Cell-free Protein Synthesis Systems for Rapid Biomanufacturing
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CFPS Technology Background and Objectives
Cell-free protein synthesis (CFPS) represents a paradigm shift in biotechnology, evolving from a research tool for understanding fundamental translation mechanisms to a powerful platform for rapid biomanufacturing. The technology originated in the 1960s with Nirenberg and Matthaei's groundbreaking work on deciphering the genetic code using cell extracts. Over subsequent decades, CFPS has undergone significant refinements in extract preparation, energy regeneration systems, and reaction optimization.
The evolution of CFPS technology has been marked by several key milestones. The transition from crude lysates to well-defined systems in the 1990s significantly improved protein yields. The early 2000s saw the development of more cost-effective energy regeneration systems, addressing one of the major economic barriers to large-scale implementation. Recent advances in chassis engineering and reaction optimization have further enhanced productivity and reduced costs, making CFPS increasingly viable for commercial applications.
Current CFPS platforms utilize extracts from various organisms including Escherichia coli, wheat germ, rabbit reticulocytes, insect cells, and CHO cells. Each system offers distinct advantages in terms of protein folding capacity, post-translational modifications, scalability, and cost-effectiveness. E. coli-based systems dominate due to their high yields and relatively low costs, while eukaryotic systems excel in producing complex proteins requiring specific modifications.
The primary objective of CFPS technology development is to establish robust, scalable, and cost-effective platforms for rapid protein production that circumvent the limitations of traditional cell-based expression systems. Specific goals include increasing protein yields, extending reaction durations, enhancing folding of complex proteins, enabling efficient incorporation of non-canonical amino acids, and reducing production costs to competitive levels.
CFPS offers unique advantages for rapid biomanufacturing, including accelerated production timelines (hours versus days/weeks), elimination of cell viability constraints, simplified process development, enhanced control over reaction conditions, and improved safety profiles. These attributes position CFPS as an enabling technology for applications requiring rapid response, such as personalized medicine, point-of-care diagnostics, and emergency vaccine production.
The technology aims to address critical challenges in biomanufacturing, including reducing time-to-market for biologics, enabling decentralized production models, facilitating on-demand manufacturing of unstable proteins, and supporting the development of novel protein therapeutics with unique modifications or structures that are challenging to produce in living cells.
The evolution of CFPS technology has been marked by several key milestones. The transition from crude lysates to well-defined systems in the 1990s significantly improved protein yields. The early 2000s saw the development of more cost-effective energy regeneration systems, addressing one of the major economic barriers to large-scale implementation. Recent advances in chassis engineering and reaction optimization have further enhanced productivity and reduced costs, making CFPS increasingly viable for commercial applications.
Current CFPS platforms utilize extracts from various organisms including Escherichia coli, wheat germ, rabbit reticulocytes, insect cells, and CHO cells. Each system offers distinct advantages in terms of protein folding capacity, post-translational modifications, scalability, and cost-effectiveness. E. coli-based systems dominate due to their high yields and relatively low costs, while eukaryotic systems excel in producing complex proteins requiring specific modifications.
The primary objective of CFPS technology development is to establish robust, scalable, and cost-effective platforms for rapid protein production that circumvent the limitations of traditional cell-based expression systems. Specific goals include increasing protein yields, extending reaction durations, enhancing folding of complex proteins, enabling efficient incorporation of non-canonical amino acids, and reducing production costs to competitive levels.
CFPS offers unique advantages for rapid biomanufacturing, including accelerated production timelines (hours versus days/weeks), elimination of cell viability constraints, simplified process development, enhanced control over reaction conditions, and improved safety profiles. These attributes position CFPS as an enabling technology for applications requiring rapid response, such as personalized medicine, point-of-care diagnostics, and emergency vaccine production.
The technology aims to address critical challenges in biomanufacturing, including reducing time-to-market for biologics, enabling decentralized production models, facilitating on-demand manufacturing of unstable proteins, and supporting the development of novel protein therapeutics with unique modifications or structures that are challenging to produce in living cells.
Market Analysis for Rapid Biomanufacturing Solutions
The global market for rapid biomanufacturing solutions is experiencing significant growth, driven by increasing demand for biologics, personalized medicine, and the need for faster production methods. Cell-free protein synthesis (CFPS) systems represent a revolutionary approach that eliminates the constraints of traditional cell-based manufacturing, offering unprecedented speed and flexibility in protein production.
