Battery Pack Design for Portable Medical Device Reliability
SEP 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Medical Battery Technology Background and Objectives
Battery technology has evolved significantly over the past decades, transitioning from simple primary cells to sophisticated rechargeable systems with advanced management capabilities. In the medical device sector, this evolution has been particularly crucial as portable and implantable devices have become increasingly prevalent in healthcare delivery. The development trajectory has moved from nickel-cadmium batteries through nickel-metal hydride to the current dominance of lithium-ion and lithium-polymer technologies, each iteration bringing improvements in energy density, cycle life, and safety features.
The medical device industry presents unique battery requirements that distinguish it from consumer electronics applications. Medical devices demand exceptional reliability, as failures can have life-threatening consequences. They require longer service life to minimize replacement procedures, particularly for implantable devices. Additionally, medical applications often need batteries that can function across wider temperature ranges and withstand sterilization processes without degradation.
Current technical objectives in medical battery pack design center around five key areas. First, enhancing energy density to enable smaller device footprints while maintaining or extending operational duration. Second, improving safety mechanisms to prevent thermal runaway, particularly in body-worn or implanted devices. Third, extending cycle life and calendar life to reduce replacement frequency. Fourth, developing more sophisticated battery management systems that can predict failures before they occur. Fifth, reducing charging times while maintaining battery health.
Regulatory considerations significantly shape the development landscape for medical battery technology. Standards such as IEC 60601-1 for medical electrical equipment and ISO 13485 for quality management systems impose rigorous requirements on battery performance, documentation, and risk management. These standards have become increasingly stringent over time, reflecting growing awareness of potential failure modes and their consequences.
The intersection of battery technology with other emerging technologies presents promising avenues for advancement. Integration with energy harvesting systems could enable self-charging capabilities. Wireless charging technologies may eliminate connector-related failures. Advanced materials science is yielding new electrode and electrolyte compositions with superior performance characteristics. Artificial intelligence algorithms are being deployed to optimize charging protocols and predict remaining useful life with greater accuracy.
The ultimate goal of medical battery pack design is to create power systems that become essentially transparent to the end user—reliable enough that healthcare providers and patients can focus entirely on treatment outcomes rather than power management concerns. This requires balancing sometimes competing objectives of safety, longevity, size, and cost in ways that are optimized for specific medical applications.
The medical device industry presents unique battery requirements that distinguish it from consumer electronics applications. Medical devices demand exceptional reliability, as failures can have life-threatening consequences. They require longer service life to minimize replacement procedures, particularly for implantable devices. Additionally, medical applications often need batteries that can function across wider temperature ranges and withstand sterilization processes without degradation.
Current technical objectives in medical battery pack design center around five key areas. First, enhancing energy density to enable smaller device footprints while maintaining or extending operational duration. Second, improving safety mechanisms to prevent thermal runaway, particularly in body-worn or implanted devices. Third, extending cycle life and calendar life to reduce replacement frequency. Fourth, developing more sophisticated battery management systems that can predict failures before they occur. Fifth, reducing charging times while maintaining battery health.
Regulatory considerations significantly shape the development landscape for medical battery technology. Standards such as IEC 60601-1 for medical electrical equipment and ISO 13485 for quality management systems impose rigorous requirements on battery performance, documentation, and risk management. These standards have become increasingly stringent over time, reflecting growing awareness of potential failure modes and their consequences.
The intersection of battery technology with other emerging technologies presents promising avenues for advancement. Integration with energy harvesting systems could enable self-charging capabilities. Wireless charging technologies may eliminate connector-related failures. Advanced materials science is yielding new electrode and electrolyte compositions with superior performance characteristics. Artificial intelligence algorithms are being deployed to optimize charging protocols and predict remaining useful life with greater accuracy.
The ultimate goal of medical battery pack design is to create power systems that become essentially transparent to the end user—reliable enough that healthcare providers and patients can focus entirely on treatment outcomes rather than power management concerns. This requires balancing sometimes competing objectives of safety, longevity, size, and cost in ways that are optimized for specific medical applications.
Market Analysis for Portable Medical Device Batteries
The portable medical device battery market is experiencing robust growth, driven by increasing adoption of wearable health monitors, portable diagnostic equipment, and home healthcare devices. The global market for medical device batteries reached approximately $1.9 billion in 2022 and is projected to grow at a CAGR of 7.8% through 2028, potentially reaching $3.0 billion. This growth trajectory is particularly pronounced in regions with aging populations and developed healthcare infrastructure, notably North America, Europe, and parts of Asia-Pacific.
