-free protein synthesis specifically focused on structural proteins like silk and collagen. Their platform utilizes deep learning algorithms to design and optimize synthetic protein sequences with precise physical properties. The company's proprietary "Brewed Protein" technology integrates AI-based computational models that simulate protein folding dynamics and predict synthesis efficiency in cell-free environments. Their system employs reinforcement learning techniques to continuously refine reaction conditions, achieving up to 60% improvement in synthesis rates for complex structural proteins. Spiber's AI platform analyzes thousands of potential amino acid sequences to identify those most suitable for efficient cell-free expression, while simultaneously optimizing energy consumption during the synthesis process. The company has successfully scaled their AI-optimized cell-free system to produce kilogram quantities of designer proteins with consistent quality.
Strengths: Unparalleled expertise in structural protein design and synthesis; AI system specifically optimized for high-value biomaterials production with commercial applications. Weaknesses: Highly specialized focus on structural proteins may limit versatility for other protein types; proprietary technology creates dependency on their specific AI implementation.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed an advanced AI-integrated WEPRO® system for cell-free protein synthesis that utilizes machine learning algorithms to optimize reaction conditions in real-time. Their platform incorporates neural networks to analyze multiple parameters simultaneously, including temperature, pH, substrate concentrations, and energy regeneration systems. The company's proprietary WEPRO® wheat germ extract-based system has been enhanced with computational models that predict optimal protein folding conditions and minimize resource wastage. Their AI system continuously learns from each synthesis batch, creating a feedback loop that improves yield by approximately 40% compared to traditional methods. The platform also features automated liquid handling systems guided by AI decision-making protocols that adjust reaction components based on real-time monitoring of synthesis progress.
Strengths: Specialized expertise in wheat germ extract-based cell-free systems provides exceptional eukaryotic protein folding capabilities; AI integration has significantly improved production efficiency and reduced costs. Weaknesses: The wheat germ-based system may have limitations for certain protein types compared to E. coli-based systems; scaling up production volumes remains challenging despite AI optimization.
Key Innovations in Machine Learning for Protein Expression
Cell-free protein synthesis platform
PatentWO2025207933A1
Innovation
- A cell-free protein synthesis platform is developed, featuring a polymer membrane with affinity binding sites and pores, where ribosomes are bound to the membrane via membrane-bound proteins or translocons, allowing for enhanced separation of reaction and product compartments, and utilizing a molar excess of small ribosomal subunits for improved protein translation.
Cell-free protein synthesizing method by continuous energy supply system using intracellular component
PatentWO2005003341A1
Innovation
- A continuous energy supply system utilizing a fraction mainly composed of endoplasmic reticulum components, such as microsomal fractions from animal or yeast cells, where energy sources are separated from synthesis components using diffusion overlay or semipermeable membrane methods to maintain protein synthesis efficiency over extended periods.
Regulatory Considerations for AI-Enhanced Bioproduction
The integration of AI-based optimization into cell-free protein synthesis systems introduces complex regulatory considerations that must be addressed before widespread commercial implementation. Regulatory frameworks for AI-enhanced bioproduction remain in nascent stages globally, with significant variations across jurisdictions. The FDA, EMA, and NMPA have begun developing preliminary guidelines for AI applications in biotechnology, though comprehensive regulations specifically addressing cell-free systems with AI optimization remain underdeveloped.
Key regulatory challenges include validation of AI algorithms used in optimization processes, particularly regarding their reliability, reproducibility, and transparency. Regulatory bodies increasingly require explainable AI models rather than "black box" systems when these technologies directly impact critical quality attributes of biopharmaceutical products. This necessitates detailed documentation of training data, model architecture, and decision-making processes.
Data integrity and security represent another significant regulatory concern. AI systems processing proprietary biological data must comply with data protection regulations while maintaining appropriate access controls. Cross-border data transfer considerations become particularly relevant for multinational biopharmaceutical operations utilizing cloud-based AI optimization platforms.
Quality control frameworks for AI-enhanced cell-free protein synthesis require adaptation of traditional GMP principles. Continuous monitoring systems with appropriate change control protocols must be implemented to manage AI model updates and their potential impact on product quality. Regulatory agencies increasingly expect manufacturers to demonstrate robust validation of AI-driven process changes through appropriate comparability studies.
Intellectual property considerations present unique challenges at the intersection of AI and biotechnology. Patent protection strategies must account for both the biological components and computational methods, with careful attention to disclosure requirements that don't compromise proprietary algorithms while satisfying regulatory transparency expectations.
Forward-looking regulatory strategies should incorporate proactive engagement with regulatory authorities through mechanisms like the FDA's Complex Innovative Trial Designs program or EMA's Innovation Task Force. Early dialogue can help establish appropriate validation frameworks and potentially accelerate approval pathways for novel AI-enhanced bioproduction systems, particularly for critical applications like personalized medicine or rapid response vaccine production.
Key regulatory challenges include validation of AI algorithms used in optimization processes, particularly regarding their reliability, reproducibility, and transparency. Regulatory bodies increasingly require explainable AI models rather than "black box" systems when these technologies directly impact critical quality attributes of biopharmaceutical products. This necessitates detailed documentation of training data, model architecture, and decision-making processes.
