Cell-free Protein Synthesis Using DNA Templates and RNA Programs
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis Evolution and Objectives
Cell-free protein synthesis (CFPS) has evolved significantly since its inception in the 1950s when Nirenberg and Matthaei first demonstrated protein synthesis outside living cells. This groundbreaking technology initially served as a research tool to decipher the genetic code but has since transformed into a versatile platform for various biotechnological applications. The evolution of CFPS systems has been marked by continuous improvements in extract preparation methods, energy regeneration systems, and reaction components optimization.
Early CFPS systems derived from E. coli extracts suffered from limited protein yields and short reaction durations. The 1980s and 1990s witnessed significant advancements with the development of coupled transcription-translation systems and improved energy regeneration mechanisms. By the 2000s, researchers achieved dramatic increases in protein yields, extending reaction lifetimes from hours to days, and expanding the repertoire of expressible proteins to include complex membrane proteins and proteins with post-translational modifications.
The integration of DNA templates and RNA programs represents a pivotal advancement in CFPS technology. Traditional CFPS systems relied primarily on plasmid DNA or PCR products as templates. However, the incorporation of engineered RNA programs has enabled more precise control over protein expression dynamics, allowing for sequential or coordinated synthesis of multiple proteins within a single reaction vessel.
The primary objectives of modern CFPS using DNA templates and RNA programs encompass several dimensions. First, researchers aim to enhance production efficiency by optimizing reaction conditions, extract preparation protocols, and energy regeneration systems to achieve higher protein yields with minimal resource consumption. Second, there is a focus on expanding the functional diversity of synthesized proteins, including those requiring complex folding, post-translational modifications, or incorporation of non-canonical amino acids.
Another critical objective involves improving the scalability and reproducibility of CFPS reactions, transitioning from laboratory-scale demonstrations to industrial-scale applications. This includes developing standardized protocols and automated platforms for high-throughput protein production. Additionally, researchers are working toward creating more accessible and cost-effective CFPS systems to democratize this technology for broader research and commercial applications.
The integration of computational design tools with CFPS represents an emerging objective, enabling the rational design of genetic circuits and protein expression systems with predictable behaviors. This convergence of in silico design with in vitro synthesis creates powerful platforms for synthetic biology applications, protein engineering, and the development of novel therapeutics and diagnostics.
Early CFPS systems derived from E. coli extracts suffered from limited protein yields and short reaction durations. The 1980s and 1990s witnessed significant advancements with the development of coupled transcription-translation systems and improved energy regeneration mechanisms. By the 2000s, researchers achieved dramatic increases in protein yields, extending reaction lifetimes from hours to days, and expanding the repertoire of expressible proteins to include complex membrane proteins and proteins with post-translational modifications.
The integration of DNA templates and RNA programs represents a pivotal advancement in CFPS technology. Traditional CFPS systems relied primarily on plasmid DNA or PCR products as templates. However, the incorporation of engineered RNA programs has enabled more precise control over protein expression dynamics, allowing for sequential or coordinated synthesis of multiple proteins within a single reaction vessel.
The primary objectives of modern CFPS using DNA templates and RNA programs encompass several dimensions. First, researchers aim to enhance production efficiency by optimizing reaction conditions, extract preparation protocols, and energy regeneration systems to achieve higher protein yields with minimal resource consumption. Second, there is a focus on expanding the functional diversity of synthesized proteins, including those requiring complex folding, post-translational modifications, or incorporation of non-canonical amino acids.
Another critical objective involves improving the scalability and reproducibility of CFPS reactions, transitioning from laboratory-scale demonstrations to industrial-scale applications. This includes developing standardized protocols and automated platforms for high-throughput protein production. Additionally, researchers are working toward creating more accessible and cost-effective CFPS systems to democratize this technology for broader research and commercial applications.
The integration of computational design tools with CFPS represents an emerging objective, enabling the rational design of genetic circuits and protein expression systems with predictable behaviors. This convergence of in silico design with in vitro synthesis creates powerful platforms for synthetic biology applications, protein engineering, and the development of novel therapeutics and diagnostics.