Patient mobility requirements and the shift toward home healthcare are primary market drivers, with hospitals and clinics increasingly utilizing portable medical equipment to improve care efficiency and reduce costs. The COVID-19 pandemic accelerated this trend, creating unprecedented demand for remote patient monitoring systems and portable ventilators, consequently boosting battery requirements.
Consumer expectations for medical device batteries differ significantly from consumer electronics, with reliability and safety taking precedence over energy density and form factor. Medical professionals and patients require batteries that deliver consistent performance under varying conditions, with minimal risk of failure during critical operations. This has created a specialized market segment where premium pricing is justified by enhanced reliability features.
Regulatory considerations heavily influence market dynamics, with FDA, CE, and other regulatory bodies imposing stringent requirements on medical device batteries. These include extensive documentation of safety testing, traceability throughout the supply chain, and adherence to standards such as IEC 60601 for medical electrical equipment. Compliance with these regulations represents a significant barrier to entry but also provides established manufacturers with competitive protection.
Battery chemistry preferences in this sector show lithium-ion dominating with approximately 65% market share, followed by nickel-metal hydride and lead-acid variants for specific applications. Emerging chemistries like solid-state batteries are gaining attention for their enhanced safety profiles, though commercial deployment remains limited.
Market segmentation reveals distinct requirements across different device categories. Implantable devices demand extremely long life and biocompatibility, while diagnostic equipment prioritizes high power delivery capability. Patient monitoring devices require balanced performance with emphasis on reliability during extended use periods.
Key market challenges include balancing extended runtime with size constraints, managing thermal characteristics to ensure patient comfort and safety, and addressing environmental concerns through sustainable design approaches. These challenges present significant opportunities for innovation in battery management systems, thermal design, and materials science applications specific to medical contexts.
Patient mobility requirements and the shift toward home healthcare are primary market drivers, with hospitals and clinics increasingly utilizing portable medical equipment to improve care efficiency and reduce costs. The COVID-19 pandemic accelerated this trend, creating unprecedented demand for remote patient monitoring systems and portable ventilators, consequently boosting battery requirements.
Consumer expectations for medical device batteries differ significantly from consumer electronics, with reliability and safety taking precedence over energy density and form factor. Medical professionals and patients require batteries that deliver consistent performance under varying conditions, with minimal risk of failure during critical operations. This has created a specialized market segment where premium pricing is justified by enhanced reliability features.
Regulatory considerations heavily influence market dynamics, with FDA, CE, and other regulatory bodies imposing stringent requirements on medical device batteries. These include extensive documentation of safety testing, traceability throughout the supply chain, and adherence to standards such as IEC 60601 for medical electrical equipment. Compliance with these regulations represents a significant barrier to entry but also provides established manufacturers with competitive protection.
Battery chemistry preferences in this sector show lithium-ion dominating with approximately 65% market share, followed by nickel-metal hydride and lead-acid variants for specific applications. Emerging chemistries like solid-state batteries are gaining attention for their enhanced safety profiles, though commercial deployment remains limited.
Market segmentation reveals distinct requirements across different device categories. Implantable devices demand extremely long life and biocompatibility, while diagnostic equipment prioritizes high power delivery capability. Patient monitoring devices require balanced performance with emphasis on reliability during extended use periods.
Key market challenges include balancing extended runtime with size constraints, managing thermal characteristics to ensure patient comfort and safety, and addressing environmental concerns through sustainable design approaches. These challenges present significant opportunities for innovation in battery management systems, thermal design, and materials science applications specific to medical contexts.
Current Challenges in Medical Battery Reliability
Despite significant advancements in battery technology, portable medical devices continue to face critical reliability challenges that directly impact patient safety and treatment efficacy. The most pressing concern remains energy density limitations, as medical devices require increasingly sophisticated functionality while maintaining compact form factors. Current lithium-ion technologies struggle to meet the demanding power requirements of advanced diagnostic and therapeutic equipment without compromising device portability.
Temperature sensitivity presents another significant challenge, with performance degradation occurring in both extreme cold and heat conditions. This is particularly problematic for devices that may be used in varied environments, from refrigerated hospital settings to high-temperature emergency scenarios. Battery performance inconsistency across these conditions can lead to unpredictable device behavior when reliability is most crucial.
