Energy Module Engineering for Sustainable Cell-free Protein Synthesis
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell-free Protein Synthesis Energy Background and Objectives
Cell-free protein synthesis (CFPS) has evolved significantly since its inception in the 1950s, transitioning from a fundamental research tool to a versatile biotechnological platform. This technology eliminates the constraints of cellular viability by extracting the necessary machinery for protein production from cells and operating in a controlled environment. The energy supply system represents a critical component of CFPS, directly influencing synthesis efficiency, yield, and sustainability.
Historically, CFPS systems relied heavily on high-energy phosphate compounds such as phosphoenolpyruvate (PEP) and creatine phosphate, which provided rapid but short-lived energy. These traditional approaches faced limitations including high cost, rapid energy depletion, and accumulation of inhibitory byproducts, restricting the scalability and commercial viability of CFPS technologies.
Recent technological advancements have focused on developing more sustainable energy regeneration systems. Notable progress includes the implementation of secondary energy sources like glucose and pyruvate, coupled with enzymatic regeneration pathways that maintain ATP levels while reducing inhibitory byproduct accumulation. These developments have extended reaction durations from hours to days and significantly improved protein yields.
The primary objective of energy module engineering for CFPS is to establish robust, cost-effective, and environmentally sustainable energy systems that can support high-yield protein production at both laboratory and industrial scales. This involves optimizing energy flux, reducing substrate costs, minimizing waste generation, and enhancing overall system efficiency.
Current research aims to develop energy modules that integrate seamlessly with various CFPS platforms, including those derived from E. coli, wheat germ, insect cells, and mammalian systems. Each platform presents unique energy requirements and challenges, necessitating tailored approaches to energy module design.
Looking forward, the field is moving toward completely renewable energy systems that utilize sustainable substrates and incorporate circular metabolic pathways. These advanced systems aim to achieve continuous energy regeneration while maintaining optimal reaction conditions for extended periods, potentially revolutionizing applications in biomanufacturing, personalized medicine, and point-of-care diagnostics.
The convergence of synthetic biology, metabolic engineering, and systems biology approaches is expected to drive innovation in CFPS energy modules, enabling more complex and demanding protein synthesis applications. Ultimately, the development of sustainable energy modules will be crucial for transitioning CFPS technology from laboratory demonstrations to commercially viable manufacturing processes capable of addressing global challenges in healthcare, agriculture, and industrial biotechnology.
Historically, CFPS systems relied heavily on high-energy phosphate compounds such as phosphoenolpyruvate (PEP) and creatine phosphate, which provided rapid but short-lived energy. These traditional approaches faced limitations including high cost, rapid energy depletion, and accumulation of inhibitory byproducts, restricting the scalability and commercial viability of CFPS technologies.
Recent technological advancements have focused on developing more sustainable energy regeneration systems. Notable progress includes the implementation of secondary energy sources like glucose and pyruvate, coupled with enzymatic regeneration pathways that maintain ATP levels while reducing inhibitory byproduct accumulation. These developments have extended reaction durations from hours to days and significantly improved protein yields.
The primary objective of energy module engineering for CFPS is to establish robust, cost-effective, and environmentally sustainable energy systems that can support high-yield protein production at both laboratory and industrial scales. This involves optimizing energy flux, reducing substrate costs, minimizing waste generation, and enhancing overall system efficiency.
Current research aims to develop energy modules that integrate seamlessly with various CFPS platforms, including those derived from E. coli, wheat germ, insect cells, and mammalian systems. Each platform presents unique energy requirements and challenges, necessitating tailored approaches to energy module design.
Looking forward, the field is moving toward completely renewable energy systems that utilize sustainable substrates and incorporate circular metabolic pathways. These advanced systems aim to achieve continuous energy regeneration while maintaining optimal reaction conditions for extended periods, potentially revolutionizing applications in biomanufacturing, personalized medicine, and point-of-care diagnostics.
