Optimization of Nucleotide Recycling in Cell-free Protein Synthesis
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CFPS Nucleotide Recycling Background and Objectives
Cell-free protein synthesis (CFPS) has emerged as a transformative biotechnology platform over the past several decades, evolving from a fundamental research tool for understanding translation mechanisms to a versatile technology with applications spanning from diagnostics to biomanufacturing. The optimization of nucleotide recycling within CFPS systems represents a critical frontier in enhancing the efficiency and economic viability of this technology.
Historically, CFPS systems have been limited by the high cost of energy substrates, particularly nucleoside triphosphates (NTPs) which are essential for transcription and translation processes. Early CFPS systems relied on exogenous addition of these expensive components, resulting in prohibitive costs for large-scale applications. The development of nucleotide recycling mechanisms marks a significant advancement in addressing this limitation.
The evolution of nucleotide recycling in CFPS can be traced through several key technological milestones. Initial efforts focused on supplementing reaction mixtures with phosphoenolpyruvate (PEP) as an energy source, which was later improved through the introduction of creatine phosphate and acetyl phosphate systems. A paradigm shift occurred with the development of secondary energy systems that could regenerate ATP from its spent forms (ADP and AMP), thereby establishing cyclical energy regeneration pathways.
Recent advances have incorporated enzymatic cascades that efficiently recycle nucleotides, significantly extending reaction durations and improving protein yields. These developments have been complemented by metabolic engineering approaches that optimize the balance between energy consumption and regeneration within the cell-free environment.
The primary objective of nucleotide recycling optimization is to establish sustainable, cost-effective CFPS systems capable of prolonged protein production without continuous external supplementation of expensive energy components. This involves developing robust regeneration pathways that maintain optimal concentrations of ATP, GTP, UTP, and CTP throughout the reaction duration.
Secondary objectives include reducing the overall cost of CFPS reactions, enhancing scalability for industrial applications, and improving the predictability and reproducibility of protein yields. Additionally, there is significant interest in developing systems that can operate effectively under diverse reaction conditions, including temperature variations and different buffer compositions.
The technological trajectory suggests a convergence toward integrated systems that combine multiple recycling pathways, potentially incorporating synthetic biology approaches to design novel enzymatic cascades specifically optimized for cell-free environments. The ultimate goal remains the creation of self-sustaining CFPS systems that maximize energy efficiency while minimizing input costs, thereby unlocking the full potential of cell-free synthetic biology for both research and commercial applications.
Historically, CFPS systems have been limited by the high cost of energy substrates, particularly nucleoside triphosphates (NTPs) which are essential for transcription and translation processes. Early CFPS systems relied on exogenous addition of these expensive components, resulting in prohibitive costs for large-scale applications. The development of nucleotide recycling mechanisms marks a significant advancement in addressing this limitation.
The evolution of nucleotide recycling in CFPS can be traced through several key technological milestones. Initial efforts focused on supplementing reaction mixtures with phosphoenolpyruvate (PEP) as an energy source, which was later improved through the introduction of creatine phosphate and acetyl phosphate systems. A paradigm shift occurred with the development of secondary energy systems that could regenerate ATP from its spent forms (ADP and AMP), thereby establishing cyclical energy regeneration pathways.
Recent advances have incorporated enzymatic cascades that efficiently recycle nucleotides, significantly extending reaction durations and improving protein yields. These developments have been complemented by metabolic engineering approaches that optimize the balance between energy consumption and regeneration within the cell-free environment.
The primary objective of nucleotide recycling optimization is to establish sustainable, cost-effective CFPS systems capable of prolonged protein production without continuous external supplementation of expensive energy components. This involves developing robust regeneration pathways that maintain optimal concentrations of ATP, GTP, UTP, and CTP throughout the reaction duration.
