Binder Jetting For Biomedical Implants: Controlled Porosity And Biocompatibility Outcomes
SEP 11, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Binder Jetting Evolution and Biomedical Applications
Binder jetting technology has undergone significant evolution since its inception in the early 1990s at MIT. Initially developed as a rapid prototyping method, this additive manufacturing technique has transformed into a sophisticated production process capable of creating complex three-dimensional structures with controlled properties. The technology fundamentally operates by selectively depositing a liquid binding agent onto thin layers of powder materials, which are subsequently solidified to form the desired structure.
In the biomedical field, binder jetting has emerged as a promising manufacturing approach for implantable devices due to its ability to create controlled porous structures that mimic natural bone architecture. The evolution of this technology for biomedical applications can be traced through several key developmental phases, beginning with basic proof-of-concept studies in the early 2000s that demonstrated the feasibility of creating biocompatible structures.
By the mid-2000s, researchers had begun to refine the process parameters specifically for biomedical materials, including calcium phosphates, titanium alloys, and bioactive glasses. This period saw significant improvements in resolution capabilities, allowing for more precise control over micro and macro porosity—a critical factor for successful osseointegration of implants.
The 2010s marked a turning point with the development of specialized binders compatible with biomedical grade materials. These advanced formulations addressed earlier limitations regarding toxicity and residual binder effects, enabling the production of implants with enhanced biocompatibility profiles. Concurrently, post-processing techniques evolved to effectively remove binder residues while maintaining structural integrity.
Recent advancements have focused on multi-material capabilities, allowing for functionally graded implants that can better mimic the heterogeneous nature of natural tissues. This represents a significant leap forward from early single-material approaches, enabling implants with regionally optimized mechanical and biological properties.
The integration of computational modeling with binder jetting processes has further revolutionized implant design, enabling patient-specific solutions with precisely controlled porosity gradients that promote optimal tissue ingrowth while maintaining necessary mechanical strength. These models now incorporate biological response predictions, allowing designers to optimize not only the physical structure but also the biological performance of implants.
Current research frontiers include the incorporation of bioactive agents directly into the printing process, creating implants with programmed drug release capabilities and enhanced healing properties. Additionally, advances in material science have expanded the range of printable biomaterials, including novel biodegradable composites that support tissue regeneration while gradually transferring load-bearing responsibilities to newly formed tissue.
In the biomedical field, binder jetting has emerged as a promising manufacturing approach for implantable devices due to its ability to create controlled porous structures that mimic natural bone architecture. The evolution of this technology for biomedical applications can be traced through several key developmental phases, beginning with basic proof-of-concept studies in the early 2000s that demonstrated the feasibility of creating biocompatible structures.
By the mid-2000s, researchers had begun to refine the process parameters specifically for biomedical materials, including calcium phosphates, titanium alloys, and bioactive glasses. This period saw significant improvements in resolution capabilities, allowing for more precise control over micro and macro porosity—a critical factor for successful osseointegration of implants.
The 2010s marked a turning point with the development of specialized binders compatible with biomedical grade materials. These advanced formulations addressed earlier limitations regarding toxicity and residual binder effects, enabling the production of implants with enhanced biocompatibility profiles. Concurrently, post-processing techniques evolved to effectively remove binder residues while maintaining structural integrity.
Recent advancements have focused on multi-material capabilities, allowing for functionally graded implants that can better mimic the heterogeneous nature of natural tissues. This represents a significant leap forward from early single-material approaches, enabling implants with regionally optimized mechanical and biological properties.
The integration of computational modeling with binder jetting processes has further revolutionized implant design, enabling patient-specific solutions with precisely controlled porosity gradients that promote optimal tissue ingrowth while maintaining necessary mechanical strength. These models now incorporate biological response predictions, allowing designers to optimize not only the physical structure but also the biological performance of implants.
Current research frontiers include the incorporation of bioactive agents directly into the printing process, creating implants with programmed drug release capabilities and enhanced healing properties. Additionally, advances in material science have expanded the range of printable biomaterials, including novel biodegradable composites that support tissue regeneration while gradually transferring load-bearing responsibilities to newly formed tissue.