The convergence of synthetic biology, metabolic engineering, and systems biology approaches is expected to drive innovation in CFPS energy modules, enabling more complex and demanding protein synthesis applications. Ultimately, the development of sustainable energy modules will be crucial for transitioning CFPS technology from laboratory demonstrations to commercially viable manufacturing processes capable of addressing global challenges in healthcare, agriculture, and industrial biotechnology.
Market Analysis for Sustainable CFPS Technologies
The cell-free protein synthesis (CFPS) market is experiencing significant growth, driven by increasing demand for rapid protein production systems in pharmaceutical, biotechnology, and research applications. Currently valued at approximately $250 million, the CFPS market is projected to grow at a CAGR of 8-10% over the next five years, potentially reaching $400-450 million by 2028.
The sustainable CFPS technologies segment represents an emerging opportunity within this market, with energy module engineering being a critical component. This sub-segment is growing faster than the overall CFPS market at an estimated 12-15% annually, reflecting the increasing emphasis on sustainability across industries.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for roughly 45% of the total CFPS market. These companies utilize CFPS technologies primarily for drug discovery, vaccine development, and therapeutic protein production. The COVID-19 pandemic has significantly accelerated adoption in this sector, as CFPS offers rapid prototyping capabilities essential for emergency response.
Academic and research institutions form the second-largest market segment (approximately 30%), where sustainable CFPS technologies are increasingly preferred due to both environmental considerations and institutional sustainability mandates. The remaining market share is distributed among diagnostic companies, agricultural biotechnology, and emerging applications in synthetic biology.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 15-18% annually, driven by expanding biotechnology sectors in China, Japan, and South Korea, along with increasing government investments in sustainable biotechnologies.
Key market drivers include increasing demand for personalized medicine, growing emphasis on sustainable manufacturing processes, and the need for rapid protein production systems. The reduced environmental footprint of sustainable CFPS technologies compared to traditional cell-based systems represents a significant market advantage, with studies indicating up to 60% reduction in water usage and 40% reduction in energy consumption.
Market barriers include high initial costs of implementing sustainable CFPS platforms, technical challenges in scaling production, and regulatory uncertainties. Additionally, the market faces competition from improving traditional cell-based protein production systems that are implementing their own sustainability initiatives.
Customer willingness to pay premiums for sustainably produced proteins varies significantly by application, with pharmaceutical applications showing the highest premium tolerance (15-20%), while research applications demonstrate more price sensitivity with acceptable premiums of only 5-10%.
The sustainable CFPS technologies segment represents an emerging opportunity within this market, with energy module engineering being a critical component. This sub-segment is growing faster than the overall CFPS market at an estimated 12-15% annually, reflecting the increasing emphasis on sustainability across industries.
Pharmaceutical and biotechnology companies constitute the largest market segment, accounting for roughly 45% of the total CFPS market. These companies utilize CFPS technologies primarily for drug discovery, vaccine development, and therapeutic protein production. The COVID-19 pandemic has significantly accelerated adoption in this sector, as CFPS offers rapid prototyping capabilities essential for emergency response.
Academic and research institutions form the second-largest market segment (approximately 30%), where sustainable CFPS technologies are increasingly preferred due to both environmental considerations and institutional sustainability mandates. The remaining market share is distributed among diagnostic companies, agricultural biotechnology, and emerging applications in synthetic biology.
Regionally, North America dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 15-18% annually, driven by expanding biotechnology sectors in China, Japan, and South Korea, along with increasing government investments in sustainable biotechnologies.
Key market drivers include increasing demand for personalized medicine, growing emphasis on sustainable manufacturing processes, and the need for rapid protein production systems. The reduced environmental footprint of sustainable CFPS technologies compared to traditional cell-based systems represents a significant market advantage, with studies indicating up to 60% reduction in water usage and 40% reduction in energy consumption.