Secondary objectives include reducing the overall cost of CFPS reactions, enhancing scalability for industrial applications, and improving the predictability and reproducibility of protein yields. Additionally, there is significant interest in developing systems that can operate effectively under diverse reaction conditions, including temperature variations and different buffer compositions.
The technological trajectory suggests a convergence toward integrated systems that combine multiple recycling pathways, potentially incorporating synthetic biology approaches to design novel enzymatic cascades specifically optimized for cell-free environments. The ultimate goal remains the creation of self-sustaining CFPS systems that maximize energy efficiency while minimizing input costs, thereby unlocking the full potential of cell-free synthetic biology for both research and commercial applications.
Market Analysis for Cell-free Protein Synthesis Applications
The cell-free protein synthesis (CFPS) market has been experiencing significant growth, driven by increasing applications in synthetic biology, personalized medicine, and pharmaceutical development. The global CFPS market was valued at approximately $97.2 million in 2020 and is projected to reach $246.8 million by 2027, growing at a CAGR of 14.3% during the forecast period.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for over 45% of the total market share. These companies utilize CFPS technology primarily for rapid protein production, drug discovery, and development of therapeutic proteins. The ability to bypass cell viability constraints makes CFPS particularly valuable for expressing toxic proteins that would otherwise be challenging in traditional cell-based systems.
Academic and research institutions constitute the second-largest market segment, where CFPS serves as a powerful tool for fundamental research in protein science, synthetic biology, and metabolic engineering. The demand in this sector is primarily driven by research grants and increasing focus on protein engineering applications.
Regionally, North America dominates the CFPS market with approximately 40% market share, followed by Europe and Asia-Pacific. The United States leads in CFPS technology adoption due to substantial investments in biotechnology research and the presence of major market players. However, the Asia-Pacific region is expected to witness the fastest growth rate, with China and Japan emerging as key markets due to increasing government funding for biotechnology research.
The optimization of nucleotide recycling in CFPS systems addresses a critical market need, as nucleotide costs represent a significant portion of overall CFPS production expenses. Efficient nucleotide recycling can potentially reduce production costs by 30-40%, making CFPS more economically viable for commercial applications.
Market trends indicate growing demand for continuous-flow CFPS systems that incorporate efficient nucleotide recycling mechanisms. These systems are particularly attractive for industrial-scale protein production, offering improved yields and cost-effectiveness compared to batch processes.
Consumer preferences are shifting toward sustainable and environmentally friendly production methods. CFPS systems with optimized nucleotide recycling align with this trend by reducing resource consumption and waste generation, potentially opening new market opportunities in the green biotechnology sector.
Emerging applications in point-of-care diagnostics and personalized medicine are expected to create new market segments for CFPS technology. The ability to rapidly produce proteins on-demand with minimal resource requirements makes optimized CFPS systems ideal for decentralized production scenarios, particularly in resource-limited settings.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for over 45% of the total market share. These companies utilize CFPS technology primarily for rapid protein production, drug discovery, and development of therapeutic proteins. The ability to bypass cell viability constraints makes CFPS particularly valuable for expressing toxic proteins that would otherwise be challenging in traditional cell-based systems.
Academic and research institutions constitute the second-largest market segment, where CFPS serves as a powerful tool for fundamental research in protein science, synthetic biology, and metabolic engineering. The demand in this sector is primarily driven by research grants and increasing focus on protein engineering applications.
Regionally, North America dominates the CFPS market with approximately 40% market share, followed by Europe and Asia-Pacific. The United States leads in CFPS technology adoption due to substantial investments in biotechnology research and the presence of major market players. However, the Asia-Pacific region is expected to witness the fastest growth rate, with China and Japan emerging as key markets due to increasing government funding for biotechnology research.
The optimization of nucleotide recycling in CFPS systems addresses a critical market need, as nucleotide costs represent a significant portion of overall CFPS production expenses. Efficient nucleotide recycling can potentially reduce production costs by 30-40%, making CFPS more economically viable for commercial applications.