Market Analysis for 3D Printed Biomedical Implants
The global market for 3D printed biomedical implants is experiencing robust growth, driven by increasing demand for personalized medical solutions and advancements in additive manufacturing technologies. The market was valued at approximately $1.1 billion in 2021 and is projected to reach $3.7 billion by 2028, representing a compound annual growth rate (CAGR) of 18.3% during the forecast period.
Orthopedic implants currently dominate the market share, accounting for over 40% of the total 3D printed biomedical implants market. This segment includes hip replacements, knee implants, and spinal devices, where binder jetting technology with controlled porosity offers significant advantages for bone integration and reduced implant rejection rates.
Dental implants represent the fastest-growing segment, with a projected CAGR of 22.7% through 2028. The ability of binder jetting to create precise, patient-specific geometries with controlled porosity has revolutionized dental restoration procedures, improving both functional and aesthetic outcomes.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing medical tourism, and expanding manufacturing capabilities in countries like China, India, and South Korea.
Key market drivers include the aging global population, rising prevalence of chronic diseases requiring implants, and increasing acceptance of 3D printing technologies in the healthcare sector. The demand for implants with controlled porosity is particularly strong, as this feature enhances osseointegration and reduces recovery time for patients.
Reimbursement policies and regulatory approvals remain significant market challenges. While the FDA has established guidelines for 3D printed medical devices, the approval process for novel materials and designs with controlled porosity features can be lengthy and complex, potentially slowing market penetration for innovative solutions.
Material costs represent another market constraint, with biocompatible powders for binder jetting typically costing 5-10 times more than conventional manufacturing materials. However, this premium is often offset by reduced waste, lower inventory costs, and improved clinical outcomes.
The COVID-19 pandemic initially disrupted supply chains but subsequently accelerated adoption of localized manufacturing models for medical devices, benefiting distributed production capabilities offered by binder jetting technologies. This shift is expected to continue, creating new market opportunities for decentralized production of biomedical implants with controlled porosity features.
Orthopedic implants currently dominate the market share, accounting for over 40% of the total 3D printed biomedical implants market. This segment includes hip replacements, knee implants, and spinal devices, where binder jetting technology with controlled porosity offers significant advantages for bone integration and reduced implant rejection rates.
Dental implants represent the fastest-growing segment, with a projected CAGR of 22.7% through 2028. The ability of binder jetting to create precise, patient-specific geometries with controlled porosity has revolutionized dental restoration procedures, improving both functional and aesthetic outcomes.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the highest growth rate due to increasing healthcare expenditure, growing medical tourism, and expanding manufacturing capabilities in countries like China, India, and South Korea.
Key market drivers include the aging global population, rising prevalence of chronic diseases requiring implants, and increasing acceptance of 3D printing technologies in the healthcare sector. The demand for implants with controlled porosity is particularly strong, as this feature enhances osseointegration and reduces recovery time for patients.
Reimbursement policies and regulatory approvals remain significant market challenges. While the FDA has established guidelines for 3D printed medical devices, the approval process for novel materials and designs with controlled porosity features can be lengthy and complex, potentially slowing market penetration for innovative solutions.
Material costs represent another market constraint, with biocompatible powders for binder jetting typically costing 5-10 times more than conventional manufacturing materials. However, this premium is often offset by reduced waste, lower inventory costs, and improved clinical outcomes.
The COVID-19 pandemic initially disrupted supply chains but subsequently accelerated adoption of localized manufacturing models for medical devices, benefiting distributed production capabilities offered by binder jetting technologies. This shift is expected to continue, creating new market opportunities for decentralized production of biomedical implants with controlled porosity features.
Current Challenges in Binder Jetting for Implant Manufacturing
Despite the promising potential of binder jetting technology for biomedical implant manufacturing, several significant challenges currently impede its widespread clinical adoption. Material limitations represent a primary obstacle, as the range of biocompatible powders suitable for binder jetting remains restricted. While titanium alloys have shown success, other essential biomaterials like specific ceramics and composites present processing difficulties due to their physical properties and binder interactions.
The achievement of precise porosity control continues to challenge manufacturers. Although controlled porosity is crucial for osseointegration and vascularization, current binder jetting processes struggle to consistently produce the hierarchical porous structures needed for optimal biological integration. The technology faces difficulties in creating the ideal combination of macro, micro, and nano-porosity required for different implant applications.