Market barriers include high initial costs of implementing sustainable CFPS platforms, technical challenges in scaling production, and regulatory uncertainties. Additionally, the market faces competition from improving traditional cell-based protein production systems that are implementing their own sustainability initiatives.
Customer willingness to pay premiums for sustainably produced proteins varies significantly by application, with pharmaceutical applications showing the highest premium tolerance (15-20%), while research applications demonstrate more price sensitivity with acceptable premiums of only 5-10%.
Current Energy Module Challenges in CFPS Systems
Cell-free protein synthesis (CFPS) systems face significant energy-related challenges that impede their widespread industrial application. The primary obstacle lies in the inefficient regeneration of adenosine triphosphate (ATP), the cellular energy currency essential for protein synthesis. Current ATP regeneration systems typically rely on phosphoenolpyruvate (PEP), creatine phosphate, or acetyl phosphate as high-energy phosphate donors, but these substrates are expensive and often lead to rapid energy depletion during extended reactions.
The accumulation of inhibitory byproducts presents another major challenge. As energy substrates are consumed, metabolic byproducts like inorganic phosphate and protons accumulate in the reaction environment. These byproducts can significantly alter the pH and ionic strength of the reaction mixture, leading to decreased enzyme activity and premature termination of protein synthesis. This issue becomes particularly pronounced in batch reactions where there is no continuous removal of waste products.
Energy module sustainability represents a critical limitation in current CFPS systems. Most existing energy regeneration pathways support protein synthesis for only 2-4 hours before energy depletion occurs. This short productive window severely restricts yield potential and makes scaling processes economically unfeasible for many applications. The development of continuous or semi-continuous energy regeneration systems has been attempted but faces challenges in maintaining consistent reaction conditions over extended periods.
The coupling efficiency between energy consumption and protein production remains suboptimal in many CFPS platforms. Studies indicate that a significant portion of supplied energy is diverted to non-productive pathways or lost through side reactions. This energy misdirection not only reduces system efficiency but also contributes to increased operational costs, as more energy substrates must be supplied to achieve target protein yields.
Temperature sensitivity of energy regeneration enzymes creates additional complications. Many energy regeneration pathways involve multiple enzymatic steps, each with different temperature optima. This creates inherent instability in the energy module when operating at temperatures optimal for translation machinery, resulting in decreased energy availability over time and contributing to reaction termination.
Scalability concerns further complicate energy module engineering. Laboratory-scale energy regeneration strategies often fail to perform consistently at larger volumes due to mass transfer limitations, heat distribution issues, and altered reaction kinetics. The development of energy modules that maintain efficiency across different scales remains a significant engineering challenge that must be addressed for industrial implementation of CFPS technology.
The accumulation of inhibitory byproducts presents another major challenge. As energy substrates are consumed, metabolic byproducts like inorganic phosphate and protons accumulate in the reaction environment. These byproducts can significantly alter the pH and ionic strength of the reaction mixture, leading to decreased enzyme activity and premature termination of protein synthesis. This issue becomes particularly pronounced in batch reactions where there is no continuous removal of waste products.
Energy module sustainability represents a critical limitation in current CFPS systems. Most existing energy regeneration pathways support protein synthesis for only 2-4 hours before energy depletion occurs. This short productive window severely restricts yield potential and makes scaling processes economically unfeasible for many applications. The development of continuous or semi-continuous energy regeneration systems has been attempted but faces challenges in maintaining consistent reaction conditions over extended periods.
The coupling efficiency between energy consumption and protein production remains suboptimal in many CFPS platforms. Studies indicate that a significant portion of supplied energy is diverted to non-productive pathways or lost through side reactions. This energy misdirection not only reduces system efficiency but also contributes to increased operational costs, as more energy substrates must be supplied to achieve target protein yields.
Temperature sensitivity of energy regeneration enzymes creates additional complications. Many energy regeneration pathways involve multiple enzymatic steps, each with different temperature optima. This creates inherent instability in the energy module when operating at temperatures optimal for translation machinery, resulting in decreased energy availability over time and contributing to reaction termination.
