Systematic Analysis of Enzyme Activity in Cell-free Protein Synthesis
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis Background and Objectives
Cell-free protein synthesis (CFPS) has evolved significantly since its inception in the 1950s when Nirenberg and Matthaei first demonstrated protein synthesis outside living cells. This technology has transformed from a fundamental research tool for decoding the genetic code to a versatile platform for various biotechnological applications. The evolution of CFPS systems has been marked by continuous improvements in extract preparation methods, energy regeneration systems, and reaction conditions optimization.
The primary objective of systematic enzyme activity analysis in CFPS is to enhance protein yield, reduce production costs, and improve the overall efficiency of the system. By understanding the complex interplay between various enzymatic reactions in cell-free environments, researchers aim to create more robust and predictable protein production platforms. This analysis is crucial for developing standardized protocols that can be reliably implemented across different laboratories and industrial settings.
Current CFPS systems are derived from various organisms including Escherichia coli, wheat germ, rabbit reticulocytes, insect cells, and more recently, CHO cells and human cell lines. Each system offers unique advantages and limitations regarding protein folding capabilities, post-translational modifications, and scalability. The selection of an appropriate CFPS system depends largely on the specific requirements of the target protein and its intended application.
The technological trajectory of CFPS has been significantly influenced by advancements in related fields such as synthetic biology, metabolic engineering, and analytical chemistry. These interdisciplinary connections have facilitated the development of more sophisticated approaches for monitoring and controlling enzymatic activities within cell-free reactions. High-throughput screening methods, real-time monitoring techniques, and computational modeling have become essential tools in this endeavor.
Recent innovations in CFPS technology include the development of continuous-exchange cell-free systems, microfluidic platforms for miniaturized reactions, and the integration of artificial cellular components to mimic specific cellular functions. These advancements have expanded the application scope of CFPS beyond traditional protein production to include protein evolution, biosensor development, and educational tools.
The systematic analysis of enzyme activity in CFPS aims to address several critical challenges, including the limited reaction lifetime due to resource depletion, variability in extract quality, and the accumulation of inhibitory byproducts. By gaining deeper insights into these limitations, researchers can develop targeted strategies to overcome them, thereby unlocking the full potential of CFPS technology for both research and commercial applications.
Looking forward, the field is moving toward more integrated approaches that combine experimental data with computational modeling to predict and optimize enzyme performance in cell-free environments. This holistic understanding will be essential for designing next-generation CFPS systems with enhanced capabilities for complex protein production and novel biotechnological applications.
The primary objective of systematic enzyme activity analysis in CFPS is to enhance protein yield, reduce production costs, and improve the overall efficiency of the system. By understanding the complex interplay between various enzymatic reactions in cell-free environments, researchers aim to create more robust and predictable protein production platforms. This analysis is crucial for developing standardized protocols that can be reliably implemented across different laboratories and industrial settings.
Current CFPS systems are derived from various organisms including Escherichia coli, wheat germ, rabbit reticulocytes, insect cells, and more recently, CHO cells and human cell lines. Each system offers unique advantages and limitations regarding protein folding capabilities, post-translational modifications, and scalability. The selection of an appropriate CFPS system depends largely on the specific requirements of the target protein and its intended application.
The technological trajectory of CFPS has been significantly influenced by advancements in related fields such as synthetic biology, metabolic engineering, and analytical chemistry. These interdisciplinary connections have facilitated the development of more sophisticated approaches for monitoring and controlling enzymatic activities within cell-free reactions. High-throughput screening methods, real-time monitoring techniques, and computational modeling have become essential tools in this endeavor.
Recent innovations in CFPS technology include the development of continuous-exchange cell-free systems, microfluidic platforms for miniaturized reactions, and the integration of artificial cellular components to mimic specific cellular functions. These advancements have expanded the application scope of CFPS beyond traditional protein production to include protein evolution, biosensor development, and educational tools.