Market trends indicate growing demand for continuous-flow CFPS systems that incorporate efficient nucleotide recycling mechanisms. These systems are particularly attractive for industrial-scale protein production, offering improved yields and cost-effectiveness compared to batch processes.
Consumer preferences are shifting toward sustainable and environmentally friendly production methods. CFPS systems with optimized nucleotide recycling align with this trend by reducing resource consumption and waste generation, potentially opening new market opportunities in the green biotechnology sector.
Emerging applications in point-of-care diagnostics and personalized medicine are expected to create new market segments for CFPS technology. The ability to rapidly produce proteins on-demand with minimal resource requirements makes optimized CFPS systems ideal for decentralized production scenarios, particularly in resource-limited settings.
Current Challenges in Nucleotide Recycling Systems
Despite significant advancements in cell-free protein synthesis (CFPS) systems, nucleotide recycling remains a critical bottleneck that limits the efficiency and cost-effectiveness of these platforms. The primary challenge stems from the rapid depletion of adenosine triphosphate (ATP) and other high-energy nucleotides that serve as essential energy sources for translation processes. Current recycling systems struggle to maintain sufficient nucleotide concentrations throughout extended reaction periods, resulting in premature termination of protein synthesis.
Metabolic burden presents another significant challenge, as recycling pathways often compete with translation machinery for essential cofactors and intermediates. This competition creates an inherent trade-off between energy regeneration and protein production rates. Additionally, the accumulation of inhibitory byproducts such as inorganic phosphate and adenosine monophosphate (AMP) progressively impairs system performance, particularly in batch reaction formats.
Stability issues further complicate nucleotide recycling efforts. Many enzymes involved in regeneration pathways exhibit limited half-lives under CFPS reaction conditions, with activity decreasing significantly after several hours at optimal translation temperatures (30-37°C). This instability necessitates either higher initial enzyme concentrations or periodic supplementation, both of which increase system complexity and cost.
Scalability represents another unresolved challenge. While small-scale laboratory demonstrations have achieved impressive recycling efficiencies, these systems often fail to maintain performance when scaled to industrially relevant volumes. Factors including oxygen transfer limitations, mixing inefficiencies, and temperature gradients contribute to heterogeneous reaction environments that compromise recycling pathway performance.
The integration of multiple recycling pathways presents significant engineering challenges. Current systems typically employ either substrate phosphorylation (e.g., creatine phosphate/creatine kinase) or oxidative phosphorylation approaches, but rarely achieve effective coordination between complementary pathways. This lack of integration results in suboptimal energy utilization and reduced system longevity.
Cost considerations remain paramount, as many current recycling systems rely on expensive enzymes or cofactors that significantly impact the economic viability of CFPS platforms. For instance, systems utilizing pyruvate oxidase for ATP regeneration require costly thiamine pyrophosphate and flavin adenine dinucleotide cofactors, while creatine phosphate-based systems demand substantial quantities of this high-cost phosphate donor.
Regulatory challenges also exist, particularly regarding the implementation of continuous-exchange systems that could theoretically overcome many recycling limitations. These approaches require sophisticated microfluidic or membrane-based technologies that add complexity and may introduce regulatory hurdles for therapeutic protein production applications.
Metabolic burden presents another significant challenge, as recycling pathways often compete with translation machinery for essential cofactors and intermediates. This competition creates an inherent trade-off between energy regeneration and protein production rates. Additionally, the accumulation of inhibitory byproducts such as inorganic phosphate and adenosine monophosphate (AMP) progressively impairs system performance, particularly in batch reaction formats.
Stability issues further complicate nucleotide recycling efforts. Many enzymes involved in regeneration pathways exhibit limited half-lives under CFPS reaction conditions, with activity decreasing significantly after several hours at optimal translation temperatures (30-37°C). This instability necessitates either higher initial enzyme concentrations or periodic supplementation, both of which increase system complexity and cost.