Post-processing requirements present another substantial hurdle. The green parts produced through binder jetting necessitate multiple post-processing steps including debinding, sintering, and surface treatments. These processes can introduce dimensional inaccuracies, structural weaknesses, and contamination risks that compromise the final implant quality and biocompatibility.
Resolution limitations affect the production of complex anatomical geometries with fine features. Current commercial binder jetting systems typically achieve resolution in the range of 50-100 μm, which proves insufficient for replicating the intricate microstructures found in natural bone tissue. This resolution constraint limits the technology's ability to produce biomimetic implants that closely resemble native tissue architecture.
Surface finish quality remains problematic, with binder jetted parts often exhibiting roughness that requires extensive post-processing. This roughness can affect cell adhesion, protein adsorption, and ultimately tissue integration if not properly controlled. The stair-stepping effect inherent to layer-by-layer manufacturing further complicates the achievement of smooth contoured surfaces.
Reproducibility and quality control present ongoing challenges, as process parameters including powder characteristics, binder formulation, printing speed, and post-processing conditions significantly impact the final implant properties. The complex interplay between these variables makes standardization difficult, resulting in batch-to-batch variations that raise regulatory concerns.
Regulatory hurdles compound these technical challenges, as the pathway to FDA approval for additively manufactured implants remains complex. The lack of established standards specifically for binder jetted medical devices creates uncertainty in validation protocols and slows clinical translation despite promising research outcomes.
The achievement of precise porosity control continues to challenge manufacturers. Although controlled porosity is crucial for osseointegration and vascularization, current binder jetting processes struggle to consistently produce the hierarchical porous structures needed for optimal biological integration. The technology faces difficulties in creating the ideal combination of macro, micro, and nano-porosity required for different implant applications.
Post-processing requirements present another substantial hurdle. The green parts produced through binder jetting necessitate multiple post-processing steps including debinding, sintering, and surface treatments. These processes can introduce dimensional inaccuracies, structural weaknesses, and contamination risks that compromise the final implant quality and biocompatibility.
Resolution limitations affect the production of complex anatomical geometries with fine features. Current commercial binder jetting systems typically achieve resolution in the range of 50-100 μm, which proves insufficient for replicating the intricate microstructures found in natural bone tissue. This resolution constraint limits the technology's ability to produce biomimetic implants that closely resemble native tissue architecture.
Surface finish quality remains problematic, with binder jetted parts often exhibiting roughness that requires extensive post-processing. This roughness can affect cell adhesion, protein adsorption, and ultimately tissue integration if not properly controlled. The stair-stepping effect inherent to layer-by-layer manufacturing further complicates the achievement of smooth contoured surfaces.
Reproducibility and quality control present ongoing challenges, as process parameters including powder characteristics, binder formulation, printing speed, and post-processing conditions significantly impact the final implant properties. The complex interplay between these variables makes standardization difficult, resulting in batch-to-batch variations that raise regulatory concerns.
Regulatory hurdles compound these technical challenges, as the pathway to FDA approval for additively manufactured implants remains complex. The lack of established standards specifically for binder jetted medical devices creates uncertainty in validation protocols and slows clinical translation despite promising research outcomes.
Existing Approaches for Controlled Porosity in Implants
01 Porosity control in binder jetting for biomedical applications
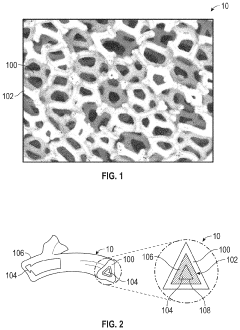
Controlling porosity in binder jetting processes is crucial for biomedical applications. Specific techniques can be employed to achieve desired porosity levels, including adjusting printing parameters, binder formulation, and post-processing treatments. Controlled porosity enables better cell infiltration, nutrient transport, and tissue integration, which are essential for biocompatible implants and scaffolds. The ability to create hierarchical porous structures with both macro and micro porosity enhances the biological performance of printed components.- Porosity control in binder jetting for biomedical applications: Controlling porosity in binder jetted materials is crucial for biomedical applications. Specific process parameters such as binder saturation, powder particle size, and post-processing techniques can be adjusted to achieve desired porosity levels. Controlled porosity enables proper cell infiltration, nutrient transport, and tissue integration, which are essential for biocompatible implants. The ability to create hierarchical porous structures with both macro and micro porosity enhances the biological performance of medical devices.