Scalability concerns further complicate energy module engineering. Laboratory-scale energy regeneration strategies often fail to perform consistently at larger volumes due to mass transfer limitations, heat distribution issues, and altered reaction kinetics. The development of energy modules that maintain efficiency across different scales remains a significant engineering challenge that must be addressed for industrial implementation of CFPS technology.
Current Energy Module Engineering Solutions
01 ATP regeneration systems for CFPS
ATP regeneration systems are crucial components in cell-free protein synthesis (CFPS) energy modules. These systems continuously replenish ATP, the primary energy source for protein synthesis, allowing for sustained production. Common regeneration approaches include using phosphoenolpyruvate (PEP) with pyruvate kinase, creatine phosphate with creatine kinase, or acetyl phosphate with acetate kinase. These systems help maintain energy levels throughout the protein synthesis process, significantly improving yield and duration of the reaction.- ATP regeneration systems for cell-free protein synthesis: ATP regeneration systems are crucial components in cell-free protein synthesis to maintain energy supply. These systems typically include enzymes like creatine kinase or acetate kinase that regenerate ATP from ADP using high-energy phosphate donors. By continuously replenishing ATP, these systems enable sustained protein synthesis reactions over extended periods, improving yield and efficiency of the cell-free protein production process.
- Secondary energy sources in cell-free protein synthesis: Secondary energy sources such as phosphoenolpyruvate, creatine phosphate, and acetyl phosphate can be incorporated into cell-free protein synthesis systems to enhance energy availability. These compounds serve as phosphate donors in enzymatic reactions that regenerate ATP. The strategic selection and combination of these secondary energy sources can significantly improve the duration and productivity of cell-free protein synthesis reactions by maintaining optimal ATP levels throughout the process.
- Continuous-exchange cell-free protein synthesis systems: Continuous-exchange cell-free protein synthesis systems involve the continuous supply of energy substrates and removal of inhibitory byproducts during the reaction. These systems utilize specialized reaction chambers with semi-permeable membranes that allow small molecules to diffuse while retaining larger components like ribosomes and enzymes. This approach significantly extends reaction lifetimes and increases protein yields by preventing energy depletion and byproduct accumulation that typically limit batch reactions.
- Optimization of energy metabolism in cell-free systems: Optimization of energy metabolism in cell-free protein synthesis involves engineering the extract preparation methods and supplementing with specific metabolic cofactors. This includes adjusting the concentration of key components like magnesium, potassium, and nucleotides, as well as incorporating metabolic regulators that enhance energy efficiency. Advanced approaches include the addition of molecular crowding agents and optimization of redox conditions to maintain enzyme activity and energy transfer efficiency throughout the reaction.
- Novel energy modules for extended-duration synthesis: Novel energy modules for extended-duration synthesis incorporate multiple energy regeneration pathways working in concert. These advanced systems may combine glycolytic enzymes with oxidative phosphorylation components or utilize synthetic metabolic circuits designed specifically for cell-free environments. Some approaches include immobilized enzyme systems, nanoparticle-based energy delivery mechanisms, or light-driven ATP regeneration systems that can maintain energy supply for significantly longer periods than conventional methods, enabling higher protein yields.