Scalability represents another unresolved challenge. While small-scale laboratory demonstrations have achieved impressive recycling efficiencies, these systems often fail to maintain performance when scaled to industrially relevant volumes. Factors including oxygen transfer limitations, mixing inefficiencies, and temperature gradients contribute to heterogeneous reaction environments that compromise recycling pathway performance.
The integration of multiple recycling pathways presents significant engineering challenges. Current systems typically employ either substrate phosphorylation (e.g., creatine phosphate/creatine kinase) or oxidative phosphorylation approaches, but rarely achieve effective coordination between complementary pathways. This lack of integration results in suboptimal energy utilization and reduced system longevity.
Cost considerations remain paramount, as many current recycling systems rely on expensive enzymes or cofactors that significantly impact the economic viability of CFPS platforms. For instance, systems utilizing pyruvate oxidase for ATP regeneration require costly thiamine pyrophosphate and flavin adenine dinucleotide cofactors, while creatine phosphate-based systems demand substantial quantities of this high-cost phosphate donor.
Regulatory challenges also exist, particularly regarding the implementation of continuous-exchange systems that could theoretically overcome many recycling limitations. These approaches require sophisticated microfluidic or membrane-based technologies that add complexity and may introduce regulatory hurdles for therapeutic protein production applications.
Established Nucleotide Recycling Methodologies
01 Enzymatic pathways for nucleotide recycling
Enzymatic pathways play a crucial role in nucleotide recycling optimization. These pathways involve specific enzymes that catalyze the conversion of nucleotide derivatives back into usable nucleotides. By enhancing the efficiency of these enzymatic reactions, the recycling process can be optimized to recover more nucleotides from cellular waste products. This approach reduces the energy required for de novo nucleotide synthesis and improves overall cellular metabolism.- Enzymatic pathways for nucleotide recycling: Enzymatic pathways play a crucial role in nucleotide recycling optimization. These pathways involve specific enzymes that catalyze the conversion of nucleotide derivatives back to their active forms. By enhancing the efficiency of these enzymatic reactions, the recycling process can be optimized to maintain adequate nucleotide pools within cells. This approach is particularly important in biological systems where nucleotide availability is limited or in high demand.
- Computational methods for nucleotide recycling analysis: Advanced computational methods are employed to analyze and optimize nucleotide recycling processes. These include simulation algorithms, machine learning approaches, and bioinformatics tools that can predict the efficiency of recycling pathways under various conditions. By modeling nucleotide metabolism and recycling, researchers can identify bottlenecks and optimize parameters to enhance the overall efficiency of the process. These computational approaches enable more targeted experimental designs and faster optimization cycles.
- Synthetic biology approaches for enhanced nucleotide recycling: Synthetic biology techniques are utilized to engineer biological systems with improved nucleotide recycling capabilities. This involves the design and construction of novel genetic circuits, metabolic pathways, or modified enzymes that can more efficiently salvage and recycle nucleotides. By introducing optimized recycling mechanisms into cells or cell-free systems, the overall yield and sustainability of nucleotide-dependent processes can be significantly enhanced.
- Nucleotide recycling in therapeutic applications: Nucleotide recycling optimization has important implications for therapeutic applications, particularly in the development of nucleoside analogs for antiviral and anticancer treatments. By understanding and manipulating the recycling pathways, researchers can enhance the efficacy of nucleoside-based drugs, reduce side effects, and overcome resistance mechanisms. This approach involves designing compounds that can effectively utilize the existing cellular recycling machinery or bypass inefficient recycling steps.
- Industrial-scale nucleotide recycling systems: Industrial applications of nucleotide recycling focus on developing large-scale, cost-effective systems for recovering and reusing nucleotides in biotechnology processes. These systems often incorporate immobilized enzymes, continuous flow reactors, or membrane separation technologies to efficiently recycle nucleotides from reaction mixtures. By implementing these advanced recycling systems, manufacturers can reduce the cost of nucleotide-dependent processes such as DNA synthesis, sequencing, and amplification while minimizing waste generation.