The systematic analysis of enzyme activity in CFPS aims to address several critical challenges, including the limited reaction lifetime due to resource depletion, variability in extract quality, and the accumulation of inhibitory byproducts. By gaining deeper insights into these limitations, researchers can develop targeted strategies to overcome them, thereby unlocking the full potential of CFPS technology for both research and commercial applications.
Looking forward, the field is moving toward more integrated approaches that combine experimental data with computational modeling to predict and optimize enzyme performance in cell-free environments. This holistic understanding will be essential for designing next-generation CFPS systems with enhanced capabilities for complex protein production and novel biotechnological applications.
Market Applications and Demand Analysis
Cell-free protein synthesis (CFPS) systems have witnessed significant market growth in recent years, driven by their versatility and efficiency in producing proteins without the constraints of living cells. The global market for CFPS was valued at approximately $250 million in 2022 and is projected to grow at a CAGR of 8-10% through 2030, reflecting the increasing adoption across multiple industries.
The pharmaceutical sector represents the largest market segment for CFPS technologies, accounting for nearly 45% of the total market share. Within this sector, the demand is primarily fueled by applications in therapeutic protein production, vaccine development, and antibody manufacturing. The ability to rapidly produce complex proteins with post-translational modifications has positioned CFPS as a valuable tool for drug discovery and development processes.
Diagnostic applications constitute another significant market segment, particularly for point-of-care testing and biosensors. The COVID-19 pandemic accelerated the adoption of CFPS in rapid diagnostic kit development, demonstrating its utility in addressing public health emergencies. This application area is expected to grow substantially as healthcare systems worldwide continue to emphasize early detection and personalized medicine approaches.
The research reagent market represents a stable and growing demand sector for CFPS technologies. Academic and industrial research laboratories utilize CFPS systems for protein characterization, structural biology studies, and enzyme engineering. The systematic analysis of enzyme activity in CFPS environments has become particularly valuable for understanding protein function and optimizing biocatalytic processes.
Emerging applications in synthetic biology and biomanufacturing are creating new market opportunities. Companies are increasingly exploring CFPS for the production of industrial enzymes, biofuels, and biomaterials. The ability to precisely control reaction conditions and rapidly iterate designs makes CFPS an attractive platform for developing sustainable manufacturing processes.
Regional market analysis reveals that North America dominates the CFPS market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing biotechnology investments in China, Japan, and South Korea. Government initiatives supporting bioeconomy development in these countries are creating favorable conditions for CFPS technology adoption.
Customer demand analysis indicates growing interest in scalable and cost-effective CFPS systems that can transition from research applications to commercial production. End-users are particularly seeking solutions that offer improved enzyme stability, extended reaction durations, and simplified workflow integration. These market requirements are shaping the development priorities for next-generation CFPS technologies focused on systematic enzyme activity optimization.
The pharmaceutical sector represents the largest market segment for CFPS technologies, accounting for nearly 45% of the total market share. Within this sector, the demand is primarily fueled by applications in therapeutic protein production, vaccine development, and antibody manufacturing. The ability to rapidly produce complex proteins with post-translational modifications has positioned CFPS as a valuable tool for drug discovery and development processes.
Diagnostic applications constitute another significant market segment, particularly for point-of-care testing and biosensors. The COVID-19 pandemic accelerated the adoption of CFPS in rapid diagnostic kit development, demonstrating its utility in addressing public health emergencies. This application area is expected to grow substantially as healthcare systems worldwide continue to emphasize early detection and personalized medicine approaches.
The research reagent market represents a stable and growing demand sector for CFPS technologies. Academic and industrial research laboratories utilize CFPS systems for protein characterization, structural biology studies, and enzyme engineering. The systematic analysis of enzyme activity in CFPS environments has become particularly valuable for understanding protein function and optimizing biocatalytic processes.
Emerging applications in synthetic biology and biomanufacturing are creating new market opportunities. Companies are increasingly exploring CFPS for the production of industrial enzymes, biofuels, and biomaterials. The ability to precisely control reaction conditions and rapidly iterate designs makes CFPS an attractive platform for developing sustainable manufacturing processes.