Market Analysis for Cell-free Expression Systems
The cell-free protein synthesis (CFPS) market has experienced significant growth in recent years, driven by increasing applications in synthetic biology, personalized medicine, and pharmaceutical research. The global market for cell-free expression systems was valued at approximately $208 million in 2020 and is projected to reach $310 million by 2025, representing a compound annual growth rate (CAGR) of 8.3%.
The pharmaceutical and biotechnology sectors currently dominate the market demand, accounting for nearly 60% of the total market share. These industries utilize cell-free systems primarily for rapid protein production, drug screening, and vaccine development. Academic research institutions constitute the second-largest market segment, contributing about 25% of the overall demand, with applications focused on fundamental research and educational purposes.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to increasing investments in biotechnology research and development infrastructure.
The market is segmented by product type into reagents, expression kits, and accessories. Expression kits represent the largest segment, accounting for 45% of the market, followed by reagents at 35%. By application, protein engineering and production hold the largest market share (38%), followed by pharmaceutical and vaccine development (30%), and synthetic biology applications (22%).
Key market drivers include the advantages of cell-free systems over traditional cell-based methods, such as reduced production time, elimination of cell viability concerns, and the ability to produce difficult-to-express proteins. The COVID-19 pandemic has further accelerated market growth, highlighting the potential of cell-free systems for rapid vaccine and therapeutic protein development.
Market challenges include high costs associated with reagents and equipment, technical limitations in scaling up production, and regulatory uncertainties. The average cost per reaction remains significantly higher than traditional cell-based methods, limiting widespread adoption in cost-sensitive applications.
Customer segments show varying needs: pharmaceutical companies prioritize scalability and reproducibility, academic researchers value flexibility and cost-effectiveness, while biotechnology startups seek systems with rapid prototyping capabilities. This diversity in customer requirements has led to market fragmentation, with various specialized products targeting specific application niches.
The pharmaceutical and biotechnology sectors currently dominate the market demand, accounting for nearly 60% of the total market share. These industries utilize cell-free systems primarily for rapid protein production, drug screening, and vaccine development. Academic research institutions constitute the second-largest market segment, contributing about 25% of the overall demand, with applications focused on fundamental research and educational purposes.
Geographically, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to increasing investments in biotechnology research and development infrastructure.
The market is segmented by product type into reagents, expression kits, and accessories. Expression kits represent the largest segment, accounting for 45% of the market, followed by reagents at 35%. By application, protein engineering and production hold the largest market share (38%), followed by pharmaceutical and vaccine development (30%), and synthetic biology applications (22%).
Key market drivers include the advantages of cell-free systems over traditional cell-based methods, such as reduced production time, elimination of cell viability concerns, and the ability to produce difficult-to-express proteins. The COVID-19 pandemic has further accelerated market growth, highlighting the potential of cell-free systems for rapid vaccine and therapeutic protein development.
Market challenges include high costs associated with reagents and equipment, technical limitations in scaling up production, and regulatory uncertainties. The average cost per reaction remains significantly higher than traditional cell-based methods, limiting widespread adoption in cost-sensitive applications.
Customer segments show varying needs: pharmaceutical companies prioritize scalability and reproducibility, academic researchers value flexibility and cost-effectiveness, while biotechnology startups seek systems with rapid prototyping capabilities. This diversity in customer requirements has led to market fragmentation, with various specialized products targeting specific application niches.
Technical Barriers in DNA-RNA Based Cell-free Systems
Despite significant advancements in cell-free protein synthesis (CFPS) systems utilizing DNA templates and RNA programs, several technical barriers continue to impede their widespread application and optimal performance. The complexity of these barriers spans multiple dimensions of the cell-free environment and requires systematic approaches to overcome.
Extract preparation remains a critical challenge, with batch-to-batch variability significantly affecting reproducibility. Current extraction methods often yield inconsistent enzyme concentrations and activity levels, leading to unpredictable protein yields. This variability stems from differences in cell growth conditions, lysis efficiency, and extract processing protocols, creating a fundamental obstacle to standardization.