02 Computational methods for nucleotide recycling analysis
Advanced computational methods are employed to analyze and optimize nucleotide recycling processes. These include simulation algorithms, machine learning approaches, and bioinformatics tools that can predict the efficiency of recycling pathways under various conditions. By modeling nucleotide metabolism and recycling pathways, researchers can identify bottlenecks and optimize process parameters to enhance nucleotide recovery rates and reduce waste in biological systems.Expand Specific Solutions03 Microfluidic systems for nucleotide recovery
Microfluidic technologies offer precise control over nucleotide recycling processes at microscale levels. These systems enable efficient separation, purification, and recovery of nucleotides from complex biological samples. By manipulating fluid dynamics at small scales, microfluidic devices can achieve higher nucleotide recovery rates with minimal sample volumes. This approach is particularly valuable in laboratory settings where sample conservation and process efficiency are critical.Expand Specific Solutions04 Genetic engineering for enhanced nucleotide salvage pathways
Genetic engineering techniques are used to modify organisms for improved nucleotide recycling capabilities. By overexpressing genes involved in nucleotide salvage pathways or introducing novel genetic elements, researchers can create biological systems with enhanced ability to recycle nucleotides. These engineered organisms can more efficiently reuse nucleotide components, reducing the metabolic cost of nucleotide synthesis and improving overall cellular energy efficiency.Expand Specific Solutions05 Chemical methods for nucleotide recovery and purification
Chemical approaches to nucleotide recycling focus on developing reagents and reaction conditions that facilitate the recovery and purification of nucleotides from degraded materials. These methods include selective precipitation, chromatographic separation, and chemical modification techniques that enable the isolation of intact nucleotides from complex mixtures. By optimizing reaction parameters and developing specialized chemical agents, higher yields of recycled nucleotides can be achieved with greater purity.Expand Specific Solutions
Leading Organizations in CFPS Technology Development
The cell-free protein synthesis (CFPS) nucleotide recycling optimization landscape is currently in a growth phase, with market size expanding as applications in synthetic biology and biopharmaceuticals increase. The technology is approaching maturity with key players demonstrating varied expertise levels. Academic institutions like Tsinghua University, Cornell University, and Northwestern University provide fundamental research, while specialized companies like DNA Script, Cellfree Sciences, and Swiftscale Biologics offer commercial applications. Established corporations including Shimadzu, QIAGEN, and Thermo Fisher Scientific GENEART are integrating CFPS into broader biotechnology portfolios. The competitive dynamics show a blend of academic-industrial partnerships, with companies like Kangma Biological Technology and Biotogether developing region-specific innovations, particularly in Asia where CFPS applications are rapidly expanding.
Cellfree Sciences Co., Ltd.
Technical Solution: Cellfree Sciences has developed a proprietary PURE (Protein synthesis Using Recombinant Elements) system that addresses nucleotide recycling challenges in cell-free protein synthesis (CFPS). Their approach incorporates engineered nucleotide recycling enzymes that efficiently regenerate ATP and GTP from their spent forms (AMP, ADP, GDP) during translation. The system includes optimized concentrations of creatine phosphate and creatine kinase for primary energy regeneration, supplemented with secondary recycling pathways using pyruvate oxidase and acetate kinase. This creates a multi-tiered energy regeneration cascade that maintains optimal nucleotide ratios throughout the protein synthesis reaction. Their technology also features customized nucleotide stabilizing agents that prevent degradation during extended reaction times, allowing for sustained protein production over 24+ hours compared to conventional systems that typically decline after 2-4 hours. The company has demonstrated 40% higher protein yields and significantly improved cost-efficiency by reducing the initial nucleotide requirements by approximately 60%.