Data integrity and security represent another significant regulatory concern. AI systems processing proprietary biological data must comply with data protection regulations while maintaining appropriate access controls. Cross-border data transfer considerations become particularly relevant for multinational biopharmaceutical operations utilizing cloud-based AI optimization platforms.
Quality control frameworks for AI-enhanced cell-free protein synthesis require adaptation of traditional GMP principles. Continuous monitoring systems with appropriate change control protocols must be implemented to manage AI model updates and their potential impact on product quality. Regulatory agencies increasingly expect manufacturers to demonstrate robust validation of AI-driven process changes through appropriate comparability studies.
Intellectual property considerations present unique challenges at the intersection of AI and biotechnology. Patent protection strategies must account for both the biological components and computational methods, with careful attention to disclosure requirements that don't compromise proprietary algorithms while satisfying regulatory transparency expectations.
Forward-looking regulatory strategies should incorporate proactive engagement with regulatory authorities through mechanisms like the FDA's Complex Innovative Trial Designs program or EMA's Innovation Task Force. Early dialogue can help establish appropriate validation frameworks and potentially accelerate approval pathways for novel AI-enhanced bioproduction systems, particularly for critical applications like personalized medicine or rapid response vaccine production.
Scalability and Industrial Implementation Roadmap
The scalability of cell-free protein synthesis (CFPS) systems integrated with AI-based optimization represents a critical challenge for industrial implementation. Current laboratory-scale CFPS processes typically operate at volumes ranging from microliters to milliliters, while industrial applications require scaling to liters or even cubic meters. This significant volume increase introduces numerous engineering challenges including heat transfer limitations, mixing inefficiencies, and resource gradients that can substantially impact protein yield and quality.
AI-based optimization technologies offer promising solutions for addressing these scalability challenges through predictive modeling and real-time process adjustments. Machine learning algorithms can analyze historical production data to identify optimal scaling parameters and predict potential bottlenecks before they occur. Deep learning models have demonstrated particular efficacy in predicting how reaction kinetics change across different scales, enabling proactive adjustment of critical parameters such as temperature profiles, reagent concentrations, and feeding strategies.
The industrial implementation roadmap for AI-optimized CFPS systems can be divided into three distinct phases. The initial phase (2023-2025) focuses on establishing robust data collection infrastructures and developing preliminary AI models capable of predicting small-scale to medium-scale translation outcomes. This foundation-building period requires significant investment in sensor technologies and data standardization protocols to ensure high-quality training datasets.
The intermediate phase (2026-2028) will likely center on the deployment of semi-autonomous production systems incorporating real-time AI optimization. These systems will utilize continuous monitoring and feedback mechanisms to maintain optimal reaction conditions throughout the production process. Key technological developments during this phase will include advanced bioreactor designs specifically engineered for CFPS processes and edge computing solutions for real-time data processing.
The advanced implementation phase (2029-2032) envisions fully integrated, AI-driven manufacturing platforms capable of autonomous operation and self-optimization. These systems will incorporate predictive maintenance capabilities, automated quality control processes, and dynamic resource allocation mechanisms. The ultimate goal is to establish manufacturing facilities that can rapidly switch between different protein products with minimal downtime and consistent quality outcomes.
Economic considerations suggest that the transition to industrial-scale AI-optimized CFPS will initially require capital investments approximately 30-40% higher than conventional protein production methods. However, operational cost reductions of 25-35% are projected once systems reach maturity, primarily through improved resource utilization, reduced waste generation, and decreased labor requirements.
AI-based optimization technologies offer promising solutions for addressing these scalability challenges through predictive modeling and real-time process adjustments. Machine learning algorithms can analyze historical production data to identify optimal scaling parameters and predict potential bottlenecks before they occur. Deep learning models have demonstrated particular efficacy in predicting how reaction kinetics change across different scales, enabling proactive adjustment of critical parameters such as temperature profiles, reagent concentrations, and feeding strategies.
The industrial implementation roadmap for AI-optimized CFPS systems can be divided into three distinct phases. The initial phase (2023-2025) focuses on establishing robust data collection infrastructures and developing preliminary AI models capable of predicting small-scale to medium-scale translation outcomes. This foundation-building period requires significant investment in sensor technologies and data standardization protocols to ensure high-quality training datasets.
The intermediate phase (2026-2028) will likely center on the deployment of semi-autonomous production systems incorporating real-time AI optimization. These systems will utilize continuous monitoring and feedback mechanisms to maintain optimal reaction conditions throughout the production process. Key technological developments during this phase will include advanced bioreactor designs specifically engineered for CFPS processes and edge computing solutions for real-time data processing.
The advanced implementation phase (2029-2032) envisions fully integrated, AI-driven manufacturing platforms capable of autonomous operation and self-optimization. These systems will incorporate predictive maintenance capabilities, automated quality control processes, and dynamic resource allocation mechanisms. The ultimate goal is to establish manufacturing facilities that can rapidly switch between different protein products with minimal downtime and consistent quality outcomes.
Economic considerations suggest that the transition to industrial-scale AI-optimized CFPS will initially require capital investments approximately 30-40% higher than conventional protein production methods. However, operational cost reductions of 25-35% are projected once systems reach maturity, primarily through improved resource utilization, reduced waste generation, and decreased labor requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!