02 Secondary energy sources and cofactors
Beyond primary ATP regeneration, cell-free protein synthesis energy modules incorporate secondary energy sources and essential cofactors. These include NAD+/NADH, NADP+/NADPH, and GTP, which support various biochemical reactions during protein synthesis. Optimized ratios of these cofactors are critical for maintaining redox balance and ensuring efficient translation. Some systems also utilize glucose or maltose with appropriate enzymes to create a continuous energy supply pathway, enhancing the overall efficiency and productivity of the cell-free system.Expand Specific Solutions03 Engineered extracts for improved energy efficiency
Specially engineered cell extracts can significantly improve the energy efficiency of cell-free protein synthesis systems. These extracts are often derived from modified organisms with enhanced metabolic pathways or reduced energy-consuming side reactions. By eliminating competing pathways that deplete energy resources and optimizing the concentration of key enzymes involved in energy metabolism, these engineered extracts can sustain protein synthesis for longer periods with higher yields. Some approaches include genetic modifications to reduce phosphatase activity or enhance glycolytic flux.Expand Specific Solutions04 Continuous-exchange and flow-based energy systems
Continuous-exchange and flow-based systems represent advanced approaches to energy management in cell-free protein synthesis. These systems continuously supply fresh energy components while removing inhibitory byproducts, allowing for extended reaction times and higher protein yields. Designs include dialysis-based setups, microfluidic platforms, and membrane-separated reaction chambers. By maintaining optimal concentrations of energy substrates and preventing the accumulation of inhibitory molecules like AMP and phosphate, these systems can achieve significantly higher protein production compared to batch reactions.Expand Specific Solutions05 Novel energy module formulations
Innovative formulations for energy modules are being developed to enhance the efficiency and cost-effectiveness of cell-free protein synthesis. These include all-in-one lyophilized preparations that maintain stability during storage, polymer-encapsulated energy components for controlled release, and synthetic metabolic pathways designed specifically for cell-free systems. Some formulations incorporate non-natural energy carriers or enzymatic cascades that can operate under a wider range of conditions. These novel approaches aim to address limitations in traditional energy systems while making cell-free protein synthesis more accessible for various applications.Expand Specific Solutions
Leading Organizations in Sustainable CFPS Development
Cell-free protein synthesis (CFPS) for sustainable energy modules is in an early growth phase, with market size expanding due to increasing applications in pharmaceuticals, diagnostics, and synthetic biology. The technology is transitioning from research to commercial applications, though still maturing. Key players include established companies like QIAGEN GmbH and Roche Diagnostics providing research tools, alongside innovative startups such as Nuprotein, Nature's Toolbox, and GreenLight Biosciences developing proprietary CFPS platforms. Academic institutions including Northwestern University, Tsinghua University, and Cornell University contribute fundamental research. Kangma Biological Technology and Cellfree Sciences are advancing specialized applications, while Shimadzu and Toyobo provide supporting technologies. The competitive landscape shows a mix of established players and emerging disruptors focusing on improving energy efficiency and sustainability in CFPS systems.
Nuprotein Co. Ltd.
Technical Solution: Nuprotein has engineered a sustainable cell-free protein synthesis platform centered around their proprietary "Cyclic Energy Regeneration" technology. Their system utilizes a carefully balanced combination of phosphoenolpyruvate (PEP) and acetyl phosphate as primary energy donors, creating a dual-phase energy release profile that maintains optimal ATP levels throughout extended reactions. The company has developed specialized extract preparation methods that preserve endogenous energy regeneration pathways while removing inhibitory components that typically limit reaction duration. Their technology incorporates a unique small-molecule additive package that stabilizes key enzymes involved in energy metabolism and prevents the accumulation of inhibitory byproducts. Nuprotein's platform features a continuous-exchange bioreactor design that allows for the selective removal of waste products while replenishing energy substrates, enabling reaction durations exceeding 24 hours. Additionally, they've implemented a proprietary redox balancing system that maintains optimal NAD+/NADH ratios, critical for sustained energy production. The company has also developed computational models that predict energy consumption patterns for different protein targets, allowing for customized energy module formulations that minimize waste and maximize production efficiency.