Current market estimates value the global cell-free protein expression market at approximately $249 million in 2023, with projections indicating a compound annual growth rate (CAGR) of 6.5% through 2030. This growth trajectory is supported by substantial investments in biotechnology and pharmaceutical sectors seeking to reduce time-to-market for critical therapeutics and vaccines.
The COVID-19 pandemic served as a catalyst for market expansion, highlighting the urgent need for rapid response capabilities in biomanufacturing. CFPS systems demonstrated their value during this crisis by enabling quick production of diagnostic reagents and vaccine components, further validating their market potential.
Geographically, North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing biotechnology investments and favorable government policies supporting biomanufacturing innovation.
By application segment, therapeutic protein production represents the largest market share (45%), followed by vaccine development (25%), diagnostic reagents (15%), and research applications (15%). The therapeutic segment's dominance is attributed to the growing pipeline of biologic drugs and increasing adoption of personalized medicine approaches.
Key customer segments include pharmaceutical companies (35%), biotechnology firms (30%), academic and research institutions (20%), and contract manufacturing organizations (15%). Large pharmaceutical companies are increasingly incorporating CFPS technologies into their R&D pipelines, while biotechnology startups are leveraging these systems to accelerate development timelines.
Market drivers include the rising demand for biologics, increasing R&D investments in synthetic biology, growing focus on personalized medicine, and the need for flexible manufacturing solutions. Additionally, the push for sustainable and environmentally friendly production methods favors CFPS systems, which typically require fewer resources than traditional cell-based manufacturing.
Challenges limiting market penetration include high initial setup costs, technical complexities in scaling production, regulatory uncertainties, and competition from established manufacturing methods. However, ongoing technological advancements and increasing commercial validation are gradually addressing these barriers.
Current market estimates value the global cell-free protein expression market at approximately $249 million in 2023, with projections indicating a compound annual growth rate (CAGR) of 6.5% through 2030. This growth trajectory is supported by substantial investments in biotechnology and pharmaceutical sectors seeking to reduce time-to-market for critical therapeutics and vaccines.
The COVID-19 pandemic served as a catalyst for market expansion, highlighting the urgent need for rapid response capabilities in biomanufacturing. CFPS systems demonstrated their value during this crisis by enabling quick production of diagnostic reagents and vaccine components, further validating their market potential.
Geographically, North America dominates the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 20%. The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing biotechnology investments and favorable government policies supporting biomanufacturing innovation.
By application segment, therapeutic protein production represents the largest market share (45%), followed by vaccine development (25%), diagnostic reagents (15%), and research applications (15%). The therapeutic segment's dominance is attributed to the growing pipeline of biologic drugs and increasing adoption of personalized medicine approaches.
Key customer segments include pharmaceutical companies (35%), biotechnology firms (30%), academic and research institutions (20%), and contract manufacturing organizations (15%). Large pharmaceutical companies are increasingly incorporating CFPS technologies into their R&D pipelines, while biotechnology startups are leveraging these systems to accelerate development timelines.
Market drivers include the rising demand for biologics, increasing R&D investments in synthetic biology, growing focus on personalized medicine, and the need for flexible manufacturing solutions. Additionally, the push for sustainable and environmentally friendly production methods favors CFPS systems, which typically require fewer resources than traditional cell-based manufacturing.
Challenges limiting market penetration include high initial setup costs, technical complexities in scaling production, regulatory uncertainties, and competition from established manufacturing methods. However, ongoing technological advancements and increasing commercial validation are gradually addressing these barriers.
Current CFPS Systems and Technical Barriers
Cell-free protein synthesis (CFPS) systems have evolved significantly over the past decades, with several platforms now available for research and commercial applications. The most widely used CFPS systems are derived from Escherichia coli extracts, which offer high protein yields (up to 2 mg/mL) and relatively low production costs. These systems benefit from extensive optimization and characterization, making them suitable for various applications including high-throughput protein production and directed evolution experiments.
Wheat germ extract-based systems represent another important platform, particularly valued for their ability to produce complex eukaryotic proteins with appropriate post-translational modifications. These systems typically achieve yields of 0.1-1 mg/mL and demonstrate excellent folding capabilities for multi-domain proteins, though at significantly higher costs compared to bacterial systems.