Regional market analysis reveals that North America dominates the CFPS market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by increasing biotechnology investments in China, Japan, and South Korea. Government initiatives supporting bioeconomy development in these countries are creating favorable conditions for CFPS technology adoption.
Customer demand analysis indicates growing interest in scalable and cost-effective CFPS systems that can transition from research applications to commercial production. End-users are particularly seeking solutions that offer improved enzyme stability, extended reaction durations, and simplified workflow integration. These market requirements are shaping the development priorities for next-generation CFPS technologies focused on systematic enzyme activity optimization.
Current Enzyme Activity Analysis Challenges
The systematic analysis of enzyme activity in cell-free protein synthesis (CFPS) systems faces several significant challenges that impede progress in this rapidly evolving field. Current analytical methods often struggle with the complex, multi-component nature of CFPS environments, where numerous enzymes operate simultaneously in a dynamic biochemical milieu.
One primary challenge is the lack of standardized protocols for measuring enzyme kinetics in CFPS systems. Unlike purified enzyme assays, CFPS contains multiple competing reactions and pathways that can interfere with accurate activity measurements. This complexity makes it difficult to isolate and quantify the contribution of individual enzymes to the overall protein synthesis process.
Real-time monitoring of enzyme activities presents another substantial hurdle. Most current techniques provide only endpoint measurements or require sampling that disrupts the reaction environment. Non-invasive, continuous monitoring technologies are limited in their ability to track multiple enzymatic activities simultaneously without interfering with the synthesis process itself.
The heterogeneity of enzyme behavior in CFPS systems further complicates analysis. Enzymes may exhibit different kinetic properties in cell-free environments compared to their in vivo counterparts due to altered substrate concentrations, absence of cellular compartmentalization, and different cofactor availability. These variations make it challenging to apply traditional enzyme kinetic models developed for purified systems.
Analytical sensitivity remains problematic, particularly for low-abundance enzymes that nonetheless play critical roles in CFPS efficiency. Current detection methods often lack the sensitivity to measure subtle changes in activity that may significantly impact overall system performance. This limitation is especially pronounced when analyzing rate-limiting steps in complex enzymatic cascades.
Cross-reactivity between components in CFPS systems frequently leads to analytical interference. Substrates, intermediates, and products from various reactions can affect assay specificity, resulting in misleading activity measurements. The development of highly selective detection methods remains an ongoing challenge.
Temperature and pH stability of analytical reagents also presents difficulties, as CFPS systems often operate under conditions that may compromise the reliability of activity assays. Many fluorescent probes and coupled enzyme assays exhibit temperature-dependent behavior that can confound accurate activity determination during extended synthesis reactions.
Finally, data interpretation challenges arise from the non-linear relationships between enzyme activities and protein synthesis outcomes. Establishing clear correlations between measured enzymatic parameters and CFPS productivity requires sophisticated mathematical modeling approaches that are still being developed for these complex biological systems.
One primary challenge is the lack of standardized protocols for measuring enzyme kinetics in CFPS systems. Unlike purified enzyme assays, CFPS contains multiple competing reactions and pathways that can interfere with accurate activity measurements. This complexity makes it difficult to isolate and quantify the contribution of individual enzymes to the overall protein synthesis process.
Real-time monitoring of enzyme activities presents another substantial hurdle. Most current techniques provide only endpoint measurements or require sampling that disrupts the reaction environment. Non-invasive, continuous monitoring technologies are limited in their ability to track multiple enzymatic activities simultaneously without interfering with the synthesis process itself.
The heterogeneity of enzyme behavior in CFPS systems further complicates analysis. Enzymes may exhibit different kinetic properties in cell-free environments compared to their in vivo counterparts due to altered substrate concentrations, absence of cellular compartmentalization, and different cofactor availability. These variations make it challenging to apply traditional enzyme kinetic models developed for purified systems.