Strengths: Their dual-phase energy release system provides excellent long-term stability for extended reactions. The continuous-exchange bioreactor design significantly extends reaction lifetime and improves overall yield. Weaknesses: The specialized extract preparation methods may be more labor-intensive and less standardized than commercial alternatives. The system's dependence on proprietary additives may create supply chain vulnerabilities and increase production costs.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has pioneered a cell-free protein synthesis platform specifically engineered for energy efficiency and sustainability. Their technology utilizes a proprietary energy regeneration system based on phosphoenolpyruvate (PEP) and pyruvate oxidase to create a continuous ATP supply chain. The company has developed a unique approach that combines metabolic engineering with enzyme optimization to create a balanced redox state during protein production. Their system incorporates carefully selected glycolytic intermediates that serve as both carbon sources and energy donors, reducing the need for expensive high-energy compounds. GreenLight's platform features a novel oxygen delivery system that maintains optimal dissolved oxygen levels for oxidative phosphorylation without damaging sensitive protein products. Additionally, they've implemented a real-time monitoring system that allows for precise control of reaction conditions and energy input, enabling on-demand adjustment of energy parameters to match the specific requirements of different protein targets. This adaptive energy management significantly reduces waste and improves overall system sustainability.
Strengths: Their integrated oxygen delivery system enables more efficient energy utilization through oxidative phosphorylation pathways. The real-time monitoring and adjustment capabilities allow for customization based on specific protein production needs. Weaknesses: The system may require more sophisticated equipment and control mechanisms compared to simpler CFPS approaches. The oxygen delivery system adds complexity that could increase the risk of oxidative damage to certain sensitive proteins.
Key Innovations in ATP Regeneration Technologies
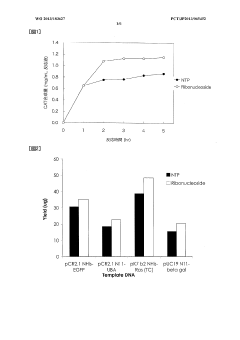
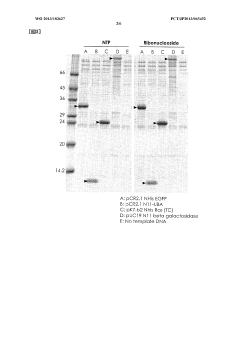
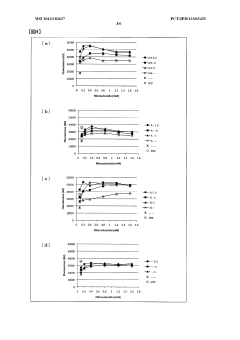
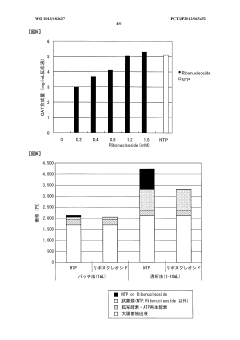
Protein synthesis method and protein synthesis kit
PatentWO2013183627A1
Innovation
- The method involves using ribonucleosides such as adenosine, guanosine, uridine, and cytidine in a cell-free protein synthesis system, where these nucleosides are converted into NTPs by enzymes present in the cell extract, eliminating the need for expensive NTPs and NMPs, thereby reducing production costs.
Cell-free protein synthesizing method by continuous energy supply system using intracellular component
PatentWO2005003341A1
Innovation
- A continuous energy supply system utilizing a fraction mainly composed of endoplasmic reticulum components, such as microsomal fractions from animal or yeast cells, where energy sources are separated from synthesis components using diffusion overlay or semipermeable membrane methods to maintain protein synthesis efficiency over extended periods.
Scalability and Cost Analysis of CFPS Energy Modules
The economic viability of Cell-Free Protein Synthesis (CFPS) systems heavily depends on the scalability and cost-effectiveness of their energy modules. Current laboratory-scale CFPS operations demonstrate promising protein yields but face significant challenges when scaling to industrial production levels. Analysis of production costs reveals that energy regeneration components constitute approximately 30-45% of the total operational expenses in CFPS systems, highlighting the critical importance of optimizing these modules for commercial applications.
When scaling up CFPS processes from milliliter to liter volumes, several technical challenges emerge. Oxygen transfer limitations become pronounced in larger reaction vessels, affecting the efficiency of oxidative phosphorylation-based energy regeneration systems. Additionally, heat dissipation issues in scaled-up reactions can lead to temperature gradients that compromise the stability and activity of energy-generating enzymes, resulting in decreased overall system performance.