Rabbit reticulocyte lysate and insect cell extract systems are employed for specialized applications requiring mammalian-like protein processing. However, these systems generally produce lower yields (typically 0.01-0.1 mg/mL) and involve more complex preparation protocols, limiting their scalability for industrial applications.
Despite significant advances, CFPS systems face several critical technical barriers. Energy supply limitations represent a fundamental challenge, as protein synthesis is highly ATP-dependent, and current energy regeneration systems often become deplematic during extended reactions. Most CFPS reactions exhaust their energy capacity within 4-6 hours, restricting total protein yield.
Extract quality inconsistency presents another major hurdle, with batch-to-batch variations significantly affecting reproducibility and scalability. The complex nature of cell extracts, containing thousands of components, makes standardization difficult and complicates regulatory approval processes for biopharmaceutical applications.
Scale-up challenges persist as most CFPS systems are optimized for microliter to milliliter reaction volumes. When scaled to industrial levels, issues including oxygen transfer limitations, heat dissipation problems, and mixing inefficiencies emerge, reducing productivity and increasing costs.
Post-translational modification capabilities remain limited in most CFPS platforms, particularly for complex modifications like glycosylation, which are essential for many therapeutic proteins. While eukaryotic systems offer some modification capacity, they typically cannot replicate the full spectrum of modifications achieved in living cells.
Cost factors continue to constrain widespread industrial adoption, with reagent expenses (particularly for energy substrates, nucleotides, and amino acids) remaining prohibitively high for many applications. Current production costs for CFPS-derived proteins typically range from $100-1000/g, significantly higher than conventional fermentation-based methods.
Wheat germ extract-based systems represent another important platform, particularly valued for their ability to produce complex eukaryotic proteins with appropriate post-translational modifications. These systems typically achieve yields of 0.1-1 mg/mL and demonstrate excellent folding capabilities for multi-domain proteins, though at significantly higher costs compared to bacterial systems.
Rabbit reticulocyte lysate and insect cell extract systems are employed for specialized applications requiring mammalian-like protein processing. However, these systems generally produce lower yields (typically 0.01-0.1 mg/mL) and involve more complex preparation protocols, limiting their scalability for industrial applications.
Despite significant advances, CFPS systems face several critical technical barriers. Energy supply limitations represent a fundamental challenge, as protein synthesis is highly ATP-dependent, and current energy regeneration systems often become deplematic during extended reactions. Most CFPS reactions exhaust their energy capacity within 4-6 hours, restricting total protein yield.
Extract quality inconsistency presents another major hurdle, with batch-to-batch variations significantly affecting reproducibility and scalability. The complex nature of cell extracts, containing thousands of components, makes standardization difficult and complicates regulatory approval processes for biopharmaceutical applications.
Scale-up challenges persist as most CFPS systems are optimized for microliter to milliliter reaction volumes. When scaled to industrial levels, issues including oxygen transfer limitations, heat dissipation problems, and mixing inefficiencies emerge, reducing productivity and increasing costs.
Post-translational modification capabilities remain limited in most CFPS platforms, particularly for complex modifications like glycosylation, which are essential for many therapeutic proteins. While eukaryotic systems offer some modification capacity, they typically cannot replicate the full spectrum of modifications achieved in living cells.
Cost factors continue to constrain widespread industrial adoption, with reagent expenses (particularly for energy substrates, nucleotides, and amino acids) remaining prohibitively high for many applications. Current production costs for CFPS-derived proteins typically range from $100-1000/g, significantly higher than conventional fermentation-based methods.
Contemporary CFPS Platforms and Methodologies
01 Cell-free protein synthesis system components and optimization
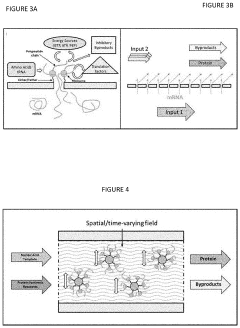
Cell-free protein synthesis systems consist of essential components extracted from cells, including ribosomes, enzymes, tRNAs, and other factors necessary for translation. These systems can be optimized by adjusting the concentration of components, energy regeneration systems, and reaction conditions to enhance protein yield and functionality. Various cell extracts can be used as the basis for these systems, including those from E. coli, wheat germ, rabbit reticulocytes, and insect cells, each offering different advantages for specific applications.- Cell-free protein synthesis system components and optimization: Cell-free protein synthesis systems consist of essential components extracted from cells, including ribosomes, translation factors, aminoacyl-tRNA synthetases, and energy regeneration systems. These systems can be optimized by adjusting component concentrations, energy sources, and reaction conditions to enhance protein yield and functionality. Various approaches to system optimization include supplementation with specific cofactors, optimization of amino acid mixtures, and development of continuous-exchange cell-free systems that extend reaction lifetimes.