Analytical sensitivity remains problematic, particularly for low-abundance enzymes that nonetheless play critical roles in CFPS efficiency. Current detection methods often lack the sensitivity to measure subtle changes in activity that may significantly impact overall system performance. This limitation is especially pronounced when analyzing rate-limiting steps in complex enzymatic cascades.
Cross-reactivity between components in CFPS systems frequently leads to analytical interference. Substrates, intermediates, and products from various reactions can affect assay specificity, resulting in misleading activity measurements. The development of highly selective detection methods remains an ongoing challenge.
Temperature and pH stability of analytical reagents also presents difficulties, as CFPS systems often operate under conditions that may compromise the reliability of activity assays. Many fluorescent probes and coupled enzyme assays exhibit temperature-dependent behavior that can confound accurate activity determination during extended synthesis reactions.
Finally, data interpretation challenges arise from the non-linear relationships between enzyme activities and protein synthesis outcomes. Establishing clear correlations between measured enzymatic parameters and CFPS productivity requires sophisticated mathematical modeling approaches that are still being developed for these complex biological systems.
Established Enzyme Activity Measurement Methods
01 Cell-free protein synthesis systems and components
Cell-free protein synthesis systems utilize extracted cellular components to produce proteins outside of living cells. These systems typically include ribosomes, translation factors, aminoacyl-tRNA synthetases, and other enzymes necessary for transcription and translation. The optimization of these components can significantly enhance protein yield and activity. Various approaches involve using extracts from different organisms, supplementing with specific enzymes, or engineering the components to improve stability and efficiency.- Cell-free protein synthesis systems and components: Cell-free protein synthesis systems utilize extracted cellular components to produce proteins outside of living cells. These systems typically include ribosomes, translation factors, aminoacyl-tRNA synthetases, and other enzymes necessary for transcription and translation. The optimization of these components can enhance the efficiency and yield of protein production. Various formulations have been developed to maintain enzyme activity and stability during the synthesis process.
- Methods for enhancing enzyme activity in cell-free systems: Various methods have been developed to enhance enzyme activity in cell-free protein synthesis systems. These include the optimization of reaction conditions such as pH, temperature, and ionic strength, as well as the addition of stabilizing agents and cofactors. Some approaches involve the use of engineered enzymes with improved stability or activity. Continuous-exchange cell-free systems that remove inhibitory byproducts and replenish substrates can also maintain enzyme activity for extended periods.
- Monitoring and measuring enzyme activity in cell-free systems: Techniques for monitoring and measuring enzyme activity in cell-free protein synthesis systems are essential for optimizing and controlling the synthesis process. These methods include spectrophotometric assays, fluorescence-based techniques, and real-time monitoring systems. Advanced analytical tools can detect changes in enzyme activity during the synthesis process, allowing for adjustments to maintain optimal conditions. Some systems incorporate biosensors or reporter proteins to provide immediate feedback on synthesis efficiency.
- Applications of cell-free protein synthesis: Cell-free protein synthesis systems have diverse applications in biotechnology, pharmaceuticals, and research. They are used for the production of difficult-to-express proteins, toxic proteins, and proteins containing non-natural amino acids. These systems enable rapid protein production for drug discovery, vaccine development, and diagnostic applications. The ability to maintain enzyme activity in these systems is crucial for their practical applications in producing functional proteins for therapeutic and industrial purposes.
- Novel formulations and additives for stabilizing enzyme activity: Novel formulations and additives have been developed to stabilize enzyme activity in cell-free protein synthesis systems. These include the use of crowding agents, chaperones, and antioxidants to protect enzymes from denaturation and oxidative damage. Some formulations incorporate liposomes or nanodiscs to mimic cellular environments and enhance enzyme stability. Cryoprotectants and lyophilization techniques allow for the preservation of enzyme activity during storage and transport of cell-free systems, enabling their use in various settings.
