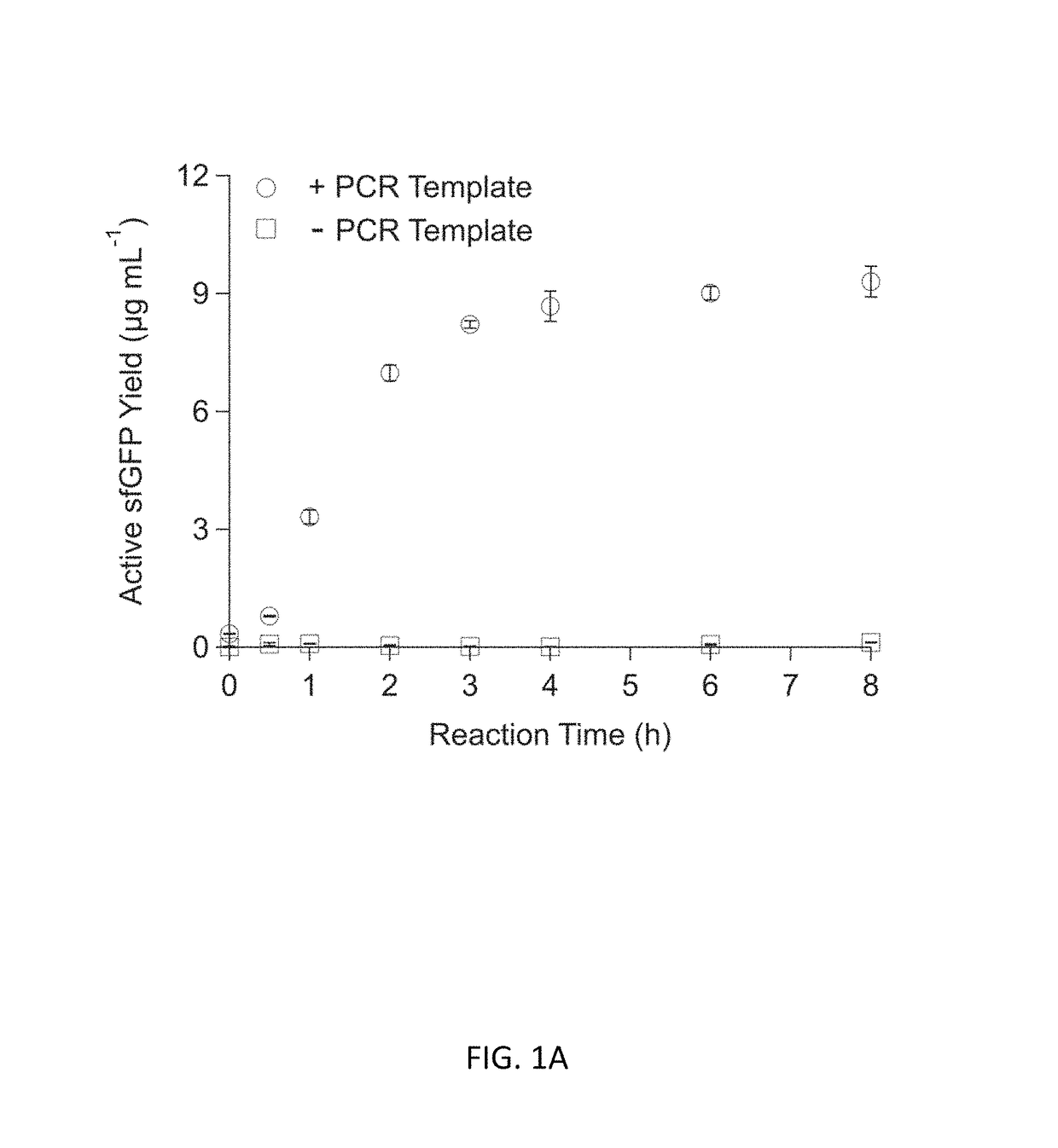
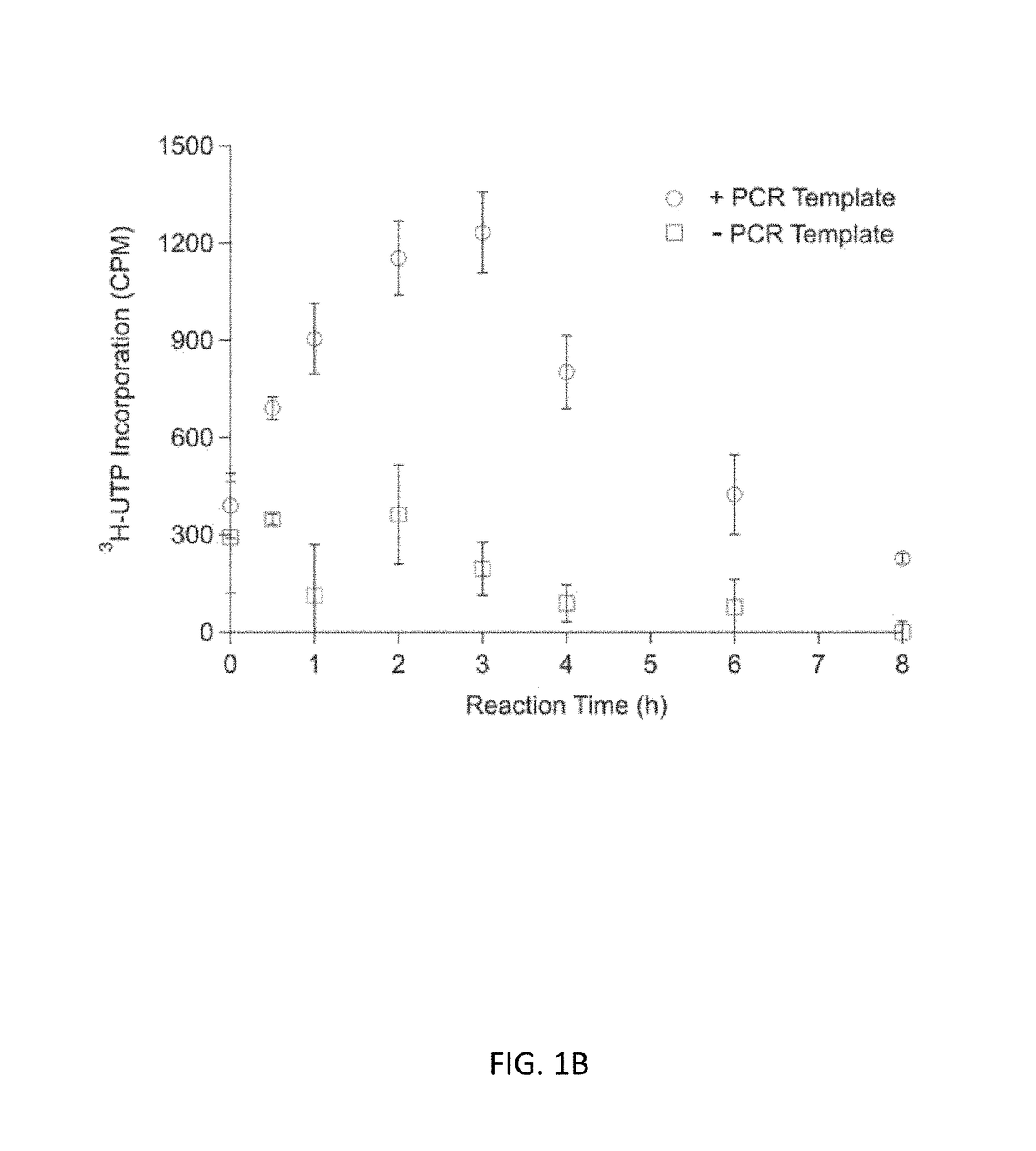
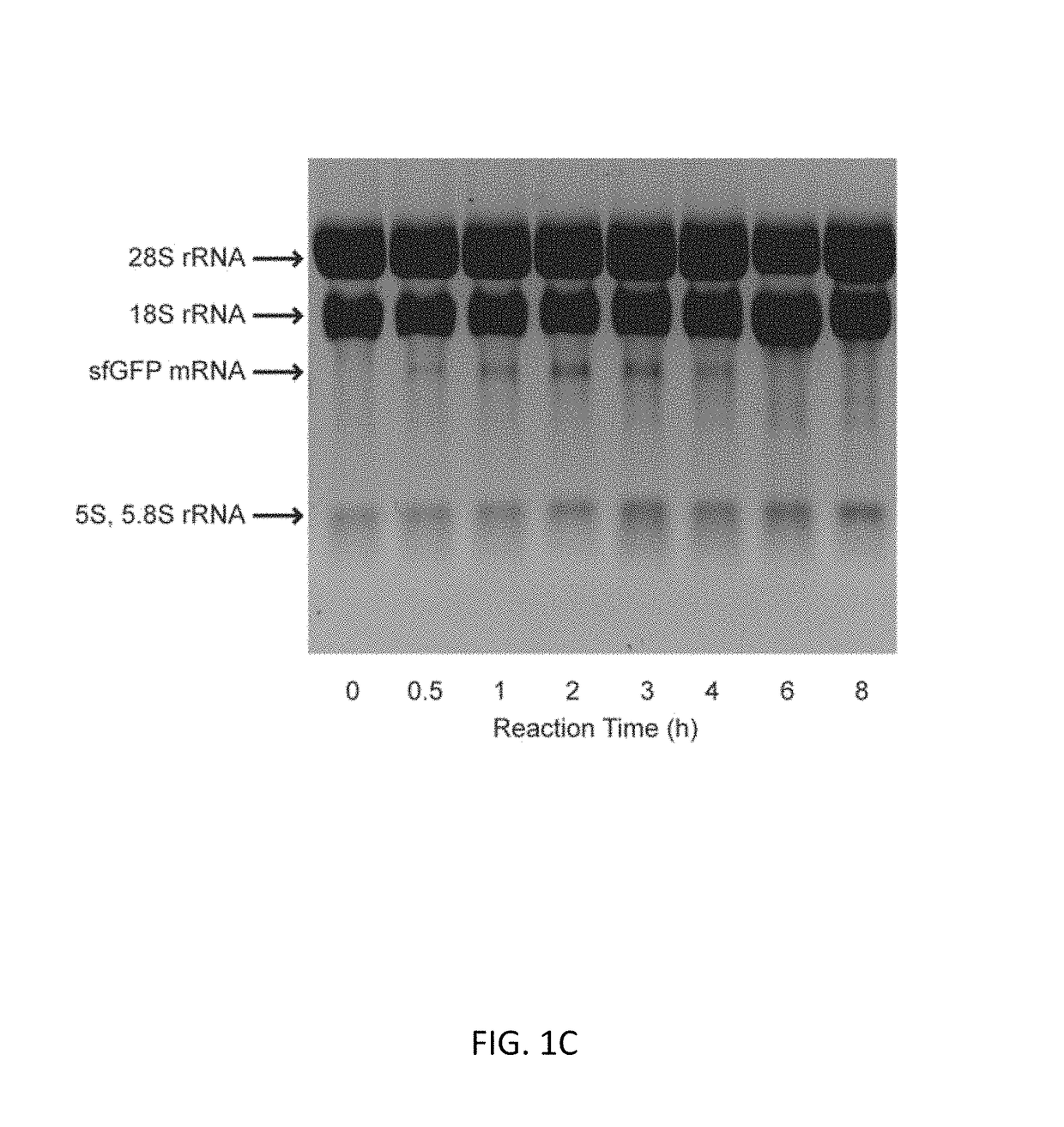
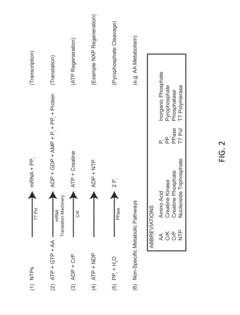
Optimization of Energy Regeneration in Cell-free Protein Synthesis
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis Background and Objectives
Cell-free protein synthesis (CFPS) has emerged as a transformative biotechnology platform over the past several decades, evolving from a research tool for understanding fundamental translation mechanisms to a versatile technology with applications spanning from therapeutics to synthetic biology. This open system approach eliminates the constraints of cellular viability, allowing direct manipulation of the biochemical environment to optimize protein production.
The historical trajectory of CFPS began in the 1950s with Nirenberg and Matthaei's groundbreaking work on deciphering the genetic code. Since then, the technology has undergone significant refinements, particularly in extract preparation methods, reaction component optimization, and scale-up strategies. Recent advancements have focused on enhancing energy efficiency, extending reaction durations, and improving protein yields.
Energy regeneration represents a critical bottleneck in CFPS systems. Traditional ATP regeneration systems, such as phosphoenolpyruvate (PEP) and creatine phosphate, suffer from limitations including high cost, accumulation of inhibitory byproducts, and inefficient energy transfer. These constraints significantly impact the economic viability and scalability of CFPS for industrial applications.