Cycle life degradation continues to plague medical battery systems, with capacity diminishing over repeated charge-discharge cycles. For critical care devices, this necessitates conservative replacement schedules that increase operational costs and environmental impact. The degradation patterns often accelerate unpredictably, creating reliability concerns that can compromise treatment protocols.
Safety mechanisms remain inadequate for the unique demands of medical environments. While consumer electronics can tolerate occasional battery failures, medical devices require near-perfect reliability. Current protection circuits and thermal management systems are not sufficiently robust for devices that may be used in oxygen-rich environments or during emergency procedures where physical damage risks are elevated.
Regulatory compliance adds another layer of complexity, with standards like IEC 60601 imposing stringent requirements that limit design options. These standards, while necessary for patient safety, often restrict the implementation of newer battery technologies that haven't undergone extensive validation in medical contexts.
Charging infrastructure compatibility presents operational challenges in healthcare settings. Medical devices must function reliably across various care environments with different charging capabilities, from well-equipped hospitals to remote care settings with limited infrastructure. This necessitates compromise between optimal charging protocols and practical field usability.
Biocompatibility and sterilization compatibility remain unresolved challenges, as battery packs must withstand repeated sterilization procedures without performance degradation. Current encapsulation technologies often fail to provide adequate protection against sterilization chemicals and high-temperature autoclaving processes, leading to premature battery failure.
End-of-life prediction algorithms lack sufficient accuracy for medical applications, where unexpected power loss could have severe consequences. Current battery management systems struggle to account for the complex usage patterns typical in medical settings, where devices may experience irregular duty cycles and varying load profiles.
Temperature sensitivity presents another significant challenge, with performance degradation occurring in both extreme cold and heat conditions. This is particularly problematic for devices that may be used in varied environments, from refrigerated hospital settings to high-temperature emergency scenarios. Battery performance inconsistency across these conditions can lead to unpredictable device behavior when reliability is most crucial.
Cycle life degradation continues to plague medical battery systems, with capacity diminishing over repeated charge-discharge cycles. For critical care devices, this necessitates conservative replacement schedules that increase operational costs and environmental impact. The degradation patterns often accelerate unpredictably, creating reliability concerns that can compromise treatment protocols.
Safety mechanisms remain inadequate for the unique demands of medical environments. While consumer electronics can tolerate occasional battery failures, medical devices require near-perfect reliability. Current protection circuits and thermal management systems are not sufficiently robust for devices that may be used in oxygen-rich environments or during emergency procedures where physical damage risks are elevated.
Regulatory compliance adds another layer of complexity, with standards like IEC 60601 imposing stringent requirements that limit design options. These standards, while necessary for patient safety, often restrict the implementation of newer battery technologies that haven't undergone extensive validation in medical contexts.
Charging infrastructure compatibility presents operational challenges in healthcare settings. Medical devices must function reliably across various care environments with different charging capabilities, from well-equipped hospitals to remote care settings with limited infrastructure. This necessitates compromise between optimal charging protocols and practical field usability.
Biocompatibility and sterilization compatibility remain unresolved challenges, as battery packs must withstand repeated sterilization procedures without performance degradation. Current encapsulation technologies often fail to provide adequate protection against sterilization chemicals and high-temperature autoclaving processes, leading to premature battery failure.
End-of-life prediction algorithms lack sufficient accuracy for medical applications, where unexpected power loss could have severe consequences. Current battery management systems struggle to account for the complex usage patterns typical in medical settings, where devices may experience irregular duty cycles and varying load profiles.
Current Battery Pack Design Solutions
01 Thermal management systems for battery packs
Effective thermal management systems are crucial for ensuring battery pack reliability. These systems help maintain optimal operating temperatures, prevent overheating, and ensure uniform temperature distribution across cells. Solutions include liquid cooling circuits, heat sinks, thermal interface materials, and advanced air cooling designs that efficiently dissipate heat generated during charging and discharging cycles, thereby extending battery life and improving overall reliability.- Thermal management systems for battery reliability: Effective thermal management systems are crucial for maintaining battery pack reliability. These systems help regulate temperature distribution within the battery pack, preventing overheating and thermal runaway conditions that can lead to failure. Solutions include cooling channels, heat sinks, thermal interface materials, and active cooling mechanisms that ensure optimal operating temperatures are maintained across all cells, extending battery life and improving safety.