Cost analysis of current CFPS energy modules indicates that substrate-level phosphorylation systems utilizing phosphoenolpyruvate (PEP) remain the most expensive option at approximately $1.50-2.00 per gram of produced protein. In contrast, glucose-based systems have reduced costs to $0.60-0.90 per gram, while more recent developments in glycolytic cascade systems have achieved costs as low as $0.30-0.50 per gram of protein. These economic improvements demonstrate the significant progress in making CFPS technology more commercially viable.
Economies of scale present both opportunities and challenges for CFPS energy modules. While bulk purchasing of reagents can reduce per-unit costs by 30-50%, specialized enzymes required for certain energy regeneration systems remain expensive even at larger scales. Mathematical modeling suggests that optimal economic efficiency occurs at production volumes between 100-1000 liters, beyond which diminishing returns on cost reduction are observed due to increasing complexity in maintaining reaction homogeneity.
Recent innovations in continuous-flow CFPS systems have demonstrated promising results for improving scalability. These systems allow for the continuous addition of energy substrates and removal of inhibitory byproducts, extending reaction lifetimes from hours to days. Economic projections indicate that such continuous systems could potentially reduce energy module costs by 40-60% compared to batch processes, representing a significant advancement toward industrial viability.
Sustainability metrics reveal that glucose-based energy systems have the lowest environmental impact, with carbon footprints approximately 65% lower than PEP-based systems. However, the environmental benefits must be balanced against slightly lower protein yields in some applications. Future economic models suggest that integrating renewable energy sources and bio-derived substrates could further reduce costs by 15-25% while simultaneously improving the sustainability profile of CFPS energy modules.
When scaling up CFPS processes from milliliter to liter volumes, several technical challenges emerge. Oxygen transfer limitations become pronounced in larger reaction vessels, affecting the efficiency of oxidative phosphorylation-based energy regeneration systems. Additionally, heat dissipation issues in scaled-up reactions can lead to temperature gradients that compromise the stability and activity of energy-generating enzymes, resulting in decreased overall system performance.
Cost analysis of current CFPS energy modules indicates that substrate-level phosphorylation systems utilizing phosphoenolpyruvate (PEP) remain the most expensive option at approximately $1.50-2.00 per gram of produced protein. In contrast, glucose-based systems have reduced costs to $0.60-0.90 per gram, while more recent developments in glycolytic cascade systems have achieved costs as low as $0.30-0.50 per gram of protein. These economic improvements demonstrate the significant progress in making CFPS technology more commercially viable.
Economies of scale present both opportunities and challenges for CFPS energy modules. While bulk purchasing of reagents can reduce per-unit costs by 30-50%, specialized enzymes required for certain energy regeneration systems remain expensive even at larger scales. Mathematical modeling suggests that optimal economic efficiency occurs at production volumes between 100-1000 liters, beyond which diminishing returns on cost reduction are observed due to increasing complexity in maintaining reaction homogeneity.
Recent innovations in continuous-flow CFPS systems have demonstrated promising results for improving scalability. These systems allow for the continuous addition of energy substrates and removal of inhibitory byproducts, extending reaction lifetimes from hours to days. Economic projections indicate that such continuous systems could potentially reduce energy module costs by 40-60% compared to batch processes, representing a significant advancement toward industrial viability.
Sustainability metrics reveal that glucose-based energy systems have the lowest environmental impact, with carbon footprints approximately 65% lower than PEP-based systems. However, the environmental benefits must be balanced against slightly lower protein yields in some applications. Future economic models suggest that integrating renewable energy sources and bio-derived substrates could further reduce costs by 15-25% while simultaneously improving the sustainability profile of CFPS energy modules.