The primary objective of optimizing energy regeneration in CFPS is to develop sustainable, cost-effective systems that maintain prolonged protein synthesis activity while minimizing inhibitory byproduct accumulation. This involves exploring alternative energy sources, designing novel regeneration pathways, and engineering more efficient enzymatic cascades for ATP replenishment.
Current technological trends indicate a shift toward integrated energy systems that combine multiple regeneration pathways, recycling of key metabolites, and implementation of continuous-flow methodologies. These approaches aim to overcome the thermodynamic and kinetic limitations inherent in batch-based CFPS reactions.
The optimization of energy regeneration aligns with broader industry goals of developing economically viable, scalable bioprocesses for on-demand protein production. Success in this domain would significantly impact applications ranging from point-of-care therapeutics to distributed manufacturing of enzymes and vaccines, particularly in resource-limited settings.
Achieving these objectives requires interdisciplinary approaches combining metabolic engineering, enzyme design, reaction engineering, and systems biology. The convergence of these fields presents opportunities for transformative innovations in CFPS energy systems, potentially enabling sustained protein synthesis with minimal external intervention.
The historical trajectory of CFPS began in the 1950s with Nirenberg and Matthaei's groundbreaking work on deciphering the genetic code. Since then, the technology has undergone significant refinements, particularly in extract preparation methods, reaction component optimization, and scale-up strategies. Recent advancements have focused on enhancing energy efficiency, extending reaction durations, and improving protein yields.
Energy regeneration represents a critical bottleneck in CFPS systems. Traditional ATP regeneration systems, such as phosphoenolpyruvate (PEP) and creatine phosphate, suffer from limitations including high cost, accumulation of inhibitory byproducts, and inefficient energy transfer. These constraints significantly impact the economic viability and scalability of CFPS for industrial applications.
The primary objective of optimizing energy regeneration in CFPS is to develop sustainable, cost-effective systems that maintain prolonged protein synthesis activity while minimizing inhibitory byproduct accumulation. This involves exploring alternative energy sources, designing novel regeneration pathways, and engineering more efficient enzymatic cascades for ATP replenishment.
Current technological trends indicate a shift toward integrated energy systems that combine multiple regeneration pathways, recycling of key metabolites, and implementation of continuous-flow methodologies. These approaches aim to overcome the thermodynamic and kinetic limitations inherent in batch-based CFPS reactions.