- Battery management systems for monitoring and control: Advanced battery management systems (BMS) play a vital role in ensuring battery pack reliability by continuously monitoring parameters such as voltage, current, temperature, and state of charge. These systems implement protective measures against overcharging, over-discharging, and other harmful conditions. They can also balance cell voltages within the pack, identify potential failures before they occur, and provide diagnostic information for maintenance, all contributing to extended battery life and improved reliability.
- Structural design and mechanical protection: The structural design of battery packs significantly impacts their reliability. This includes robust housing materials, shock absorption systems, and vibration dampening mechanisms that protect cells from physical damage. Proper cell arrangement and secure mounting within the pack prevent movement during operation. Additionally, reinforced casings and protective enclosures shield the battery from environmental factors such as moisture, dust, and impact, enhancing overall durability and reliability in various operating conditions.
- Cell balancing and connection technologies: Cell balancing technologies ensure uniform performance across all cells in a battery pack, preventing individual cells from experiencing stress that could lead to premature failure. Advanced connection methods, including bus bars, welded connections, and flexible circuits, maintain reliable electrical pathways between cells. These technologies minimize resistance, reduce heat generation at connection points, and accommodate thermal expansion, all of which contribute to improved pack reliability and longevity.
- Failure detection and safety mechanisms: Implementing robust failure detection systems and safety mechanisms is essential for battery pack reliability. These include sensors that detect abnormal conditions, isolation devices that prevent fault propagation, and emergency shutdown systems that activate during critical failures. Some designs incorporate pressure relief mechanisms, thermal fuses, and redundant safety circuits. Advanced diagnostic algorithms can predict potential failures before they occur, allowing for preventive maintenance and ensuring safe operation throughout the battery pack's lifecycle.
02 Battery management systems (BMS) for monitoring and control
Advanced battery management systems play a vital role in enhancing battery pack reliability by continuously monitoring critical parameters such as voltage, current, temperature, and state of charge. These systems implement protective measures against overcharging, over-discharging, and short circuits. They also provide real-time diagnostics, predictive maintenance capabilities, and can balance cell voltages to prevent premature degradation, ultimately extending the operational life of battery packs.Expand Specific Solutions03 Mechanical design and structural integrity
The mechanical design of battery packs significantly impacts their reliability. Robust structural components, shock-absorbing materials, and reinforced casings protect cells from physical damage due to vibration, impact, or thermal expansion. Advanced mounting systems, cell-to-cell connections, and pack enclosures are engineered to withstand environmental stresses while maintaining electrical connectivity. These design elements ensure long-term structural integrity and reliable performance under various operating conditions.Expand Specific Solutions04 Cell balancing and voltage regulation techniques
Cell balancing technologies are essential for maintaining uniform performance across all cells in a battery pack. Active and passive balancing circuits redistribute energy between cells to equalize voltage levels, preventing individual cells from experiencing stress due to overcharging or deep discharging. Advanced voltage regulation algorithms monitor cell performance over time, compensating for aging effects and manufacturing variations. These techniques significantly improve pack reliability by ensuring all cells operate within their optimal parameters.Expand Specific Solutions05 Safety mechanisms and fault detection systems
Comprehensive safety mechanisms and fault detection systems are critical components for reliable battery pack operation. These include integrated circuit protection, emergency disconnect features, and redundant safety systems that can identify and respond to abnormal conditions. Advanced diagnostic algorithms continuously monitor for potential failure modes, thermal runaway precursors, and electrical anomalies. Early detection of potential issues allows for preventive measures to be implemented before catastrophic failures occur, significantly enhancing overall battery pack reliability.Expand Specific Solutions
Key Medical Battery Manufacturers and Competitors
The battery pack design for portable medical devices is in a mature growth phase, with a market size exceeding $3 billion annually and growing at 7-9% CAGR. The competitive landscape features established electronics giants (Samsung SDI, Sony, Panasonic, LG Energy Solution) leveraging their consumer electronics expertise alongside specialized medical technology companies (Medtronic, Cardiac Pacemakers, Stryker). Technical maturity varies across applications, with companies like Apple and Philips driving innovation in miniaturization and reliability features, while Samsung and LG lead in energy density improvements. The industry is witnessing increased focus on safety protocols, extended lifecycle management, and integration of smart battery management systems, with companies like Murata Manufacturing providing specialized components for medical-grade reliability.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed MediBatt technology specifically for portable medical device applications, focusing on reliability and safety. Their battery packs utilize high-energy density lithium-ion cells with proprietary electrolyte formulations that minimize degradation over thousands of charge cycles. Samsung's design incorporates multi-layered safety features including ceramic-coated separators that prevent internal short circuits even under mechanical stress or puncture. Their battery management system employs AI-driven predictive analytics to forecast potential failures before they occur, allowing for preventive maintenance. Samsung's thermal management solution uses a micro-channel cooling system that maintains uniform temperature distribution across the battery pack, preventing hotspots that could lead to premature degradation. The company has also developed specialized manufacturing processes that ensure extremely low defect rates (below 1 PPM) for medical-grade batteries, critical for life-supporting devices. Their latest generation incorporates self-diagnostic capabilities that continuously monitor internal resistance changes to detect early signs of capacity fade.