02 Methods for enhancing enzyme activity in cell-free systems
Various methods have been developed to enhance enzyme activity in cell-free protein synthesis systems. These include optimizing reaction conditions such as temperature, pH, and ionic strength; adding stabilizing agents; incorporating chaperones to assist in protein folding; and using continuous-exchange systems to remove inhibitory byproducts. Additionally, the addition of specific cofactors, energy regeneration systems, and protease inhibitors can significantly improve the activity and yield of synthesized enzymes.Expand Specific Solutions03 Energy regeneration systems for sustained protein synthesis
Energy regeneration systems are crucial for maintaining ATP levels and extending the duration of cell-free protein synthesis reactions. These systems typically involve enzymes such as pyruvate kinase, creatine kinase, or acetate kinase that regenerate ATP from ADP using high-energy phosphate donors. Advanced systems may incorporate multiple energy sources and recycling pathways to maximize efficiency. The continuous supply of energy enables higher protein yields and maintains enzyme activity over extended periods.Expand Specific Solutions04 Applications of cell-free enzyme systems in diagnostics and therapeutics
Cell-free enzyme systems have diverse applications in diagnostics and therapeutics. They can be used for rapid production of diagnostic proteins, antibodies, and enzymes for point-of-care testing. In therapeutics, these systems enable the synthesis of difficult-to-express proteins, personalized medicines, and vaccines. The ability to quickly produce active enzymes without cellular constraints makes these systems valuable for responding to emerging diseases and for developing novel biocatalysts for pharmaceutical production.Expand Specific Solutions05 Novel formulations and stabilization techniques
Novel formulations and stabilization techniques have been developed to preserve enzyme activity in cell-free protein synthesis systems. These include lyophilization methods that allow for long-term storage of reaction components, microfluidic platforms that optimize reaction conditions, and the incorporation of synthetic polymers or nanoparticles that stabilize enzymes. Additionally, the use of non-standard amino acids and modified tRNAs can produce enzymes with enhanced stability or novel catalytic properties, expanding the utility of cell-free systems.Expand Specific Solutions
Leading Research Groups and Industry Players
Cell-free protein synthesis (CFPS) technology is currently in a growth phase, with the market expanding due to increasing applications in synthetic biology, pharmaceuticals, and diagnostics. The global CFPS market is estimated to reach $3.8 billion by 2027, growing at a CAGR of approximately 8%. Technologically, the field shows varying maturity levels across different applications. Leading players include Sutro Biopharma, which has pioneered CFPS for therapeutic protein development, and Cellfree Sciences, specializing in high-throughput protein expression systems. Academic institutions like Cornell University and Tsinghua University contribute significant research advancements, while companies like Shimadzu Corp. and Toyobo provide essential supporting technologies. Spiber has demonstrated commercial viability through its Brewed Protein materials, while Kangma Biological Technology focuses on diagnostic applications, indicating the technology's expanding commercial potential across multiple sectors.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed the WEPRO® system, a wheat germ extract-based cell-free protein synthesis platform specifically optimized for systematic enzyme activity analysis. Their technology utilizes eukaryotic translation machinery that maintains proper folding and post-translational modifications critical for accurate enzyme function assessment. The company employs a high-throughput screening approach with their WEPRO® system that allows simultaneous analysis of multiple enzymes under varying conditions, enabling comprehensive kinetic parameter determination. Their proprietary RTS (Rapid Translation System) technology incorporates real-time monitoring capabilities through fluorescent or colorimetric reporters that track enzyme activity during the synthesis process itself, providing dynamic data on enzyme formation and function. Cellfree Sciences has also developed specialized reaction chambers with controlled microenvironments that mimic cellular conditions for more physiologically relevant enzyme activity measurements.
Strengths: Superior eukaryotic protein folding capabilities compared to bacterial systems, allowing analysis of complex mammalian enzymes. Their wheat germ extract shows remarkable stability at room temperature, enabling extended reaction times for slow-catalyzing enzymes. Weaknesses: Higher cost compared to bacterial cell-free systems, potentially limiting large-scale applications. The wheat germ extract may contain endogenous enzymes that could interfere with certain activity assays.