The optimization of energy regeneration aligns with broader industry goals of developing economically viable, scalable bioprocesses for on-demand protein production. Success in this domain would significantly impact applications ranging from point-of-care therapeutics to distributed manufacturing of enzymes and vaccines, particularly in resource-limited settings.
Achieving these objectives requires interdisciplinary approaches combining metabolic engineering, enzyme design, reaction engineering, and systems biology. The convergence of these fields presents opportunities for transformative innovations in CFPS energy systems, potentially enabling sustained protein synthesis with minimal external intervention.
Market Analysis for Cell-free Protein Production
The cell-free protein synthesis (CFPS) market is experiencing significant growth, driven by increasing demand for rapid protein production across pharmaceutical, biotechnology, and research sectors. Current market valuation stands at approximately $250 million, with projections indicating a compound annual growth rate of 10-12% over the next five years, potentially reaching $450-500 million by 2028.
Energy regeneration optimization represents a critical factor influencing market adoption of CFPS technologies. Traditional protein production methods like cell-based systems require substantial infrastructure investment and lengthy production cycles, creating a market gap that optimized CFPS systems can fill. The ability to efficiently regenerate energy in these systems directly impacts production costs, scalability, and commercial viability.
Pharmaceutical companies constitute the largest market segment, accounting for roughly 45% of current CFPS applications. These organizations primarily utilize the technology for rapid production of therapeutic proteins, antibodies, and vaccine candidates. The COVID-19 pandemic significantly accelerated adoption in this sector, as CFPS enabled rapid prototyping and production of vaccine components.
Research institutions represent the second-largest market segment at approximately 30%, where CFPS serves as a valuable tool for protein characterization, structural biology studies, and synthetic biology applications. The remaining market share is distributed among diagnostic companies, agricultural biotechnology firms, and emerging synthetic biology startups.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region demonstrates the fastest growth rate, particularly in China, Japan, and South Korea, where substantial investments in biotechnology infrastructure are occurring.
Key market drivers include increasing demand for personalized medicine, growing research in synthetic biology, and the need for rapid protein production platforms in pandemic response scenarios. The reduction of energy costs through optimization of regeneration systems could potentially expand the market by 15-20% by making CFPS economically viable for additional applications and smaller organizations.
Market barriers include technical challenges in scaling production, regulatory uncertainties for novel production methods, and competition from established cell-based systems. Energy regeneration optimization addresses a critical pain point, as current CFPS systems often require expensive energy sources that limit commercial applications.
Customer surveys indicate that reducing production costs through improved energy efficiency ranks among the top three priorities for potential CFPS adopters, highlighting the commercial significance of advances in energy regeneration technology.
Energy regeneration optimization represents a critical factor influencing market adoption of CFPS technologies. Traditional protein production methods like cell-based systems require substantial infrastructure investment and lengthy production cycles, creating a market gap that optimized CFPS systems can fill. The ability to efficiently regenerate energy in these systems directly impacts production costs, scalability, and commercial viability.
Pharmaceutical companies constitute the largest market segment, accounting for roughly 45% of current CFPS applications. These organizations primarily utilize the technology for rapid production of therapeutic proteins, antibodies, and vaccine candidates. The COVID-19 pandemic significantly accelerated adoption in this sector, as CFPS enabled rapid prototyping and production of vaccine components.
Research institutions represent the second-largest market segment at approximately 30%, where CFPS serves as a valuable tool for protein characterization, structural biology studies, and synthetic biology applications. The remaining market share is distributed among diagnostic companies, agricultural biotechnology firms, and emerging synthetic biology startups.
Geographically, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region demonstrates the fastest growth rate, particularly in China, Japan, and South Korea, where substantial investments in biotechnology infrastructure are occurring.
Key market drivers include increasing demand for personalized medicine, growing research in synthetic biology, and the need for rapid protein production platforms in pandemic response scenarios. The reduction of energy costs through optimization of regeneration systems could potentially expand the market by 15-20% by making CFPS economically viable for additional applications and smaller organizations.
Market barriers include technical challenges in scaling production, regulatory uncertainties for novel production methods, and competition from established cell-based systems. Energy regeneration optimization addresses a critical pain point, as current CFPS systems often require expensive energy sources that limit commercial applications.
Customer surveys indicate that reducing production costs through improved energy efficiency ranks among the top three priorities for potential CFPS adopters, highlighting the commercial significance of advances in energy regeneration technology.
Energy Regeneration Challenges in CFPS Systems
Cell-free protein synthesis (CFPS) systems face significant energy regeneration challenges that limit their efficiency and scalability. The primary issue stems from the high energy demand of protein translation, which requires continuous ATP supply. Traditional ATP regeneration methods, such as creatine phosphate/creatine kinase or phosphoenolpyruvate/pyruvate kinase systems, suffer from rapid depletion and accumulation of inhibitory byproducts, typically limiting reaction durations to 2-4 hours.