Strengths: Exceptional manufacturing consistency with industry-leading quality control, superior energy density enabling smaller form factors, and advanced safety features exceeding medical device regulatory requirements. Weaknesses: Higher initial cost compared to conventional battery solutions, limited customization options for very specialized medical applications, and proprietary BMS architecture requiring specific integration protocols.
Cardiac Pacemakers, Inc.
Technical Solution: Cardiac Pacemakers, Inc. has developed specialized battery pack solutions specifically for implantable and wearable cardiac monitoring devices. Their proprietary LongevityPlus battery technology utilizes lithium-carbon monofluoride (Li/CFx) chemistry optimized for extremely low self-discharge rates and predictable discharge curves essential for life-critical applications. Their battery packs incorporate hermetically sealed titanium casings that prevent moisture ingress even in implanted environments. The company's power management system features ultra-low quiescent current circuits that maximize device longevity while maintaining continuous monitoring capabilities. Their battery designs include proprietary electrolyte formulations that resist degradation even after years of continuous operation at body temperature. Cardiac Pacemakers has pioneered hybrid power systems that combine primary batteries with energy harvesting technologies to extend operational life in certain applications. Their battery packs undergo extensive reliability testing including accelerated aging under simulated physiological conditions and are validated to maintain performance characteristics for up to 10 years of continuous operation.
Strengths: Unmatched longevity for implantable applications (8-12 years depending on device parameters), exceptional reliability with documented failure rates below 0.05% over operational lifetime, and specialized form factors optimized for anatomical constraints. Weaknesses: Limited energy density compared to consumer-grade batteries, higher production costs due to specialized materials and manufacturing processes, and limited applicability to non-implantable medical devices requiring higher power outputs.
Critical Patents in Medical Battery Reliability
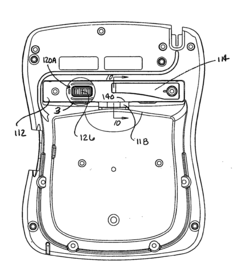
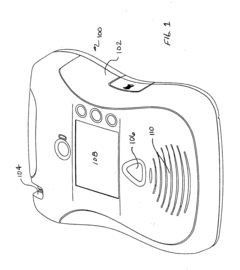
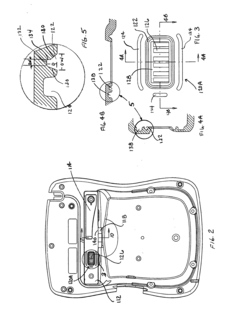
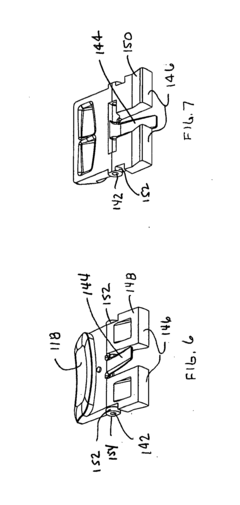
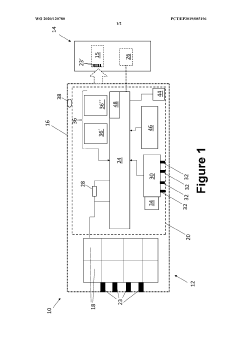
Battery pack for an electronic device
PatentInactiveUS20120150248A1
Innovation
- A removable battery pack design for portable medical devices that uses an electrical connector with a gasket creating a watertight connection without compressing the gasket during insertion, reducing the force needed for engagement and enhancing seal integrity through a tapered gasket groove and self-locking latch mechanism.