Toyobo Co., Ltd.
Technical Solution: Toyobo has developed the PURESYSTEM®, a reconstituted cell-free protein synthesis platform composed entirely of purified components for systematic enzyme activity analysis. This defined system eliminates background enzymatic activities present in extract-based systems, providing an exceptionally clean background for precise enzyme characterization. Their technology incorporates all necessary translation factors, aminoacyl-tRNA synthetases, ribosomes, and energy regeneration components in optimized ratios. Toyobo's PURESYSTEM® features customizable reaction conditions that can be tailored to specific enzyme requirements, including redox potential, cofactor concentrations, and pH optimization. The company has integrated their cell-free platform with microfluidic devices for miniaturized, high-throughput enzyme activity screening. Their system allows for the direct incorporation of isotopically labeled amino acids, facilitating NMR-based structural analysis of synthesized enzymes in correlation with activity measurements. Toyobo has also developed specialized formulations that enhance the synthesis of membrane-associated enzymes while maintaining their native conformations and activities.
Strengths: Exceptional signal-to-noise ratio for enzyme activity measurements due to the absence of contaminating activities. Complete control over reaction components allows precise manipulation of conditions affecting enzyme function. Weaknesses: Higher cost compared to extract-based systems due to the need for purified components. Lower protein yields compared to some extract-based systems, potentially limiting applications requiring substantial enzyme quantities.
Key Innovations in Enzyme Kinetics Monitoring
Method for synthesizing cell-free protein while adjusting ph using enzyme
PatentWO2015190857A1
Innovation
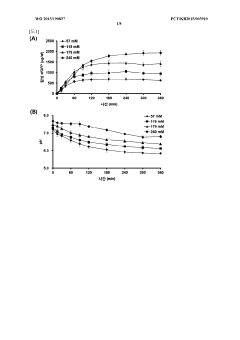
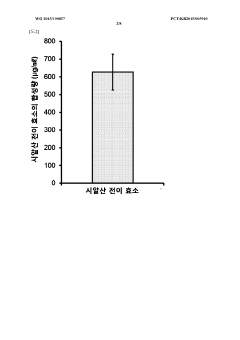
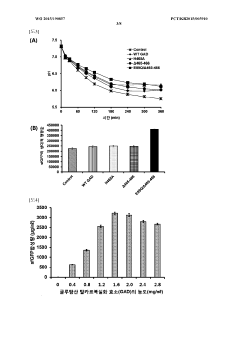
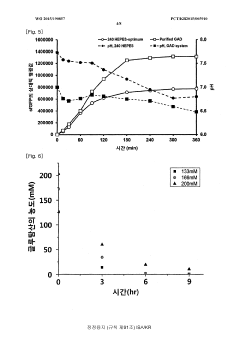
- Employing mutant amino acid decarboxylases, such as glutamic acid decarboxylase, to control pH by consuming glutamic acid and maintaining a buffer-free state, thereby stabilizing the reaction environment and enhancing protein synthesis productivity.
Cell-Free Protein Synthesis
PatentInactiveUS20080153128A1
Innovation
- The method involves amplifying DNA molecules with a stem-loop structure at their 3′-end to prevent degradation and conducting protein synthesis in a cell extract lacking endoribonuclease E (RNase E) activity, enhancing mRNA stability and protein expression.
Regulatory Considerations for CFPS Applications
Cell-free protein synthesis (CFPS) applications are increasingly moving from research settings to commercial and clinical environments, necessitating careful consideration of regulatory frameworks. The regulatory landscape for CFPS technologies spans multiple domains including pharmaceutical regulations, biosafety protocols, and quality control standards. In the United States, the FDA has begun developing specific guidance for cell-free systems used in therapeutic protein production, focusing on consistency, purity, and safety profiles of the final products.