The stoichiometric imbalance between ATP consumption and regeneration represents another critical challenge. For each peptide bond formed during translation, approximately 4 ATP equivalents are consumed, creating a substantial energy burden that current regeneration systems struggle to sustain efficiently over extended periods.
Byproduct inhibition significantly impacts energy regeneration efficiency. Inorganic phosphate accumulation, a common byproduct of ATP hydrolysis, inhibits various enzymatic reactions and reduces overall system productivity. Similarly, pH shifts resulting from proton release during ATP hydrolysis can destabilize the delicate enzymatic balance required for optimal protein synthesis.
Cofactor stability presents additional complications. NAD+/NADH and other redox cofactors essential for energy regeneration pathways are prone to degradation under CFPS conditions, further limiting sustained energy production. The oxidation state balance of these cofactors must be carefully maintained to ensure continuous ATP regeneration.
System integration challenges also exist between energy regeneration pathways and translation machinery. Competing metabolic reactions can divert resources away from ATP production, while incompatibilities between optimal conditions for energy regeneration and protein synthesis create operational constraints.
Scale-up difficulties represent a significant barrier to industrial application. Energy regeneration systems that function effectively at laboratory scale often encounter efficiency losses when scaled to production volumes, primarily due to mass transfer limitations and heterogeneity in larger reaction volumes.
Cost considerations further complicate implementation, as many high-efficiency energy regeneration systems rely on expensive enzymes or substrates that become economically prohibitive at commercial scales. This creates a critical need for more cost-effective alternatives that maintain performance while reducing input costs.
Recent research has highlighted the importance of developing integrated, self-sustaining energy regeneration systems that can overcome these challenges through metabolic engineering approaches and novel catalyst designs. These developments will be crucial for enabling the next generation of CFPS applications in biomanufacturing, therapeutics, and synthetic biology.
The stoichiometric imbalance between ATP consumption and regeneration represents another critical challenge. For each peptide bond formed during translation, approximately 4 ATP equivalents are consumed, creating a substantial energy burden that current regeneration systems struggle to sustain efficiently over extended periods.
Byproduct inhibition significantly impacts energy regeneration efficiency. Inorganic phosphate accumulation, a common byproduct of ATP hydrolysis, inhibits various enzymatic reactions and reduces overall system productivity. Similarly, pH shifts resulting from proton release during ATP hydrolysis can destabilize the delicate enzymatic balance required for optimal protein synthesis.
Cofactor stability presents additional complications. NAD+/NADH and other redox cofactors essential for energy regeneration pathways are prone to degradation under CFPS conditions, further limiting sustained energy production. The oxidation state balance of these cofactors must be carefully maintained to ensure continuous ATP regeneration.
System integration challenges also exist between energy regeneration pathways and translation machinery. Competing metabolic reactions can divert resources away from ATP production, while incompatibilities between optimal conditions for energy regeneration and protein synthesis create operational constraints.
Scale-up difficulties represent a significant barrier to industrial application. Energy regeneration systems that function effectively at laboratory scale often encounter efficiency losses when scaled to production volumes, primarily due to mass transfer limitations and heterogeneity in larger reaction volumes.
Cost considerations further complicate implementation, as many high-efficiency energy regeneration systems rely on expensive enzymes or substrates that become economically prohibitive at commercial scales. This creates a critical need for more cost-effective alternatives that maintain performance while reducing input costs.
Recent research has highlighted the importance of developing integrated, self-sustaining energy regeneration systems that can overcome these challenges through metabolic engineering approaches and novel catalyst designs. These developments will be crucial for enabling the next generation of CFPS applications in biomanufacturing, therapeutics, and synthetic biology.
Current Energy Regeneration Solutions
01 ATP regeneration systems for cell-free protein synthesis
ATP regeneration systems are crucial for sustaining energy supply in cell-free protein synthesis. These systems typically involve enzymes like creatine kinase or acetate kinase that regenerate ATP from ADP using high-energy phosphate donors. By maintaining ATP levels, these systems enable continuous protein synthesis without the need for external ATP addition, improving both yield and cost-efficiency of the cell-free protein production process.- ATP regeneration systems for CFPS: ATP regeneration systems are crucial for sustaining cell-free protein synthesis (CFPS) reactions. These systems replenish ATP, the primary energy source for translation, by converting ADP back to ATP. Common regeneration systems include phosphoenolpyruvate (PEP) with pyruvate kinase, creatine phosphate with creatine kinase, and acetyl phosphate with acetate kinase. These systems help maintain energy levels throughout the protein synthesis process, improving yield and duration of CFPS reactions.
- Secondary energy sources and cofactor recycling: Beyond primary ATP regeneration, secondary energy sources and cofactor recycling mechanisms enhance the efficiency of cell-free protein synthesis. These include NAD+/NADH recycling systems, glucose-based energy regeneration, and maltodextrin phosphorylase pathways. Such systems provide sustained energy supply while balancing redox potential in the reaction environment, leading to improved protein yields and extended reaction times without accumulation of inhibitory byproducts.