Energy regeneration systems present another substantial barrier. The ATP-dependent nature of protein synthesis demands continuous energy supply, yet current regeneration systems suffer from limited longevity. Phosphate accumulation from ATP hydrolysis inhibits translation machinery, while substrate depletion rapidly diminishes synthesis rates, typically limiting reaction durations to 2-4 hours.
RNA stability in cell-free environments poses significant challenges, as endogenous nucleases rapidly degrade mRNA templates. While nuclease inhibitors can partially address this issue, they often interfere with other essential enzymatic activities in the system, creating a delicate balance between RNA protection and overall system functionality.
Translation efficiency barriers include suboptimal ribosome loading onto mRNA, codon usage biases, and inefficient termination processes. These factors collectively reduce protein yield and increase resource consumption, particularly affecting the synthesis of complex or large proteins that require extended translation times.
Post-translational modifications represent perhaps the most significant limitation in current systems. While prokaryotic CFPS systems excel at basic protein production, they lack the sophisticated machinery for glycosylation, phosphorylation, and other modifications essential for many eukaryotic proteins' functionality. Hybrid systems incorporating microsomes or specific enzymes show promise but remain inefficient and incomplete.
Scale-up challenges persist as reactions moving from microliter to liter scale encounter oxygen transfer limitations, heat dissipation issues, and mixing inefficiencies. These physical constraints often result in decreased productivity at larger volumes, hampering industrial applications.
DNA template quality and design significantly impact system performance. Impurities in DNA preparations can inhibit transcription and translation machinery, while suboptimal regulatory elements (promoters, ribosome binding sites) lead to inefficient expression. Additionally, secondary structures in mRNA can impede ribosome progression, further reducing synthesis efficiency.
Extract preparation remains a critical challenge, with batch-to-batch variability significantly affecting reproducibility. Current extraction methods often yield inconsistent enzyme concentrations and activity levels, leading to unpredictable protein yields. This variability stems from differences in cell growth conditions, lysis efficiency, and extract processing protocols, creating a fundamental obstacle to standardization.
Energy regeneration systems present another substantial barrier. The ATP-dependent nature of protein synthesis demands continuous energy supply, yet current regeneration systems suffer from limited longevity. Phosphate accumulation from ATP hydrolysis inhibits translation machinery, while substrate depletion rapidly diminishes synthesis rates, typically limiting reaction durations to 2-4 hours.
RNA stability in cell-free environments poses significant challenges, as endogenous nucleases rapidly degrade mRNA templates. While nuclease inhibitors can partially address this issue, they often interfere with other essential enzymatic activities in the system, creating a delicate balance between RNA protection and overall system functionality.
Translation efficiency barriers include suboptimal ribosome loading onto mRNA, codon usage biases, and inefficient termination processes. These factors collectively reduce protein yield and increase resource consumption, particularly affecting the synthesis of complex or large proteins that require extended translation times.
Post-translational modifications represent perhaps the most significant limitation in current systems. While prokaryotic CFPS systems excel at basic protein production, they lack the sophisticated machinery for glycosylation, phosphorylation, and other modifications essential for many eukaryotic proteins' functionality. Hybrid systems incorporating microsomes or specific enzymes show promise but remain inefficient and incomplete.
Scale-up challenges persist as reactions moving from microliter to liter scale encounter oxygen transfer limitations, heat dissipation issues, and mixing inefficiencies. These physical constraints often result in decreased productivity at larger volumes, hampering industrial applications.
DNA template quality and design significantly impact system performance. Impurities in DNA preparations can inhibit transcription and translation machinery, while suboptimal regulatory elements (promoters, ribosome binding sites) lead to inefficient expression. Additionally, secondary structures in mRNA can impede ribosome progression, further reducing synthesis efficiency.