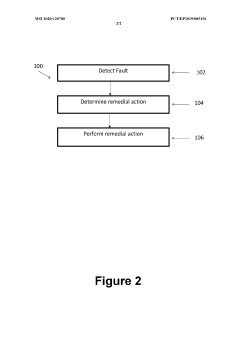
System to improve safety and reliability of a lithium-ion (li-ion) battery pack
PatentWO2020120780A1
Innovation
- Incorporating shock sensors and gas sensors into the battery management system to detect impending failures such as impacts or gas leaks, with the system capable of performing remediation actions like shutting down the battery pack or alerting medical personnel, and optionally transmitting data wirelessly for remote monitoring.
Safety Standards and Regulatory Compliance
Portable medical devices must adhere to stringent safety standards and regulatory requirements to ensure patient safety and device reliability. For battery pack designs, compliance with IEC 60601-1 (Medical Electrical Equipment Safety) serves as the cornerstone standard, establishing fundamental safety requirements including protection against electric shock, mechanical hazards, and excessive temperatures. This standard specifically addresses battery management systems and requires comprehensive risk assessment documentation.
Battery-specific standards such as IEC 62133 for secondary lithium cells and IEC 61960 for lithium battery performance are equally critical. These standards mandate specific testing protocols for thermal stability, short circuit protection, overcharge protection, and mechanical durability. Manufacturers must demonstrate compliance through extensive testing and documentation before market approval.
Regional regulatory frameworks impose additional requirements. In the United States, FDA regulations under 21 CFR Part 820 govern quality management systems for medical devices, with specific provisions for battery-powered equipment. The FDA's guidance document on battery-powered medical devices outlines expectations for safety features, labeling, and post-market surveillance. Similarly, the European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) establish comprehensive requirements for CE marking, with particular attention to risk management for energy sources.
Transportation regulations present another critical compliance area. UN 38.3 testing requirements for lithium batteries establish protocols for safe shipping and handling, including altitude simulation, thermal testing, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests. Medical device manufacturers must obtain certification that their battery packs meet these requirements before shipping internationally.
Emerging standards are addressing specific reliability concerns in portable medical applications. IEC 62368-1 introduces a hazard-based approach to safety engineering that is increasingly relevant for medical devices with multiple power sources. Additionally, sustainability regulations like the EU Battery Directive 2006/66/EC impose requirements for battery collection, recycling, and substance restrictions that impact design decisions.
Compliance strategies must be integrated early in the design process rather than treated as an afterthought. Successful battery pack designs incorporate safety features like thermal fuses, current limiters, cell balancing circuits, and redundant protection mechanisms. Documentation requirements include detailed test reports, risk analyses, technical files, and declarations of conformity that demonstrate how the design meets all applicable standards.
Battery-specific standards such as IEC 62133 for secondary lithium cells and IEC 61960 for lithium battery performance are equally critical. These standards mandate specific testing protocols for thermal stability, short circuit protection, overcharge protection, and mechanical durability. Manufacturers must demonstrate compliance through extensive testing and documentation before market approval.
Regional regulatory frameworks impose additional requirements. In the United States, FDA regulations under 21 CFR Part 820 govern quality management systems for medical devices, with specific provisions for battery-powered equipment. The FDA's guidance document on battery-powered medical devices outlines expectations for safety features, labeling, and post-market surveillance. Similarly, the European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) establish comprehensive requirements for CE marking, with particular attention to risk management for energy sources.
Transportation regulations present another critical compliance area. UN 38.3 testing requirements for lithium batteries establish protocols for safe shipping and handling, including altitude simulation, thermal testing, vibration, shock, external short circuit, impact, overcharge, and forced discharge tests. Medical device manufacturers must obtain certification that their battery packs meet these requirements before shipping internationally.
Emerging standards are addressing specific reliability concerns in portable medical applications. IEC 62368-1 introduces a hazard-based approach to safety engineering that is increasingly relevant for medical devices with multiple power sources. Additionally, sustainability regulations like the EU Battery Directive 2006/66/EC impose requirements for battery collection, recycling, and substance restrictions that impact design decisions.
Compliance strategies must be integrated early in the design process rather than treated as an afterthought. Successful battery pack designs incorporate safety features like thermal fuses, current limiters, cell balancing circuits, and redundant protection mechanisms. Documentation requirements include detailed test reports, risk analyses, technical files, and declarations of conformity that demonstrate how the design meets all applicable standards.