- Applications of cell-free protein synthesis in therapeutics and diagnostics: Cell-free protein synthesis systems are increasingly used for rapid production of therapeutic proteins, vaccines, and diagnostic reagents. These systems allow for the expression of difficult-to-produce proteins, including membrane proteins, toxic proteins, and proteins requiring post-translational modifications. The technology enables point-of-care protein production, personalized medicine applications, and rapid response to emerging pathogens by producing diagnostic antigens or therapeutic antibodies on demand without the constraints of traditional cell culture methods.
- Engineering and modification of cell-free systems: Advanced engineering approaches have been developed to enhance cell-free protein synthesis systems, including genetic modification of source organisms, incorporation of non-natural amino acids, and development of synthetic or semi-synthetic systems. These engineered systems can produce proteins with novel functions, expanded chemical diversity, and improved properties. Modifications may include the addition of chaperones for proper protein folding, redox components for disulfide bond formation, and glycosylation machinery for post-translational modifications.
- Miniaturization and high-throughput applications: Cell-free protein synthesis systems have been miniaturized for high-throughput applications, including protein microarrays, microfluidic devices, and automated screening platforms. These miniaturized formats enable rapid prototyping of proteins, directed evolution experiments, and screening of protein variants with desired properties. The technology allows for parallel synthesis of multiple proteins in small volumes, reducing reagent costs and increasing experimental throughput for applications in drug discovery, protein engineering, and synthetic biology.
- Commercial and industrial scale production: Advances in cell-free protein synthesis have enabled scaling up from laboratory to commercial and industrial production levels. These developments include improved extract preparation methods, enhanced energy regeneration systems, and novel bioreactor designs specifically adapted for cell-free reactions. Large-scale cell-free protein synthesis offers advantages including rapid production cycles, simplified purification processes, and the ability to produce proteins that are toxic to living cells. The technology has been commercialized for applications ranging from research reagents to biopharmaceutical manufacturing.
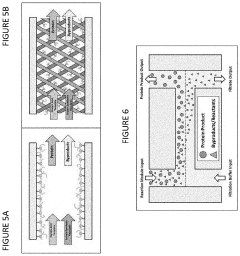
02 Energy regeneration and continuous-flow systems
Energy regeneration is critical for sustained protein synthesis in cell-free systems. Advanced systems incorporate ATP regeneration mechanisms, such as creatine phosphate/creatine kinase or phosphoenolpyruvate/pyruvate kinase pathways, to maintain energy levels during protein production. Continuous-flow cell-free systems allow for the constant supply of substrates and removal of inhibitory byproducts, significantly extending reaction lifetimes and increasing protein yields compared to batch reactions.Expand Specific Solutions03 Applications in therapeutic protein production
Cell-free protein synthesis systems offer advantages for producing therapeutic proteins, including difficult-to-express proteins, cytotoxic proteins, and those requiring post-translational modifications. These systems allow for rapid production of proteins for drug discovery, personalized medicine, and vaccine development. The open nature of cell-free systems enables direct incorporation of non-natural amino acids and other modifications that can enhance therapeutic efficacy or stability.Expand Specific Solutions04 Membrane protein synthesis and stabilization
Cell-free systems have been adapted for the synthesis of membrane proteins, which are traditionally challenging to produce in conventional expression systems. These specialized cell-free platforms incorporate lipids, detergents, or nanodiscs to provide a suitable environment for proper folding and stabilization of membrane proteins. This approach enables structural studies and functional characterization of important drug targets and cellular receptors.Expand Specific Solutions05 Miniaturized and high-throughput cell-free systems
Recent advances have led to the development of miniaturized cell-free protein synthesis platforms suitable for high-throughput applications. These systems can be integrated with microfluidic devices or droplet-based technologies to enable parallel synthesis of multiple proteins with minimal reagent consumption. Such platforms are valuable for protein engineering, directed evolution, and rapid screening of protein variants, significantly accelerating the discovery and optimization of novel proteins for various applications.Expand Specific Solutions