Strengths: Superior longevity of protein synthesis reactions with sustained activity over 24+ hours; reduced nucleotide input requirements leading to significant cost savings; higher protein yields compared to conventional systems. Weaknesses: Proprietary system components may increase initial setup costs; complex enzyme balancing requires precise optimization for different protein targets; potential intellectual property restrictions limiting customization.
Nanjing Biotogether Co., Ltd.
Technical Solution: Nanjing Biotogether has developed an innovative approach to nucleotide recycling in cell-free protein synthesis through their BioT-CFPS platform. Their technology centers on a novel dual-phase energy regeneration system that combines traditional phosphate-based energy sources with a secondary lipid-based energy reservoir. The core innovation lies in their engineered nucleotide recycling enzymes that have been optimized for function in the interface between aqueous and lipid phases, creating microenvironments that enhance catalytic efficiency and stability. Their system incorporates modified versions of adenylate kinase and nucleoside diphosphate kinase with improved thermal stability and reduced product inhibition, allowing for more efficient ATP and GTP regeneration from their respective monophosphate forms. Biotogether has also developed a proprietary nucleotide stabilization technology that prevents degradation during extended reaction times by sequestering nucleotides in protective lipid structures when not actively participating in protein synthesis. This approach has demonstrated the ability to maintain productive protein synthesis for over 20 hours with minimal additional nucleotide supplementation. The company reports achieving protein yields of up to 1.8 mg/mL in their optimized system, representing a significant improvement over conventional CFPS approaches that typically yield 0.5-0.8 mg/mL.
Strengths: Innovative dual-phase system provides unique advantages for nucleotide stability and recycling efficiency; extended reaction duration enables higher protein yields; reduced nucleotide consumption improves cost-effectiveness. Weaknesses: Novel lipid-phase components may introduce additional complexity in reaction setup and optimization; potential challenges in scaling up the dual-phase system; may require specialized equipment or expertise to implement effectively.
Key Patents in CFPS Energy Regeneration Systems
Optimized bioprocessing for scalable cell-free protein synthesis
PatentWO2023196608A1
Innovation
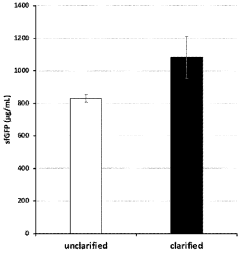
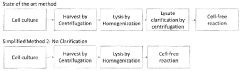
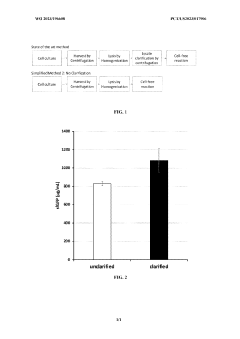
- The method involves using unclarified cellular lysates for protein synthesis, eliminating the need for clarification steps, and includes a system for preparing and processing the lysates using various lysis methods and components such as nucleic acid templates, amino acids, and energy sources, while maintaining substantial protein yields.
Reaction mixture for cell-free protein synthesis, cell-free protein synthesis method in which same is used, and kit for cell-free protein synthesis
PatentWO2018198542A1
Innovation
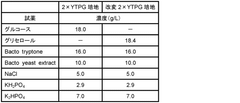
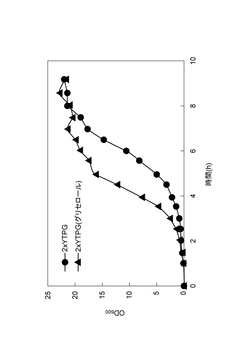
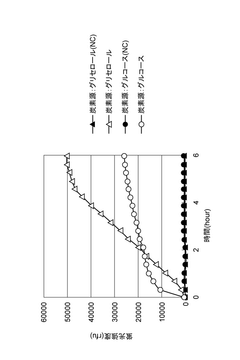
- A reaction mixture for cell-free protein synthesis containing a cell extract from E. coli and glycerol, which includes a template nucleic acid and an energy source, is used to increase protein synthesis efficiency by adding glycerol, allowing for longer reaction duration and improved protein production.