Thermal Management Strategies
Thermal management is a critical aspect of battery pack design for portable medical devices, as temperature fluctuations can significantly impact battery performance, reliability, and safety. Effective thermal management strategies must balance the competing demands of device miniaturization, extended battery life, and operational safety. The primary challenge lies in dissipating heat generated during charging and discharging cycles while maintaining the battery cells within their optimal temperature range of 15-35°C.
Passive thermal management approaches represent the most widely implemented solutions in portable medical devices due to their simplicity and reliability. These include strategic placement of thermal insulation materials, incorporation of phase change materials (PCMs) that absorb excess heat during peak operation, and the use of thermally conductive paths to direct heat away from critical components. Recent advancements in nano-enhanced PCMs have demonstrated up to 40% improvement in thermal regulation efficiency compared to traditional materials.
Active thermal management systems, though less common in portable applications due to power consumption concerns, offer more precise temperature control for high-demand medical devices. These systems typically incorporate miniaturized fans, liquid cooling channels, or thermoelectric coolers. The latest generation of micro-fans designed specifically for medical device applications can provide effective cooling while consuming less than 100mW of power, making them viable for integration into larger portable devices where thermal loads are significant.
Hybrid approaches combining both passive and active elements represent the cutting edge of thermal management for medical battery packs. These systems employ intelligent control algorithms that activate active cooling components only when passive measures are insufficient, thereby optimizing energy efficiency. Sensors distributed throughout the battery pack continuously monitor temperature gradients, enabling predictive thermal management rather than reactive responses to overheating events.
Design considerations must account for various operational scenarios, including extended use in diverse environments, storage conditions, and emergency situations. Medical devices used in field settings may experience ambient temperatures ranging from 0°C to 40°C, necessitating robust thermal management solutions. Additionally, thermal design must address heat dissipation during rapid charging protocols, which are increasingly demanded by healthcare providers to minimize device downtime.
Regulatory compliance adds another layer of complexity to thermal management strategies. IEC 60601-1 standards specify strict temperature limits for medical device surfaces that contact patients or operators, while IEC 62133 outlines safety requirements specifically for batteries, including thermal runaway prevention. Comprehensive thermal modeling and simulation during the design phase, followed by rigorous thermal testing under various operational conditions, are essential steps in developing compliant and reliable battery packs for portable medical applications.
Passive thermal management approaches represent the most widely implemented solutions in portable medical devices due to their simplicity and reliability. These include strategic placement of thermal insulation materials, incorporation of phase change materials (PCMs) that absorb excess heat during peak operation, and the use of thermally conductive paths to direct heat away from critical components. Recent advancements in nano-enhanced PCMs have demonstrated up to 40% improvement in thermal regulation efficiency compared to traditional materials.
Active thermal management systems, though less common in portable applications due to power consumption concerns, offer more precise temperature control for high-demand medical devices. These systems typically incorporate miniaturized fans, liquid cooling channels, or thermoelectric coolers. The latest generation of micro-fans designed specifically for medical device applications can provide effective cooling while consuming less than 100mW of power, making them viable for integration into larger portable devices where thermal loads are significant.
Hybrid approaches combining both passive and active elements represent the cutting edge of thermal management for medical battery packs. These systems employ intelligent control algorithms that activate active cooling components only when passive measures are insufficient, thereby optimizing energy efficiency. Sensors distributed throughout the battery pack continuously monitor temperature gradients, enabling predictive thermal management rather than reactive responses to overheating events.
Design considerations must account for various operational scenarios, including extended use in diverse environments, storage conditions, and emergency situations. Medical devices used in field settings may experience ambient temperatures ranging from 0°C to 40°C, necessitating robust thermal management solutions. Additionally, thermal design must address heat dissipation during rapid charging protocols, which are increasingly demanded by healthcare providers to minimize device downtime.
Regulatory compliance adds another layer of complexity to thermal management strategies. IEC 60601-1 standards specify strict temperature limits for medical device surfaces that contact patients or operators, while IEC 62133 outlines safety requirements specifically for batteries, including thermal runaway prevention. Comprehensive thermal modeling and simulation during the design phase, followed by rigorous thermal testing under various operational conditions, are essential steps in developing compliant and reliable battery packs for portable medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!