Leading Organizations in CFPS Research and Development
Cell-free Protein Synthesis (CFPS) systems for rapid biomanufacturing are in the growth phase of industry development, with an expanding market estimated to reach $100-150 million by 2025. The technology is advancing from early-stage research to commercial applications, particularly in pharmaceuticals and diagnostics. Technical maturity varies significantly among key players: established research institutions like Fraunhofer-Gesellschaft, Riken, and universities (Tsinghua, Cornell, Northwestern) provide foundational research, while commercial entities demonstrate different specialization levels. Companies like Cellfree Sciences and Swiftscale Biologics offer specialized CFPS platforms, while Shimadzu and Toyobo integrate CFPS into broader biotechnology portfolios. Emerging players such as Kangma Biological Technology and Jiangsu Fulcrum Biotechnology represent the growing commercial interest in this transformative biomanufacturing approach.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed the WEPRO® system, a wheat germ extract-based cell-free protein synthesis platform that enables high-yield production of complex proteins. Their proprietary technology utilizes the eukaryotic translation machinery from wheat germ extract, which lacks inhibitory factors present in other systems, allowing for continuous protein synthesis for up to 72 hours. The company has optimized their system for both analytical and preparative scale production, with yields reaching up to 10 mg/ml of target protein. Their bilayer reaction format separates the translation reaction from the energy supply, enabling sustained synthesis without inhibition by reaction byproducts[1]. Additionally, they've developed specialized vectors and expression kits that facilitate high-throughput protein production for structural biology and drug discovery applications[2].
Strengths: Superior protein yield compared to many competing systems; excellent for producing complex eukaryotic proteins with proper folding and post-translational modifications; scalable from microliter to liter volumes. Weaknesses: Higher cost compared to bacterial systems; wheat germ extract preparation requires specialized expertise; limited application for certain membrane proteins.
Swiftscale Biologics, Inc.
Technical Solution: Swiftscale Biologics has pioneered an advanced E. coli-based cell-free protein synthesis platform specifically optimized for rapid biomanufacturing of therapeutic proteins and vaccines. Their technology combines highly optimized cell extracts with proprietary genetic elements and metabolic engineering to achieve unprecedented protein yields exceeding 2.5 g/L in batch reactions. The company has developed a continuous exchange cell-free (CECF) system that extends reaction duration from hours to days, dramatically improving economics. Their platform incorporates real-time monitoring capabilities using fluorescent reporters to track synthesis progress and optimize production parameters dynamically[3]. Swiftscale's technology enables rapid production of complex biologics including antibodies, cytokines, and vaccine antigens in less than 24 hours from gene sequence to purified protein, representing a 10-50x acceleration compared to conventional cell-based methods[4].
Strengths: Extremely rapid production timeline (hours vs weeks); highly scalable from micrograms to grams; flexibility to produce toxic proteins impossible in living cells; minimal equipment requirements. Weaknesses: Higher cost of goods compared to traditional bioreactors for large-scale production; challenges with certain post-translational modifications; limited track record for GMP manufacturing of clinical products.
Key Innovations in Extract Preparation and Reaction Optimization
Optimized bioprocessing for scalable cell-free protein synthesis
PatentWO2023196608A1
Innovation
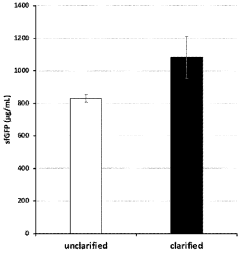
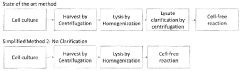
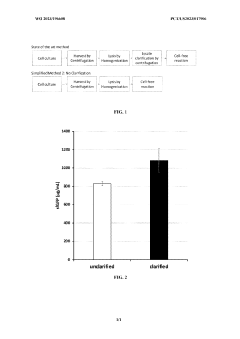
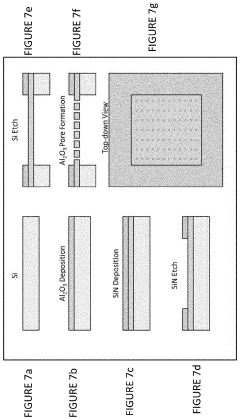
- The method involves using unclarified cellular lysates for protein synthesis, eliminating the need for clarification steps, and includes a system for preparing and processing the lysates using various lysis methods and components such as nucleic acid templates, amino acids, and energy sources, while maintaining substantial protein yields.