Environmental Impact Assessment of CFPS Technologies
Cell-free protein synthesis (CFPS) technologies, while offering significant advantages over traditional cell-based systems, present unique environmental considerations that warrant comprehensive assessment. The environmental footprint of CFPS systems begins with raw material sourcing, where extraction of cellular components often requires energy-intensive processes and chemical reagents. These extraction methods can generate hazardous waste streams containing organic solvents, detergents, and biological materials that require specialized disposal protocols to prevent environmental contamination.
Energy consumption represents a critical environmental factor in CFPS operations. Current systems typically demand substantial energy inputs for reaction maintenance, temperature control, and mixing operations. This energy dependency creates a significant carbon footprint, particularly when powered by non-renewable energy sources. Comparative lifecycle analyses indicate that CFPS systems may consume 30-45% more energy per gram of protein produced than optimized cell-based systems, though this gap narrows considerably when accounting for downstream processing requirements.
Water usage in CFPS technologies presents another environmental concern. The production of high-purity water for reaction buffers and subsequent purification steps consumes substantial water resources. Additionally, the disposal of reaction mixtures containing unmetabolized substrates, enzymes, and cofactors can potentially impact aquatic ecosystems if not properly managed. Recent advancements in recycling reaction components have demonstrated potential reductions in water consumption by up to 60%, though implementation remains limited.
Chemical waste generation from CFPS operations includes spent energy substrates, buffer components, and stabilizing agents. The environmental persistence of these compounds varies significantly, with some nucleotide derivatives and modified amino acids showing resistance to conventional wastewater treatment processes. Emerging research indicates that certain CFPS waste streams may contain bioactive compounds capable of disrupting microbial communities in receiving environments, necessitating advanced treatment approaches.
Sustainable engineering approaches for CFPS energy modules show promising environmental benefits. Systems utilizing renewable energy sources for reaction maintenance can reduce carbon emissions by 70-85% compared to conventional setups. Furthermore, the development of recyclable ATP regeneration systems and enzyme immobilization techniques has demonstrated potential for reducing resource consumption and waste generation. These innovations, coupled with process intensification strategies, suggest pathways toward environmentally sustainable CFPS technologies that align with circular economy principles and reduced environmental impact across the technology lifecycle.
Energy consumption represents a critical environmental factor in CFPS operations. Current systems typically demand substantial energy inputs for reaction maintenance, temperature control, and mixing operations. This energy dependency creates a significant carbon footprint, particularly when powered by non-renewable energy sources. Comparative lifecycle analyses indicate that CFPS systems may consume 30-45% more energy per gram of protein produced than optimized cell-based systems, though this gap narrows considerably when accounting for downstream processing requirements.
Water usage in CFPS technologies presents another environmental concern. The production of high-purity water for reaction buffers and subsequent purification steps consumes substantial water resources. Additionally, the disposal of reaction mixtures containing unmetabolized substrates, enzymes, and cofactors can potentially impact aquatic ecosystems if not properly managed. Recent advancements in recycling reaction components have demonstrated potential reductions in water consumption by up to 60%, though implementation remains limited.
Chemical waste generation from CFPS operations includes spent energy substrates, buffer components, and stabilizing agents. The environmental persistence of these compounds varies significantly, with some nucleotide derivatives and modified amino acids showing resistance to conventional wastewater treatment processes. Emerging research indicates that certain CFPS waste streams may contain bioactive compounds capable of disrupting microbial communities in receiving environments, necessitating advanced treatment approaches.
Sustainable engineering approaches for CFPS energy modules show promising environmental benefits. Systems utilizing renewable energy sources for reaction maintenance can reduce carbon emissions by 70-85% compared to conventional setups. Furthermore, the development of recyclable ATP regeneration systems and enzyme immobilization techniques has demonstrated potential for reducing resource consumption and waste generation. These innovations, coupled with process intensification strategies, suggest pathways toward environmentally sustainable CFPS technologies that align with circular economy principles and reduced environmental impact across the technology lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!