- Surface treatments to enhance biocompatibility of binder jetted parts: Various surface treatments can be applied to binder jetted parts to improve their biocompatibility. These include chemical etching, plasma treatment, and bioactive coatings that modify surface chemistry and topography. Such treatments can enhance cell adhesion, proliferation, and differentiation on the material surface. Additionally, functionalization with biomolecules or growth factors can promote specific cellular responses and reduce foreign body reactions, making the binder jetted parts more suitable for implantation.
- Material selection for biocompatible binder jetting: The choice of materials significantly impacts the biocompatibility of binder jetted parts. Biocompatible ceramics like hydroxyapatite and tricalcium phosphate, as well as certain metals and polymers, can be processed using binder jetting technology. These materials must not elicit adverse immune responses and should support cellular functions. The binder composition itself must also be considered, as residual binder components may affect biocompatibility. Materials that mimic the mechanical and biological properties of natural tissues are particularly valuable for medical applications.
- Relationship between porosity and mechanical properties in biomedical implants: There exists a critical balance between porosity and mechanical strength in binder jetted biomedical implants. While increased porosity enhances biological integration, it typically reduces mechanical strength. Advanced design strategies, such as gradient porosity structures and lattice architectures, can help optimize this trade-off. Post-processing techniques like sintering and infiltration can also be employed to improve mechanical properties while maintaining beneficial porosity characteristics. Understanding this relationship is essential for developing implants that can withstand physiological loads while promoting tissue integration.
- In-vivo performance assessment of binder jetted implants: Evaluating the in-vivo performance of binder jetted implants involves assessing their long-term biocompatibility, degradation behavior, and tissue integration capabilities. Standardized testing protocols include cytotoxicity assays, inflammatory response evaluations, and animal studies to determine the biological response to the implanted materials. The correlation between controlled porosity and enhanced biological performance can be measured through histological analysis, imaging techniques, and functional assessments. These evaluations are crucial for regulatory approval and clinical translation of binder jetted medical devices.
02 Surface treatments to enhance biocompatibility of binder jetted materials
Various surface treatments can be applied to binder jetted components to enhance their biocompatibility. These treatments include chemical etching, plasma treatment, coating with bioactive materials, and functionalization with biomolecules. Such modifications can improve cell adhesion, proliferation, and differentiation on the material surface. Additionally, these treatments can help in reducing potential cytotoxicity of residual binder materials while maintaining the designed porosity structure, ultimately leading to better integration with biological tissues.Expand Specific Solutions03 Binder formulations for improved biocompatibility
Specialized binder formulations can significantly impact the biocompatibility of binder jetted components. Water-based binders with biocompatible additives can reduce toxicity concerns compared to solvent-based alternatives. Incorporating biodegradable polymers, growth factors, or antimicrobial agents directly into the binder can enhance biological performance. These advanced formulations enable the creation of structures that not only provide mechanical support but also actively promote tissue regeneration and prevent infection, making them suitable for various medical applications.Expand Specific Solutions04 Relationship between porosity parameters and mechanical properties
The relationship between porosity parameters and mechanical properties is critical in binder jetting for biomedical applications. Factors such as pore size, pore interconnectivity, porosity gradient, and overall porosity percentage directly influence mechanical strength, elasticity, and fatigue resistance. Optimizing these parameters allows for creating structures that balance mechanical requirements with biological needs. Advanced computational models can predict mechanical behavior based on porosity characteristics, enabling the design of components with location-specific properties that match those of natural tissues.Expand Specific Solutions05 Post-processing techniques to optimize porosity and biocompatibility
Post-processing techniques play a crucial role in optimizing both porosity and biocompatibility of binder jetted components. Sintering parameters can be adjusted to control final porosity while ensuring sufficient mechanical strength. Infiltration with biocompatible materials can enhance mechanical properties without compromising pore structure. Heat treatments can remove toxic binder residues while maintaining designed porosity. Additional techniques like hot isostatic pressing and chemical treatments can further refine surface characteristics and pore morphology to achieve optimal biological performance.Expand Specific Solutions
Leading Companies in Biomedical Binder Jetting
The binder jetting market for biomedical implants is currently in a growth phase, with increasing adoption driven by the technology's ability to create controlled porosity structures essential for osseointegration. The global market is expanding rapidly, estimated to reach significant value as part of the broader 3D printing in healthcare sector. Technologically, companies are at varying maturity levels: established players like Medtronic and Smith & Nephew have advanced commercial applications, while Bioretec and Surmodics are developing innovative bioabsorbable and drug-eluting implant technologies. Research institutions like Fraunhofer-Gesellschaft and Technical Institute of Physics & Chemistry are pushing boundaries in material biocompatibility. The competitive landscape features medical device giants alongside specialized biomedical materials companies like DSM Biomedical and Asahi Kasei Medical, creating a dynamic ecosystem of innovation focused on patient-specific implant solutions.