Scalability and Cost Analysis of CFPS Systems
The economic viability of Cell-Free Protein Synthesis (CFPS) systems remains a significant challenge for industrial applications. Current cost estimates for CFPS range from $0.03 to $1.00 per milligram of protein produced, substantially higher than traditional cell-based expression systems. This cost differential presents a major barrier to widespread commercial adoption, particularly for large-scale production of commodity proteins and therapeutics.
Energy regeneration and nucleotide recycling represent critical cost factors in CFPS operations. Traditional ATP regeneration systems using phosphoenolpyruvate can account for up to 30% of reaction costs. Implementation of more efficient nucleotide recycling mechanisms has demonstrated potential cost reductions of 25-40% in laboratory settings, though these improvements have not yet been fully realized at industrial scale.
Scaling CFPS from microliter laboratory reactions to industrial volumes introduces significant engineering challenges. Oxygen transfer limitations, heat dissipation issues, and mixing inefficiencies can substantially reduce protein yields in larger reaction vessels. Current maximum effective batch sizes typically range from 100mL to 1L, with productivity declining markedly beyond these volumes. Continuous-flow and semi-continuous systems offer promising alternatives but require additional capital investment and process development.
Equipment requirements for CFPS present another scalability consideration. While small-scale reactions require minimal specialized equipment, industrial implementation demands sophisticated bioreactors with precise temperature control, mixing capabilities, and monitoring systems. The capital expenditure for such equipment can range from $50,000 to several million dollars depending on scale and automation level, necessitating significant production volumes to achieve reasonable return on investment.
Labor costs also impact CFPS economics substantially. The preparation of cell extracts and reaction components remains labor-intensive, with skilled technician time representing 15-25% of total production costs. Automation opportunities exist but require initial capital investment that may only be justified at larger production scales.
Comparative economic analyses suggest that CFPS becomes cost-competitive with traditional expression systems primarily for high-value, complex proteins where conventional production methods struggle with yield or functionality. For standard recombinant proteins, CFPS currently remains 3-5 times more expensive than optimized cell-based systems. However, this gap continues to narrow as nucleotide recycling technologies and other process optimizations advance.
Energy regeneration and nucleotide recycling represent critical cost factors in CFPS operations. Traditional ATP regeneration systems using phosphoenolpyruvate can account for up to 30% of reaction costs. Implementation of more efficient nucleotide recycling mechanisms has demonstrated potential cost reductions of 25-40% in laboratory settings, though these improvements have not yet been fully realized at industrial scale.
Scaling CFPS from microliter laboratory reactions to industrial volumes introduces significant engineering challenges. Oxygen transfer limitations, heat dissipation issues, and mixing inefficiencies can substantially reduce protein yields in larger reaction vessels. Current maximum effective batch sizes typically range from 100mL to 1L, with productivity declining markedly beyond these volumes. Continuous-flow and semi-continuous systems offer promising alternatives but require additional capital investment and process development.
Equipment requirements for CFPS present another scalability consideration. While small-scale reactions require minimal specialized equipment, industrial implementation demands sophisticated bioreactors with precise temperature control, mixing capabilities, and monitoring systems. The capital expenditure for such equipment can range from $50,000 to several million dollars depending on scale and automation level, necessitating significant production volumes to achieve reasonable return on investment.
Labor costs also impact CFPS economics substantially. The preparation of cell extracts and reaction components remains labor-intensive, with skilled technician time representing 15-25% of total production costs. Automation opportunities exist but require initial capital investment that may only be justified at larger production scales.
Comparative economic analyses suggest that CFPS becomes cost-competitive with traditional expression systems primarily for high-value, complex proteins where conventional production methods struggle with yield or functionality. For standard recombinant proteins, CFPS currently remains 3-5 times more expensive than optimized cell-based systems. However, this gap continues to narrow as nucleotide recycling technologies and other process optimizations advance.