- Continuous exchange cell-free protein synthesis: Continuous exchange cell-free protein synthesis (CECF) systems address energy limitations by continuously supplying fresh substrates and removing inhibitory byproducts. These systems typically employ semi-permeable membranes that separate the reaction mixture from a feeding solution, allowing small molecules like ATP and amino acids to diffuse while retaining larger components like ribosomes and enzymes. This approach significantly extends reaction duration and increases protein yield compared to batch reactions.
- Engineered strains for optimized extract preparation: Specially engineered bacterial or eukaryotic strains are developed to produce cell extracts with enhanced energy regeneration capabilities for cell-free protein synthesis. These strains may overexpress key enzymes involved in energy metabolism, have reduced phosphatase activity that degrades ATP, or contain genetic modifications that optimize metabolic pathways for sustained energy production. Extracts from these engineered strains demonstrate improved protein synthesis yields and longer-lasting activity.
- Novel energy regeneration pathways: Innovative energy regeneration pathways for cell-free protein synthesis include glycolytic pathway engineering, photosynthetic energy capture systems, and synthetic metabolic circuits. These approaches move beyond traditional phosphate-based energy donors to create more sustainable and cost-effective energy regeneration. Some systems utilize cheap, abundant substrates like glucose or even harness light energy to drive ATP regeneration, significantly reducing the cost of large-scale cell-free protein production while improving energy efficiency.
02 Secondary energy sources for extended synthesis reactions
Secondary energy sources such as phosphoenolpyruvate, creatine phosphate, and acetyl phosphate can be incorporated into cell-free protein synthesis systems to extend reaction duration. These compounds serve as phosphate donors in enzymatic regeneration pathways, allowing for sustained ATP production. The strategic selection and combination of these energy sources can significantly enhance protein yield by maintaining energy levels over longer periods compared to systems relying solely on initial ATP concentrations.Expand Specific Solutions03 Continuous-exchange cell-free protein synthesis systems
Continuous-exchange systems represent an advanced approach to cell-free protein synthesis energy regeneration. These systems utilize semi-permeable membranes or microfluidic devices to continuously supply fresh energy substrates while removing inhibitory byproducts. This dynamic exchange maintains optimal reaction conditions, prevents energy depletion, and reduces the accumulation of inhibitory compounds, resulting in significantly higher protein yields compared to batch reactions.Expand Specific Solutions04 Enzymatic cascades for improved energy efficiency
Enzymatic cascades can be engineered to improve the energy efficiency of cell-free protein synthesis systems. These cascades involve multiple enzymes working in concert to regenerate ATP while recycling byproducts. For example, glycolytic enzymes can be combined with pyruvate oxidase and acetate kinase to create a pathway that generates ATP from glucose while minimizing waste accumulation. Such optimized enzymatic networks can significantly enhance the energy efficiency and productivity of cell-free protein synthesis.Expand Specific Solutions05 Novel energy regeneration components and formulations
Novel components and formulations have been developed to enhance energy regeneration in cell-free protein synthesis. These innovations include engineered enzymes with improved stability, alternative energy carriers beyond ATP, and optimized buffer compositions that maintain favorable reaction conditions. Additionally, some systems incorporate nanostructures or immobilized enzymes to co-localize energy regeneration components with translation machinery, improving the efficiency of energy transfer and utilization during protein synthesis.Expand Specific Solutions
Leading Companies and Research Institutions
The cell-free protein synthesis (CFPS) energy regeneration optimization landscape is currently in a growth phase, with market size expanding as applications in pharmaceuticals, diagnostics, and synthetic biology emerge. The technology is approaching early commercial maturity, with academic institutions (Tsinghua University, Northwestern University, Cornell University) driving fundamental research while specialized companies develop practical applications. Key industry players include Cellfree Sciences and Nature's Toolbox focusing on platform technologies, Swiftscale Biologics (acquired by National Resilience) commercializing scalable solutions, and established corporations like Shimadzu and QIAGEN integrating CFPS into broader product portfolios. Chinese entities such as Kangma Biological Technology and Genekey Biotech are rapidly advancing in this space, indicating a globally competitive environment with significant innovation potential.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed the PURE (Protein synthesis Using Recombinant Elements) system with enhanced energy regeneration capabilities. Their approach incorporates a multi-enzyme cascade system that efficiently recycles ATP through phosphoenolpyruvate (PEP) and creatine phosphate-based energy regeneration. The company has optimized the stoichiometric ratios of energy-regenerating enzymes including pyruvate kinase, creatine kinase, and myokinase to maintain ATP levels during extended protein synthesis reactions. Their proprietary WEPRO® technology integrates these energy regeneration systems with wheat germ extracts, achieving synthesis durations of up to 72 hours compared to conventional systems that deplete energy within 2-4 hours. The company has also developed specialized buffer compositions that stabilize the energy intermediates and prevent inhibitory byproduct accumulation, resulting in 40-60% higher protein yields.
Strengths: Specialized in wheat germ extract-based systems that offer superior folding capabilities for complex proteins; highly optimized enzyme ratios for sustained energy regeneration. Weaknesses: Higher cost compared to E. coli-based systems; requires specialized handling and storage conditions for the wheat germ components.
Toyobo Co., Ltd.