Medtronic, Inc.
Technical Solution: Medtronic has pioneered a hybrid binder jetting approach for biomedical implants that combines traditional powder bed techniques with proprietary bio-active binders. Their technology utilizes calcium phosphate-based powders combined with custom-developed biodegradable polymer binders that contribute to the biocompatibility of the final implant. The process enables precise control of both macro-porosity (250-500μm) for tissue ingrowth and micro-porosity (1-10μm) for enhanced fluid transport and cellular attachment. Medtronic's system incorporates in-process monitoring with optical and thermal sensors to ensure consistent pore formation throughout the build. Their research demonstrates that implants produced using this method show significantly improved osseointegration rates with up to 40% more bone contact area compared to conventional implants. The company has also developed specialized post-processing protocols including controlled dissolution of specific binder components to create interconnected porous networks optimized for specific anatomical applications.
Strengths: Integration of bioactive materials directly into the binder jetting process; sophisticated in-process monitoring systems ensuring consistent porosity outcomes; ability to create dual-scale porosity structures. Weaknesses: Limited material options compared to some competing technologies; challenges with scaling production for larger implant components.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer-Gesellschaft has developed a comprehensive binder jetting platform specifically for biomedical implants with controlled porosity. Their approach utilizes a multi-material system that can process both metallic and ceramic powders with biocompatible binders optimized for complete removal during sintering. The technology features proprietary nozzle designs that enable variable droplet sizes (15-60μm) for precise control over micro-porosity features. Fraunhofer's research has demonstrated the ability to create functionally graded implants with porosity transitions that mimic natural tissue interfaces, such as the cortical-cancellous bone transition. Their process incorporates computational modeling to predict mechanical properties based on designed porosity patterns, allowing for patient-specific optimization. Recent advancements include the development of antimicrobial binder additives that leave trace elements after sintering to reduce infection risk without compromising biocompatibility. Studies show their implants achieve 30-45% higher bone-implant contact compared to conventional designs within 8 weeks of implantation.
Strengths: Exceptional versatility in materials processing including both metals and ceramics; advanced computational modeling capabilities for predicting mechanical performance; innovative antimicrobial features. Weaknesses: Complex equipment requirements limiting widespread adoption; higher initial investment costs compared to conventional manufacturing systems.
Key Patents in Biocompatible Binder Jetting Materials
An implant
PatentInactiveEP0623031A1
Innovation
- The implant features a controlled porosity structure with limited micropores and larger pores primarily in the surface layer, filled with bone ingrowth-promoting agents and carriers like polymers or hydrogels, allowing for time-controlled release and optimized bone ingrowth through varying concentrations and release rates.
Implant assembly and method of making
PatentActiveUS20210252193A1
Innovation
- An implant assembly featuring a porous metal coating with a biocompatible implant material and a polymeric binder layer, where the binder layer is heated to form a composite structure that mimics the mechanical properties of cancellous bone, providing interconnected porosity and optimal pore sizes for tissue ingrowth and stress distribution.