Cell-free protein synthesis systems
PatentActiveUS20200255881A1
Innovation
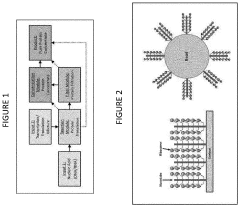
- A cell-free protein synthesis (CFPS) system with localized ribosomes attached to structures, allowing for repeated use in in vitro translation reactions, maximizing surface area for biomolecular interactions and enabling continuous operation with efficient product collection and replacement, thereby enhancing protein production rates.
Regulatory Framework for CFPS-derived Biologics
The regulatory landscape for Cell-free Protein Synthesis (CFPS) derived biologics remains in a developmental stage, with significant variations across different jurisdictions. Currently, the U.S. Food and Drug Administration (FDA) evaluates CFPS-derived products primarily under existing frameworks for biologics and recombinant proteins, focusing on product quality, consistency, and safety rather than the manufacturing process itself. This approach aligns with the FDA's increasing emphasis on Quality by Design (QbD) principles.
In the European Union, the European Medicines Agency (EMA) has begun developing specific guidelines for cell-free expression systems, recognizing their unique characteristics compared to traditional cell-based manufacturing. These guidelines emphasize comprehensive characterization of starting materials, process validation, and product consistency across batches.
Regulatory challenges specific to CFPS include the characterization of complex extract compositions, which may contain thousands of components from lysed cells. Regulatory bodies increasingly require manufacturers to demonstrate thorough understanding of these components and their potential impact on final product quality and safety. Additionally, the absence of cellular containment raises questions about potential contaminants that differ from those in traditional bioprocessing.
The accelerated timeline of CFPS manufacturing presents another regulatory consideration. While rapid production offers advantages for personalized medicine and emergency response scenarios, it necessitates the development of equally rapid analytical methods for quality control and release testing. Regulatory frameworks are evolving to accommodate these compressed timelines while maintaining rigorous safety standards.
International harmonization efforts, led by organizations such as the International Council for Harmonisation (ICH), are working to establish consistent global standards for CFPS-derived biologics. These initiatives aim to reduce regulatory barriers to market entry while ensuring product safety across different regions.
For emerging applications like point-of-care manufacturing using CFPS, regulatory bodies are developing novel frameworks that address the unique challenges of decentralized production. These frameworks focus on standardization of portable systems, validation protocols for on-site production, and appropriate quality control measures for products with extremely short shelf-lives.
Companies developing CFPS-derived biologics are advised to engage with regulatory authorities early through mechanisms like the FDA's Emerging Technology Program or the EMA's Innovation Task Force to navigate this evolving landscape effectively and establish appropriate development pathways for their specific products.
In the European Union, the European Medicines Agency (EMA) has begun developing specific guidelines for cell-free expression systems, recognizing their unique characteristics compared to traditional cell-based manufacturing. These guidelines emphasize comprehensive characterization of starting materials, process validation, and product consistency across batches.
Regulatory challenges specific to CFPS include the characterization of complex extract compositions, which may contain thousands of components from lysed cells. Regulatory bodies increasingly require manufacturers to demonstrate thorough understanding of these components and their potential impact on final product quality and safety. Additionally, the absence of cellular containment raises questions about potential contaminants that differ from those in traditional bioprocessing.
The accelerated timeline of CFPS manufacturing presents another regulatory consideration. While rapid production offers advantages for personalized medicine and emergency response scenarios, it necessitates the development of equally rapid analytical methods for quality control and release testing. Regulatory frameworks are evolving to accommodate these compressed timelines while maintaining rigorous safety standards.
International harmonization efforts, led by organizations such as the International Council for Harmonisation (ICH), are working to establish consistent global standards for CFPS-derived biologics. These initiatives aim to reduce regulatory barriers to market entry while ensuring product safety across different regions.
For emerging applications like point-of-care manufacturing using CFPS, regulatory bodies are developing novel frameworks that address the unique challenges of decentralized production. These frameworks focus on standardization of portable systems, validation protocols for on-site production, and appropriate quality control measures for products with extremely short shelf-lives.