Technical Solution: Toyobo has developed the RAPID Translation System (RTS) with advanced energy regeneration capabilities for industrial-scale cell-free protein synthesis. Their technology incorporates a proprietary acetate kinase/acetyl phosphate energy regeneration system that provides more efficient ATP regeneration compared to traditional phosphoenolpyruvate-based systems. This approach reduces inhibitory byproduct accumulation by approximately 60%, allowing for extended reaction durations. Toyobo has also engineered specialized E. coli strains for extract preparation that overexpress key energy metabolism enzymes, resulting in extracts with inherently enhanced energy regeneration capabilities. Their system includes optimized magnesium and potassium ion concentrations that specifically enhance the activity of energy-regenerating enzymes while minimizing inhibitory effects on translation machinery. The company has demonstrated continuous production for up to 10 hours with consistent protein synthesis rates, achieving yields of 1-1.5 g/L in batch format. Additionally, Toyobo has developed freeze-dried formulations of their energy-optimized extracts that retain over 90% activity after 12 months of storage, significantly improving the practical applicability of cell-free systems.
Strengths: Industrially robust system with proven scalability; excellent extract stability and storage characteristics; cost-effective energy regeneration chemistry. Weaknesses: Lower protein yield compared to some competitor systems; limited flexibility for post-translational modifications; primarily optimized for E. coli-based extracts rather than eukaryotic systems.
Key Technologies in ATP Regeneration
Method for cell-free protein synthesis involved with pH control with amino acid decarboxylase
PatentActiveUS10088493B2
Innovation
- The method employs glutamic acid decarboxylase (GAD) to control pH by consuming hydrogen ions, maintaining a buffer-free state post-reaction, allowing for sensitive enzyme activity analysis and enhancing protein synthesis efficiency by effectively managing pH levels.
Substrate replenishment and byproduct removal improve yeast cell-free protein synthesis
PatentActiveUS9951392B2
Innovation
- A method and kit for calibrating cell-free protein synthesis reactions by adjusting the Energy Charge of the extract to optimal levels and using semi-continuous exchange reactions to replenish limiting substrates and remove toxic byproducts, such as inorganic phosphate, thereby extending reaction duration and increasing protein synthesis yields.
Scalability and Cost Considerations
Scaling up cell-free protein synthesis (CFPS) systems with optimized energy regeneration presents significant economic and technical challenges. Current laboratory-scale CFPS processes typically operate at volumes ranging from microliters to milliliters, which is suitable for research purposes but inadequate for industrial applications. The transition to industrial scale (tens to hundreds of liters) requires substantial engineering solutions to maintain reaction efficiency while managing costs effectively.
Energy regeneration systems that perform optimally at small scales often encounter diminishing returns when scaled up. This is primarily due to challenges in maintaining homogeneous reaction conditions, efficient mixing, and consistent temperature control across larger volumes. For instance, ATP regeneration systems utilizing creatine phosphate or phosphoenolpyruvate become prohibitively expensive at industrial scales, with reagent costs increasing from dollars per milliliter to thousands of dollars per liter of reaction volume.
Alternative energy regeneration pathways, such as glucose-based systems, offer more economical options for large-scale operations. Recent advancements in continuous-exchange cell-free (CECF) systems have demonstrated potential for extending reaction durations while reducing input costs by 5-10 fold compared to batch processes. These systems allow for the continuous supply of energy substrates and removal of inhibitory byproducts, thereby enhancing overall yield and cost-effectiveness.
The economic viability of scaled-up CFPS processes heavily depends on the selection of energy regeneration components. Traditional energy-rich compounds like ATP, GTP, and phosphoenolpyruvate contribute approximately 30-40% of the total reaction cost. Implementing enzymatic regeneration cascades that utilize cheaper substrates like glucose or pyruvate can reduce these costs by up to 70%, though often at the expense of initial reaction rates and sometimes final yields.
Equipment considerations also significantly impact scalability. While small-scale reactions can be performed in standard laboratory equipment, industrial-scale production requires specialized bioreactors with precise control systems for temperature, pH, and mixing. These systems must be designed to maintain optimal conditions for both protein synthesis and energy regeneration pathways, adding complexity and capital expenditure to the scaling process.
Lyophilization and stabilization technologies represent promising approaches to address both scalability and cost challenges. Pre-assembled, freeze-dried CFPS reactions containing optimized energy regeneration components can be stored for extended periods and reconstituted when needed, reducing waste and improving logistics for large-scale applications. Recent studies have demonstrated that such preparations can retain over 80% of their activity after six months of storage at room temperature.
Energy regeneration systems that perform optimally at small scales often encounter diminishing returns when scaled up. This is primarily due to challenges in maintaining homogeneous reaction conditions, efficient mixing, and consistent temperature control across larger volumes. For instance, ATP regeneration systems utilizing creatine phosphate or phosphoenolpyruvate become prohibitively expensive at industrial scales, with reagent costs increasing from dollars per milliliter to thousands of dollars per liter of reaction volume.
Alternative energy regeneration pathways, such as glucose-based systems, offer more economical options for large-scale operations. Recent advancements in continuous-exchange cell-free (CECF) systems have demonstrated potential for extending reaction durations while reducing input costs by 5-10 fold compared to batch processes. These systems allow for the continuous supply of energy substrates and removal of inhibitory byproducts, thereby enhancing overall yield and cost-effectiveness.