Current DNA Template and RNA Program Methodologies
01 Cell-free protein synthesis systems and components
Cell-free protein synthesis systems utilize cellular extracts or purified components to produce proteins without intact cells. These systems typically include ribosomes, tRNAs, aminoacyl-tRNA synthetases, translation factors, and energy regeneration components. Various extracts can be derived from different organisms such as E. coli, wheat germ, rabbit reticulocytes, or insect cells, each with specific advantages for different applications. These systems allow for rapid protein production and are particularly useful for expressing toxic proteins or those requiring specific modifications.- Cell-free protein synthesis systems and components: Cell-free protein synthesis systems utilize cellular extracts or purified components to produce proteins without intact cells. These systems typically include ribosomes, translation factors, tRNAs, amino acids, energy sources, and nucleotides. Various extracts can be derived from different organisms such as E. coli, wheat germ, rabbit reticulocytes, or insect cells, each with specific advantages for different applications. These systems allow for rapid protein production and are particularly useful for expressing toxic or membrane proteins that might be challenging in traditional cell-based systems.
- Enhanced yield and efficiency in cell-free protein synthesis: Various approaches have been developed to improve the yield and efficiency of cell-free protein synthesis. These include optimizing reaction conditions such as temperature, pH, and ionic strength; supplementing with additional components like chaperones to assist protein folding; developing continuous-exchange cell-free systems that allow for the replenishment of substrates and removal of inhibitory byproducts; and engineering the translation machinery components for improved performance. These enhancements have significantly increased protein yields and extended reaction durations.
- Applications in therapeutic protein production: Cell-free protein synthesis has emerged as a valuable platform for producing therapeutic proteins and vaccines. This approach enables rapid production of proteins with precise control over modifications and folding, which is crucial for maintaining biological activity. The system allows for the incorporation of non-natural amino acids and site-specific modifications that can enhance stability, half-life, or targeting capabilities of therapeutic proteins. Additionally, the absence of cellular contaminants simplifies downstream purification processes, making it particularly suitable for producing pharmaceuticals that require high purity.
- Microfluidic and miniaturized cell-free systems: Miniaturized cell-free protein synthesis platforms integrate microfluidic technologies to enable high-throughput protein production with minimal reagent consumption. These systems feature microscale reaction chambers, controlled fluid handling, and often incorporate sensors for real-time monitoring of synthesis progress. The miniaturization allows for parallel processing of multiple proteins simultaneously, making them ideal for protein engineering, directed evolution experiments, and rapid prototyping of protein variants. Additionally, these platforms can be automated to reduce labor requirements and increase reproducibility.
- Genetic circuit design and synthetic biology applications: Cell-free protein synthesis serves as a platform for testing and optimizing genetic circuits and synthetic biology constructs. By eliminating cellular complexity, researchers can directly observe the behavior of genetic elements without interference from cellular processes. This approach enables rapid prototyping of genetic circuits, characterization of regulatory elements, and testing of biosensors before implementation in living cells. The open nature of cell-free systems also allows for precise manipulation of reaction components to study fundamental aspects of gene expression and regulation.
02 Energy regeneration systems for sustained protein synthesis
Efficient energy regeneration is crucial for sustained protein synthesis in cell-free systems. Various approaches include the use of phosphoenolpyruvate (PEP), creatine phosphate, or glucose-based energy regeneration systems. These systems continuously supply ATP and GTP required for translation, extending the duration of protein synthesis reactions. Advanced energy regeneration methods incorporate enzymes like pyruvate kinase or creatine kinase to recycle ADP back to ATP, significantly improving protein yields and reaction longevity.Expand Specific Solutions03 Optimization of reaction conditions and additives
Optimizing reaction conditions is essential for maximizing protein yield in cell-free systems. This includes adjusting parameters such as temperature, pH, ion concentrations (particularly magnesium and potassium), and adding specific additives. Stabilizing agents like polyethylene glycol, chaperones, and protease inhibitors can enhance protein folding and prevent degradation. Additionally, supplementing with amino acids, nucleotides, and cofactors can overcome resource limitations. These optimizations can significantly improve both the quantity and quality of synthesized proteins.Expand Specific Solutions04 Continuous-flow and microfluidic cell-free protein synthesis