The European Medicines Agency (EMA) has established working groups to address the unique regulatory challenges posed by CFPS technologies, particularly when used for personalized medicine applications. These regulatory bodies emphasize validation of enzyme activity measurement methods, as inconsistent enzyme performance can significantly impact product quality and safety profiles.
Biosafety regulations present another critical consideration, as CFPS systems may contain components derived from pathogenic organisms or genetically modified sources. Comprehensive documentation of enzyme sources, purification methods, and activity characterization is mandatory for regulatory compliance. The WHO has published guidelines specifically addressing biosafety concerns in cell-free systems, recommending standardized testing protocols for enzyme preparations.
Quality control regulations require robust analytical methods for enzyme activity monitoring throughout the CFPS process. Regulatory bodies increasingly demand real-time monitoring capabilities and validation of analytical methods used to characterize enzymatic performance. The International Council for Harmonisation (ICH) guidelines provide frameworks for analytical method validation that are being adapted specifically for CFPS applications.
Intellectual property considerations also influence regulatory approaches, as many CFPS enzyme systems are protected by patents. Regulatory submissions must address potential IP conflicts and licensing requirements, particularly for novel enzyme formulations or proprietary activity enhancement methods.
For diagnostic applications of CFPS, regulations focus on reproducibility and clinical validity. The IVDR (In Vitro Diagnostic Regulation) in Europe and similar frameworks in other regions establish specific requirements for enzyme activity characterization in diagnostic CFPS applications, with particular emphasis on lot-to-lot consistency and shelf-life stability.
Emerging regulatory trends indicate movement toward harmonized international standards for CFPS applications, with collaborative efforts between the FDA, EMA, and regulatory bodies in Asia to establish consistent approaches to enzyme activity assessment and validation. Companies developing CFPS technologies are advised to engage with regulatory authorities early in development processes to align their enzyme activity analysis methods with evolving regulatory expectations.
The European Medicines Agency (EMA) has established working groups to address the unique regulatory challenges posed by CFPS technologies, particularly when used for personalized medicine applications. These regulatory bodies emphasize validation of enzyme activity measurement methods, as inconsistent enzyme performance can significantly impact product quality and safety profiles.
Biosafety regulations present another critical consideration, as CFPS systems may contain components derived from pathogenic organisms or genetically modified sources. Comprehensive documentation of enzyme sources, purification methods, and activity characterization is mandatory for regulatory compliance. The WHO has published guidelines specifically addressing biosafety concerns in cell-free systems, recommending standardized testing protocols for enzyme preparations.
Quality control regulations require robust analytical methods for enzyme activity monitoring throughout the CFPS process. Regulatory bodies increasingly demand real-time monitoring capabilities and validation of analytical methods used to characterize enzymatic performance. The International Council for Harmonisation (ICH) guidelines provide frameworks for analytical method validation that are being adapted specifically for CFPS applications.
Intellectual property considerations also influence regulatory approaches, as many CFPS enzyme systems are protected by patents. Regulatory submissions must address potential IP conflicts and licensing requirements, particularly for novel enzyme formulations or proprietary activity enhancement methods.
For diagnostic applications of CFPS, regulations focus on reproducibility and clinical validity. The IVDR (In Vitro Diagnostic Regulation) in Europe and similar frameworks in other regions establish specific requirements for enzyme activity characterization in diagnostic CFPS applications, with particular emphasis on lot-to-lot consistency and shelf-life stability.
Emerging regulatory trends indicate movement toward harmonized international standards for CFPS applications, with collaborative efforts between the FDA, EMA, and regulatory bodies in Asia to establish consistent approaches to enzyme activity assessment and validation. Companies developing CFPS technologies are advised to engage with regulatory authorities early in development processes to align their enzyme activity analysis methods with evolving regulatory expectations.