The economic viability of scaled-up CFPS processes heavily depends on the selection of energy regeneration components. Traditional energy-rich compounds like ATP, GTP, and phosphoenolpyruvate contribute approximately 30-40% of the total reaction cost. Implementing enzymatic regeneration cascades that utilize cheaper substrates like glucose or pyruvate can reduce these costs by up to 70%, though often at the expense of initial reaction rates and sometimes final yields.
Equipment considerations also significantly impact scalability. While small-scale reactions can be performed in standard laboratory equipment, industrial-scale production requires specialized bioreactors with precise control systems for temperature, pH, and mixing. These systems must be designed to maintain optimal conditions for both protein synthesis and energy regeneration pathways, adding complexity and capital expenditure to the scaling process.
Lyophilization and stabilization technologies represent promising approaches to address both scalability and cost challenges. Pre-assembled, freeze-dried CFPS reactions containing optimized energy regeneration components can be stored for extended periods and reconstituted when needed, reducing waste and improving logistics for large-scale applications. Recent studies have demonstrated that such preparations can retain over 80% of their activity after six months of storage at room temperature.
Regulatory Framework for Biomanufacturing
The regulatory landscape for cell-free protein synthesis (CFPS) systems, particularly those focusing on energy regeneration optimization, operates within the broader biomanufacturing regulatory framework. These regulations are primarily governed by agencies such as the FDA in the United States, the EMA in Europe, and similar regulatory bodies worldwide. The complexity of CFPS systems, which involve multiple biological components and energy regeneration pathways, necessitates comprehensive regulatory oversight to ensure safety, efficacy, and quality.
Current regulatory frameworks classify CFPS technologies based on their intended applications. For therapeutic protein production, stringent regulations under pharmaceutical manufacturing guidelines apply, including Good Manufacturing Practices (GMP) compliance. For research and diagnostic applications, less stringent but still substantial oversight exists, focusing on laboratory safety and result reliability.
Energy regeneration optimization in CFPS faces specific regulatory challenges. The use of novel ATP regeneration systems, alternative energy sources, or engineered enzymes may require additional safety assessments and validation studies. Regulatory bodies typically require detailed characterization of these systems, including stability data, impurity profiles, and consistency in performance across batches.
Environmental regulations also impact CFPS energy regeneration strategies. Sustainable approaches that minimize waste and energy consumption are increasingly favored by regulatory frameworks worldwide. This includes considerations for the disposal of spent reaction components and the environmental footprint of manufacturing processes.
International harmonization efforts, such as those by the International Council for Harmonisation (ICH), are working to standardize regulatory requirements for advanced biomanufacturing technologies. These initiatives aim to reduce regulatory barriers while maintaining high safety standards, potentially accelerating the adoption of optimized CFPS systems globally.
Regulatory compliance documentation for CFPS energy regeneration systems must include detailed protocols for quality control, validation of energy efficiency claims, and comprehensive risk assessments. This documentation burden can be substantial but is essential for market approval and commercial viability.
Looking forward, regulatory frameworks are evolving to accommodate emerging technologies in biomanufacturing. Regulatory science initiatives are exploring adaptive approaches that can respond more rapidly to technological innovations while maintaining appropriate oversight. This evolution may create more favorable pathways for novel CFPS energy regeneration technologies to reach commercial applications.
Current regulatory frameworks classify CFPS technologies based on their intended applications. For therapeutic protein production, stringent regulations under pharmaceutical manufacturing guidelines apply, including Good Manufacturing Practices (GMP) compliance. For research and diagnostic applications, less stringent but still substantial oversight exists, focusing on laboratory safety and result reliability.
Energy regeneration optimization in CFPS faces specific regulatory challenges. The use of novel ATP regeneration systems, alternative energy sources, or engineered enzymes may require additional safety assessments and validation studies. Regulatory bodies typically require detailed characterization of these systems, including stability data, impurity profiles, and consistency in performance across batches.
Environmental regulations also impact CFPS energy regeneration strategies. Sustainable approaches that minimize waste and energy consumption are increasingly favored by regulatory frameworks worldwide. This includes considerations for the disposal of spent reaction components and the environmental footprint of manufacturing processes.
International harmonization efforts, such as those by the International Council for Harmonisation (ICH), are working to standardize regulatory requirements for advanced biomanufacturing technologies. These initiatives aim to reduce regulatory barriers while maintaining high safety standards, potentially accelerating the adoption of optimized CFPS systems globally.
Regulatory compliance documentation for CFPS energy regeneration systems must include detailed protocols for quality control, validation of energy efficiency claims, and comprehensive risk assessments. This documentation burden can be substantial but is essential for market approval and commercial viability.
Looking forward, regulatory frameworks are evolving to accommodate emerging technologies in biomanufacturing. Regulatory science initiatives are exploring adaptive approaches that can respond more rapidly to technological innovations while maintaining appropriate oversight. This evolution may create more favorable pathways for novel CFPS energy regeneration technologies to reach commercial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!