Regulatory Considerations for CFPS-derived Products
Cell-free protein synthesis (CFPS) derived products are increasingly entering commercial and clinical applications, necessitating careful consideration of regulatory frameworks. These products face unique regulatory challenges due to their hybrid nature, combining biological components with in vitro manufacturing processes. Regulatory bodies such as the FDA, EMA, and NMPA have not yet established specific guidelines for CFPS-derived products, creating uncertainty for developers.
The regulatory classification of CFPS products depends largely on their intended use and composition. Products intended for therapeutic applications typically fall under biologics regulations, requiring extensive safety and efficacy testing. Diagnostic applications may be regulated as medical devices or in vitro diagnostics, with varying requirements based on risk classification. Research-use-only products face less stringent oversight but still require compliance with quality standards.
A critical regulatory consideration involves the sourcing of components for CFPS systems. Cell extracts and nucleotides must meet specific purity standards, particularly when derived from animal or human sources. Documentation of origin, processing methods, and quality control measures is essential for regulatory approval. The optimization of nucleotide recycling systems introduces additional regulatory complexities, as novel enzymatic components may require separate safety assessments.
Manufacturing processes for CFPS products demand robust quality control systems. Regulatory bodies expect validated methods for ensuring batch-to-batch consistency, stability testing protocols, and contamination controls. The nucleotide recycling systems must demonstrate reliability and reproducibility across production scales. Process analytical technologies (PAT) are increasingly expected for real-time monitoring of critical quality attributes.
Intellectual property considerations intersect with regulatory requirements, particularly for novel nucleotide recycling methodologies. Patent protection strategies must align with disclosure requirements for regulatory submissions. Freedom-to-operate analyses are essential before commercialization to avoid infringement issues with existing patents on CFPS components or recycling enzymes.
International regulatory harmonization efforts are gradually addressing CFPS technologies, though significant regional differences persist. The International Council for Harmonisation (ICH) guidelines provide some applicable frameworks, but product developers must navigate country-specific requirements. Early engagement with regulatory authorities through scientific advice meetings can help clarify expectations and potentially streamline the approval process for products utilizing optimized nucleotide recycling systems.
The regulatory classification of CFPS products depends largely on their intended use and composition. Products intended for therapeutic applications typically fall under biologics regulations, requiring extensive safety and efficacy testing. Diagnostic applications may be regulated as medical devices or in vitro diagnostics, with varying requirements based on risk classification. Research-use-only products face less stringent oversight but still require compliance with quality standards.
A critical regulatory consideration involves the sourcing of components for CFPS systems. Cell extracts and nucleotides must meet specific purity standards, particularly when derived from animal or human sources. Documentation of origin, processing methods, and quality control measures is essential for regulatory approval. The optimization of nucleotide recycling systems introduces additional regulatory complexities, as novel enzymatic components may require separate safety assessments.
Manufacturing processes for CFPS products demand robust quality control systems. Regulatory bodies expect validated methods for ensuring batch-to-batch consistency, stability testing protocols, and contamination controls. The nucleotide recycling systems must demonstrate reliability and reproducibility across production scales. Process analytical technologies (PAT) are increasingly expected for real-time monitoring of critical quality attributes.
Intellectual property considerations intersect with regulatory requirements, particularly for novel nucleotide recycling methodologies. Patent protection strategies must align with disclosure requirements for regulatory submissions. Freedom-to-operate analyses are essential before commercialization to avoid infringement issues with existing patents on CFPS components or recycling enzymes.
International regulatory harmonization efforts are gradually addressing CFPS technologies, though significant regional differences persist. The International Council for Harmonisation (ICH) guidelines provide some applicable frameworks, but product developers must navigate country-specific requirements. Early engagement with regulatory authorities through scientific advice meetings can help clarify expectations and potentially streamline the approval process for products utilizing optimized nucleotide recycling systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!