Continuous-flow and microfluidic approaches represent advanced implementations of cell-free protein synthesis. These systems allow for the continuous supply of substrates and removal of inhibitory byproducts, overcoming limitations of batch reactions. Microfluidic devices enable miniaturization, parallelization, and automation of protein synthesis reactions, reducing reagent consumption while increasing throughput. These technologies are particularly valuable for high-throughput screening applications and the production of proteins that are difficult to express in conventional systems.Expand Specific Solutions05 Applications in protein engineering and synthetic biology
Cell-free protein synthesis has diverse applications in protein engineering and synthetic biology. It enables rapid prototyping of engineered proteins, incorporation of non-natural amino acids, and production of proteins with site-specific modifications. These systems are valuable for directed evolution experiments, high-throughput screening of protein variants, and the development of biosensors. Additionally, cell-free systems can be used to produce therapeutic proteins, vaccines, and diagnostic reagents, offering advantages in speed, scalability, and safety compared to traditional cell-based methods.Expand Specific Solutions
Industry Leaders in Cell-free Expression Technologies
Cell-free Protein Synthesis (CFPS) using DNA templates and RNA programs is currently in the early growth phase, with an estimated market size of $100-150 million that is projected to expand significantly as applications in therapeutics and diagnostics mature. The technology has reached moderate maturity, with key players demonstrating varied capabilities across the value chain. Companies like Cellfree Sciences, NUProtein, and Shimadzu Corp have established specialized expertise in cell-free expression systems, while pharmaceutical giants such as Pfizer are exploring applications in drug discovery. Academic institutions including Tsinghua University, Northwestern University, and RIKEN are driving fundamental innovations. Emerging players like Kangma Biological Technology and Regis Biotechnology are developing novel applications in diagnostics and mRNA technologies, indicating a competitive landscape that balances established expertise with disruptive innovation.
Touchlight IP Ltd.
Technical Solution: Touchlight has developed a revolutionary doggybone™ DNA (dbDNA™) platform specifically applicable to cell-free protein synthesis. Their enzymatic manufacturing process produces linear, covalently closed DNA constructs that serve as highly efficient templates for in vitro transcription and subsequent protein synthesis [7]. The dbDNA™ technology eliminates bacterial sequences and antibiotic resistance genes found in traditional plasmids, resulting in minimal background expression and improved safety profiles. Touchlight's system incorporates specialized promoter elements and ribosome binding sites optimized for cell-free environments, enabling enhanced mRNA production and translation efficiency. Their templates feature engineered 5' and 3' untranslated regions that improve mRNA stability and ribosome recruitment. Additionally, Touchlight has developed proprietary methods for rapid template switching, allowing for quick adaptation to different protein targets without extensive re-optimization of reaction conditions [8].
Strengths: Rapid, scalable production of highly pure DNA templates (up to gram scale); elimination of bacterial contaminants improves regulatory compliance; templates show exceptional stability at room temperature. Weaknesses: Relatively higher cost compared to traditional plasmid preparation; may require specialized equipment for optimal implementation; limited historical performance data compared to established systems.
Nuprotein Co. Ltd.
Technical Solution: Nuprotein has developed an advanced cell-free protein synthesis platform that combines optimized extract preparation methods with engineered genetic elements for enhanced expression. Their technology utilizes specially designed DNA templates featuring proprietary promoter sequences and translation enhancing elements that significantly increase protein yields in cell-free environments [9]. The company has pioneered a continuous-exchange cell-free (CECF) system that removes inhibitory byproducts while replenishing energy sources, enabling extended reaction times and higher protein yields. Nuprotein's platform incorporates specialized RNA stabilizing elements and optimized codon usage patterns tailored for their extract sources, resulting in more efficient translation. Their system also features proprietary post-translational modification capabilities, allowing for the production of proteins with specific glycosylation patterns or other modifications essential for proper function. Recent advancements include the development of lyophilized reaction components that maintain activity during long-term storage, significantly improving the accessibility and convenience of their technology [10].
Strengths: Exceptional protein yields through continuous-exchange technology; versatile platform capable of expressing both prokaryotic and eukaryotic proteins; simplified workflow with pre-optimized reaction components. Weaknesses: Higher complexity of setup compared to batch reactions; potentially higher costs due to specialized equipment requirements; may require significant optimization for novel protein targets.