Scale-up and Manufacturing Implications
The transition from laboratory-scale cell-free protein synthesis (CFPS) to industrial production presents significant engineering challenges that must be addressed systematically. Current CFPS systems typically operate at milliliter volumes in research settings, while commercial applications require scaling to liters or even hundreds of liters. This scale-up process introduces numerous variables affecting enzyme activity and overall system performance that are not apparent at smaller scales.
Temperature gradients become increasingly problematic in larger reaction vessels, potentially creating heterogeneous microenvironments that affect enzyme kinetics differently throughout the reaction volume. Industrial-scale systems must incorporate sophisticated temperature control mechanisms to maintain optimal enzymatic activity across the entire reaction mixture.
Oxygen transfer limitations represent another critical factor in scaled-up operations. Many enzymes in CFPS systems require specific redox conditions to function optimally. As reaction volumes increase, maintaining consistent oxygen levels throughout becomes challenging, necessitating advanced aeration and mixing technologies that do not compromise protein integrity through excessive shear forces.
Batch-to-batch consistency emerges as a fundamental manufacturing concern for commercial CFPS applications. Enzyme preparations must demonstrate reproducible activity profiles across production runs to ensure product quality. This requires standardized enzyme production protocols, activity assays, and quality control measures specifically designed for the manufacturing environment.
Continuous processing represents a promising alternative to batch production for CFPS scale-up. Such systems allow for constant addition of substrates and removal of products, potentially extending reaction lifetimes by preventing inhibitory byproduct accumulation that can diminish enzyme activity. However, implementing continuous CFPS requires sophisticated bioprocess engineering to maintain enzyme stability over extended operational periods.
Economic considerations ultimately drive manufacturing decisions for CFPS technologies. The cost of enzyme production, particularly for complex multi-enzyme systems, remains a significant barrier to commercialization. Developing cost-effective methods for large-scale enzyme production, such as optimized recombinant expression systems or cell-based production platforms with enhanced yields, will be essential for viable manufacturing operations.
Regulatory compliance adds another layer of complexity to CFPS manufacturing. Enzyme preparations for commercial applications must meet stringent quality standards, particularly for pharmaceutical applications. This necessitates the development of validated analytical methods to characterize enzyme activity, stability, and purity at production scale.
Temperature gradients become increasingly problematic in larger reaction vessels, potentially creating heterogeneous microenvironments that affect enzyme kinetics differently throughout the reaction volume. Industrial-scale systems must incorporate sophisticated temperature control mechanisms to maintain optimal enzymatic activity across the entire reaction mixture.
Oxygen transfer limitations represent another critical factor in scaled-up operations. Many enzymes in CFPS systems require specific redox conditions to function optimally. As reaction volumes increase, maintaining consistent oxygen levels throughout becomes challenging, necessitating advanced aeration and mixing technologies that do not compromise protein integrity through excessive shear forces.
Batch-to-batch consistency emerges as a fundamental manufacturing concern for commercial CFPS applications. Enzyme preparations must demonstrate reproducible activity profiles across production runs to ensure product quality. This requires standardized enzyme production protocols, activity assays, and quality control measures specifically designed for the manufacturing environment.
Continuous processing represents a promising alternative to batch production for CFPS scale-up. Such systems allow for constant addition of substrates and removal of products, potentially extending reaction lifetimes by preventing inhibitory byproduct accumulation that can diminish enzyme activity. However, implementing continuous CFPS requires sophisticated bioprocess engineering to maintain enzyme stability over extended operational periods.
Economic considerations ultimately drive manufacturing decisions for CFPS technologies. The cost of enzyme production, particularly for complex multi-enzyme systems, remains a significant barrier to commercialization. Developing cost-effective methods for large-scale enzyme production, such as optimized recombinant expression systems or cell-based production platforms with enhanced yields, will be essential for viable manufacturing operations.
Regulatory compliance adds another layer of complexity to CFPS manufacturing. Enzyme preparations for commercial applications must meet stringent quality standards, particularly for pharmaceutical applications. This necessitates the development of validated analytical methods to characterize enzyme activity, stability, and purity at production scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!