Companies developing CFPS-derived biologics are advised to engage with regulatory authorities early through mechanisms like the FDA's Emerging Technology Program or the EMA's Innovation Task Force to navigate this evolving landscape effectively and establish appropriate development pathways for their specific products.
Economic Feasibility and Cost Analysis of CFPS Implementation
The economic viability of Cell-free Protein Synthesis (CFPS) systems represents a critical factor in their widespread adoption for rapid biomanufacturing. Current cost analyses indicate that CFPS implementation requires significant initial investment, primarily in extract preparation equipment, reaction vessels, and purification systems. These capital expenditures typically range from $100,000 to $500,000 for small-scale operations, with larger industrial setups potentially exceeding $2 million.
Operational costs present another substantial consideration, with reagents and energy consumption constituting approximately 60-70% of recurring expenses. Recent advancements have reduced reagent costs from $0.50-1.00 per mg of protein to $0.10-0.30 in optimized systems, representing a significant improvement in economic feasibility. Energy requirements for maintaining reaction conditions add approximately 15-20% to operational expenses, varying based on scale and facility location.
Labor costs contribute an additional 20-25% to operational expenses, though this percentage decreases with increasing automation and scale. Skilled personnel remain essential for system optimization and quality control, particularly in pharmaceutical and medical applications where regulatory compliance adds further complexity and cost.
Scale-up economics demonstrate promising trends, with cost-per-unit decreasing substantially at larger production volumes. Studies indicate that medium-scale CFPS operations (50-100L) can achieve cost reductions of 40-60% compared to small-scale (1-5L) systems through improved reagent utilization and operational efficiencies. This economy of scale represents a critical threshold for commercial viability in many applications.
Return on investment (ROI) calculations suggest that CFPS systems can become economically competitive with traditional cell-based manufacturing for certain high-value proteins within 2-3 years of implementation. This timeline shortens considerably for applications requiring rapid production cycles or those producing difficult-to-express proteins where traditional methods struggle.
Comparative analyses with conventional biomanufacturing methods reveal that while CFPS typically has higher per-unit production costs for standard proteins, it offers significant economic advantages in time-sensitive applications, reducing development cycles by 60-80%. This time-to-market advantage translates to substantial economic benefits, particularly in therapeutic and diagnostic fields where market timing critically impacts commercial success.
Future economic projections indicate that continued technological improvements could reduce CFPS production costs by an additional 30-50% within the next five years, potentially positioning it as the preferred manufacturing method for an expanding range of protein products beyond current niche applications.
Operational costs present another substantial consideration, with reagents and energy consumption constituting approximately 60-70% of recurring expenses. Recent advancements have reduced reagent costs from $0.50-1.00 per mg of protein to $0.10-0.30 in optimized systems, representing a significant improvement in economic feasibility. Energy requirements for maintaining reaction conditions add approximately 15-20% to operational expenses, varying based on scale and facility location.
Labor costs contribute an additional 20-25% to operational expenses, though this percentage decreases with increasing automation and scale. Skilled personnel remain essential for system optimization and quality control, particularly in pharmaceutical and medical applications where regulatory compliance adds further complexity and cost.
Scale-up economics demonstrate promising trends, with cost-per-unit decreasing substantially at larger production volumes. Studies indicate that medium-scale CFPS operations (50-100L) can achieve cost reductions of 40-60% compared to small-scale (1-5L) systems through improved reagent utilization and operational efficiencies. This economy of scale represents a critical threshold for commercial viability in many applications.
Return on investment (ROI) calculations suggest that CFPS systems can become economically competitive with traditional cell-based manufacturing for certain high-value proteins within 2-3 years of implementation. This timeline shortens considerably for applications requiring rapid production cycles or those producing difficult-to-express proteins where traditional methods struggle.
Comparative analyses with conventional biomanufacturing methods reveal that while CFPS typically has higher per-unit production costs for standard proteins, it offers significant economic advantages in time-sensitive applications, reducing development cycles by 60-80%. This time-to-market advantage translates to substantial economic benefits, particularly in therapeutic and diagnostic fields where market timing critically impacts commercial success.
Future economic projections indicate that continued technological improvements could reduce CFPS production costs by an additional 30-50% within the next five years, potentially positioning it as the preferred manufacturing method for an expanding range of protein products beyond current niche applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!