Key Patents in Cell-free Protein Production
Methods of RNA and protein synthesis
PatentInactiveUS7186525B2
Innovation
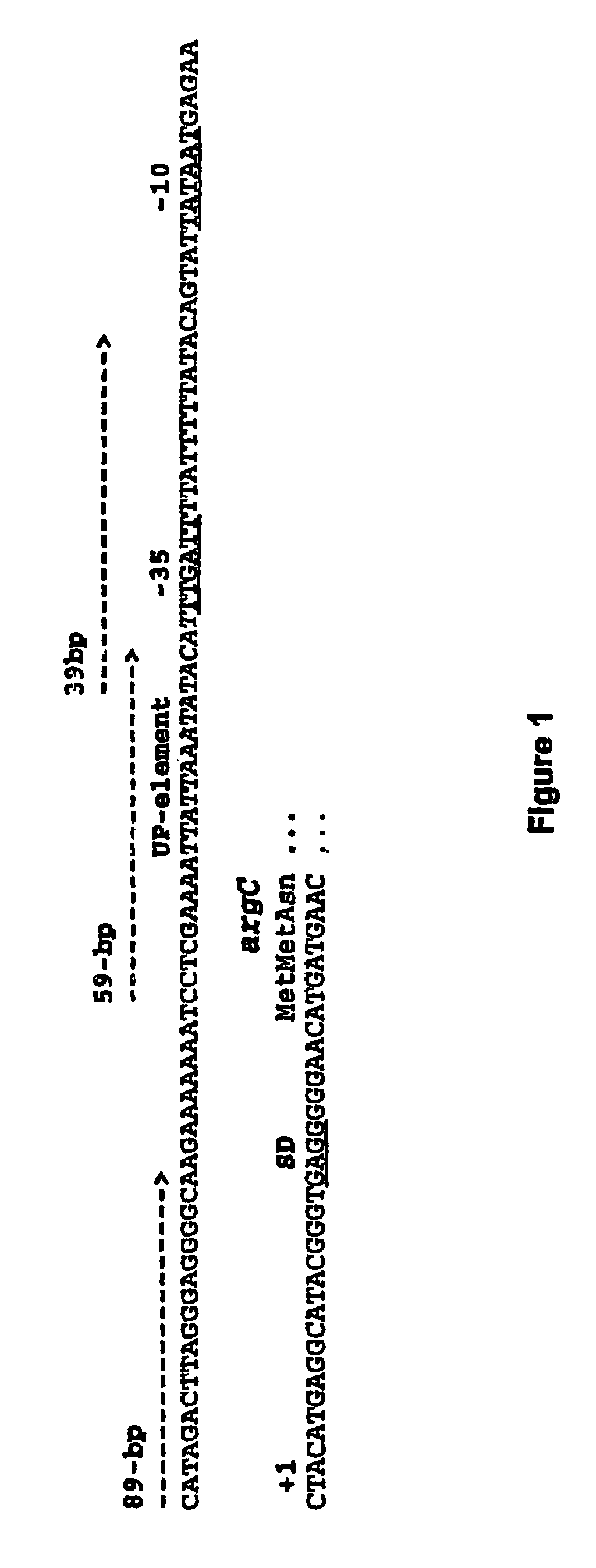
- Increasing the concentration of the α subunit of RNA polymerase in expression systems, while keeping other subunits at natural concentrations, enhances mRNA and protein synthesis by optimizing transcription initiation, using specific promoters like the argC gene from Bacillus stearothermophilus and incorporating thermostable RNA polymerase and DNA-binding regulatory proteins.
Translation enhancer, template nucleic acid, production method of translation template, and production method of protein
PatentPendingUS20220333121A1
Innovation
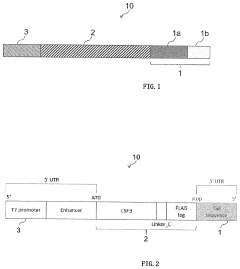
- Incorporating a translation enhancer with a 3' untranslated region that includes a first region of 10 to 40 nucleic acids adjacent to the 3' terminal of the coding region and a poly-A sequence of 2 to 40 continuous "A"s, forming a hairpin structure, which improves protein synthesis efficiency even with a shorter 3'UTR.
Regulatory Framework for Cell-free Biologics
The regulatory landscape for cell-free biologics represents a complex and evolving framework that significantly impacts the development and commercialization of cell-free protein synthesis (CFPS) technologies. Currently, cell-free biologics occupy a unique position between traditional pharmaceutical products and biotechnology innovations, creating challenges for regulatory bodies worldwide in establishing appropriate oversight mechanisms.
The U.S. Food and Drug Administration (FDA) has begun addressing CFPS products through its Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER), depending on the specific application and intended use. Products derived from cell-free systems using DNA templates and RNA programs may be classified as biologics, requiring Biologics License Applications (BLAs), or as drugs necessitating New Drug Applications (NDAs).
In Europe, the European Medicines Agency (EMA) has established the Advanced Therapy Medicinal Products (ATMP) framework, which may encompass certain cell-free biologics. However, the classification remains ambiguous for many CFPS applications, creating regulatory uncertainty for developers and manufacturers operating in this space.
Key regulatory considerations for cell-free biologics include source material validation, process consistency, product purity, and stability profiles. Regulatory bodies are particularly concerned with the characterization of DNA templates and RNA programs used in CFPS, requiring comprehensive documentation of their design, synthesis, and quality control measures.
Safety assessment frameworks for cell-free biologics are still developing, with regulatory agencies focusing on potential immunogenicity, toxicity profiles, and unintended biological effects. The absence of cellular components in CFPS products may simplify certain safety evaluations compared to cell-based therapies, potentially offering a streamlined regulatory pathway.
Intellectual property protection represents another critical regulatory dimension, with patent landscapes becoming increasingly complex as CFPS technologies advance. Companies must navigate patent thickets covering expression systems, template designs, and production methodologies when bringing cell-free biologics to market.
Global harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH), aiming to standardize regulatory approaches to novel biologics including cell-free products. However, significant regional variations persist, creating challenges for companies seeking multinational approvals for CFPS-derived therapeutics and diagnostics.
As the field evolves, regulatory frameworks will likely adapt to accommodate the unique characteristics of cell-free biologics, potentially establishing specialized pathways that recognize their distinct manufacturing processes and risk profiles compared to traditional biologics and pharmaceuticals.
The U.S. Food and Drug Administration (FDA) has begun addressing CFPS products through its Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER), depending on the specific application and intended use. Products derived from cell-free systems using DNA templates and RNA programs may be classified as biologics, requiring Biologics License Applications (BLAs), or as drugs necessitating New Drug Applications (NDAs).
In Europe, the European Medicines Agency (EMA) has established the Advanced Therapy Medicinal Products (ATMP) framework, which may encompass certain cell-free biologics. However, the classification remains ambiguous for many CFPS applications, creating regulatory uncertainty for developers and manufacturers operating in this space.
Key regulatory considerations for cell-free biologics include source material validation, process consistency, product purity, and stability profiles. Regulatory bodies are particularly concerned with the characterization of DNA templates and RNA programs used in CFPS, requiring comprehensive documentation of their design, synthesis, and quality control measures.
Safety assessment frameworks for cell-free biologics are still developing, with regulatory agencies focusing on potential immunogenicity, toxicity profiles, and unintended biological effects. The absence of cellular components in CFPS products may simplify certain safety evaluations compared to cell-based therapies, potentially offering a streamlined regulatory pathway.
Intellectual property protection represents another critical regulatory dimension, with patent landscapes becoming increasingly complex as CFPS technologies advance. Companies must navigate patent thickets covering expression systems, template designs, and production methodologies when bringing cell-free biologics to market.
Global harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH), aiming to standardize regulatory approaches to novel biologics including cell-free products. However, significant regional variations persist, creating challenges for companies seeking multinational approvals for CFPS-derived therapeutics and diagnostics.
As the field evolves, regulatory frameworks will likely adapt to accommodate the unique characteristics of cell-free biologics, potentially establishing specialized pathways that recognize their distinct manufacturing processes and risk profiles compared to traditional biologics and pharmaceuticals.
Scalability Challenges and Industrial Applications
The scalability of cell-free protein synthesis (CFPS) systems represents a significant challenge for industrial implementation. Current laboratory-scale CFPS reactions typically range from microliters to milliliters, while industrial applications require volumes in liters or even cubic meters. This volume expansion introduces numerous technical hurdles including oxygen transfer limitations, heat dissipation issues, and difficulties in maintaining homogeneous reaction conditions. Additionally, the cost of reagents—particularly nucleotides, amino acids, and energy regeneration components—increases proportionally with scale, creating economic barriers to widespread adoption.
Several companies have made notable progress in addressing these scalability challenges. Sutro Biopharma has developed a proprietary CFPS platform capable of producing therapeutic proteins at scales approaching 100 liters. Similarly, Greenlight Biosciences has engineered scalable cell-free RNA production systems that demonstrate consistent yield and quality across different production volumes. These advancements suggest that industrial-scale CFPS is becoming increasingly viable.
The industrial applications of CFPS using DNA templates and RNA programs span multiple sectors. In pharmaceuticals, CFPS enables rapid production of personalized medicines and difficult-to-express proteins that traditional cell-based systems cannot efficiently produce. Companies like Moderna and BioNTech have leveraged similar cell-free approaches for mRNA vaccine production, demonstrating the technology's potential for addressing global health challenges.
In diagnostics, CFPS systems are being integrated into point-of-care devices for rapid detection of pathogens and biomarkers. These applications benefit from the system's ability to function without requiring sterile conditions or complex infrastructure, making them suitable for resource-limited settings. The food industry has also begun exploring CFPS for producing food-grade proteins and enzymes with improved safety profiles compared to genetically modified organism-based production.
Despite promising advances, several technical hurdles remain before widespread industrial adoption becomes reality. The development of continuous-flow CFPS systems represents one promising approach to overcome volume limitations, allowing for sustained production while maintaining reaction efficiency. Additionally, the integration of microfluidic technologies with CFPS shows potential for parallelization and automation, potentially reducing costs while increasing throughput. Economic analyses suggest that further optimization of energy regeneration systems and recycling of expensive components could reduce production costs by 30-50%, potentially making CFPS economically competitive with conventional protein production methods for high-value products.
Several companies have made notable progress in addressing these scalability challenges. Sutro Biopharma has developed a proprietary CFPS platform capable of producing therapeutic proteins at scales approaching 100 liters. Similarly, Greenlight Biosciences has engineered scalable cell-free RNA production systems that demonstrate consistent yield and quality across different production volumes. These advancements suggest that industrial-scale CFPS is becoming increasingly viable.
The industrial applications of CFPS using DNA templates and RNA programs span multiple sectors. In pharmaceuticals, CFPS enables rapid production of personalized medicines and difficult-to-express proteins that traditional cell-based systems cannot efficiently produce. Companies like Moderna and BioNTech have leveraged similar cell-free approaches for mRNA vaccine production, demonstrating the technology's potential for addressing global health challenges.
In diagnostics, CFPS systems are being integrated into point-of-care devices for rapid detection of pathogens and biomarkers. These applications benefit from the system's ability to function without requiring sterile conditions or complex infrastructure, making them suitable for resource-limited settings. The food industry has also begun exploring CFPS for producing food-grade proteins and enzymes with improved safety profiles compared to genetically modified organism-based production.
Despite promising advances, several technical hurdles remain before widespread industrial adoption becomes reality. The development of continuous-flow CFPS systems represents one promising approach to overcome volume limitations, allowing for sustained production while maintaining reaction efficiency. Additionally, the integration of microfluidic technologies with CFPS shows potential for parallelization and automation, potentially reducing costs while increasing throughput. Economic analyses suggest that further optimization of energy regeneration systems and recycling of expensive components could reduce production costs by 30-50%, potentially making CFPS economically competitive with conventional protein production methods for high-value products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!