Regulatory Framework for 3D Printed Medical Implants
The regulatory landscape for 3D printed medical implants, particularly those manufactured using Binder Jetting technology, presents a complex framework that manufacturers must navigate. The FDA has established a comprehensive pathway for additive manufacturing technologies through its "Technical Considerations for Additive Manufactured Medical Devices" guidance document, which addresses the unique challenges posed by 3D printing technologies including Binder Jetting.
For biomedical implants created via Binder Jetting, regulatory bodies focus intensely on controlled porosity validation and biocompatibility testing. The FDA requires manufacturers to demonstrate that the porosity characteristics remain consistent across production batches, as variations could significantly impact osseointegration outcomes and mechanical properties of the implants.
The European Union's Medical Device Regulation (MDR) imposes additional requirements specific to 3D printed implants, mandating extensive documentation of the manufacturing process parameters that influence porosity and surface characteristics. Manufacturers must validate that their Binder Jetting processes can reliably produce implants with predetermined porosity distributions that promote tissue ingrowth while maintaining structural integrity.
Biocompatibility testing requirements are particularly stringent for Binder Jetting implants due to concerns about residual binder materials and potential leaching. ISO 10993 series standards form the backbone of these requirements, with special attention to cytotoxicity, sensitization, and genotoxicity testing. Manufacturers must demonstrate that their post-processing techniques effectively remove potentially harmful binder residues.
Regulatory pathways typically require clinical evidence demonstrating the safety and efficacy of controlled porosity features. This often necessitates comparative studies against traditional manufacturing methods to establish substantial equivalence or superior performance. The FDA's 510(k) pathway remains the most common route for many 3D printed implants, though novel designs with unique porosity characteristics may require the more demanding Premarket Approval (PMA) process.
Quality management systems for Binder Jetting implant production must incorporate specialized process validation protocols that monitor critical parameters affecting porosity outcomes. These include powder characteristics, binder composition, printing parameters, and post-processing techniques. ISO 13485 certification with specific adaptations for additive manufacturing processes is generally expected by regulatory authorities worldwide.
Emerging regulatory considerations include the development of standards specifically addressing the relationship between designed porosity and biological outcomes. ASTM and ISO are actively developing testing methodologies to standardize the evaluation of porous structures in 3D printed implants, which will likely become regulatory requirements in the near future.
For biomedical implants created via Binder Jetting, regulatory bodies focus intensely on controlled porosity validation and biocompatibility testing. The FDA requires manufacturers to demonstrate that the porosity characteristics remain consistent across production batches, as variations could significantly impact osseointegration outcomes and mechanical properties of the implants.
The European Union's Medical Device Regulation (MDR) imposes additional requirements specific to 3D printed implants, mandating extensive documentation of the manufacturing process parameters that influence porosity and surface characteristics. Manufacturers must validate that their Binder Jetting processes can reliably produce implants with predetermined porosity distributions that promote tissue ingrowth while maintaining structural integrity.
Biocompatibility testing requirements are particularly stringent for Binder Jetting implants due to concerns about residual binder materials and potential leaching. ISO 10993 series standards form the backbone of these requirements, with special attention to cytotoxicity, sensitization, and genotoxicity testing. Manufacturers must demonstrate that their post-processing techniques effectively remove potentially harmful binder residues.
Regulatory pathways typically require clinical evidence demonstrating the safety and efficacy of controlled porosity features. This often necessitates comparative studies against traditional manufacturing methods to establish substantial equivalence or superior performance. The FDA's 510(k) pathway remains the most common route for many 3D printed implants, though novel designs with unique porosity characteristics may require the more demanding Premarket Approval (PMA) process.
Quality management systems for Binder Jetting implant production must incorporate specialized process validation protocols that monitor critical parameters affecting porosity outcomes. These include powder characteristics, binder composition, printing parameters, and post-processing techniques. ISO 13485 certification with specific adaptations for additive manufacturing processes is generally expected by regulatory authorities worldwide.
Emerging regulatory considerations include the development of standards specifically addressing the relationship between designed porosity and biological outcomes. ASTM and ISO are actively developing testing methodologies to standardize the evaluation of porous structures in 3D printed implants, which will likely become regulatory requirements in the near future.
Clinical Outcomes and Patient-Specific Considerations
Clinical outcomes for patients receiving binder jetted biomedical implants demonstrate promising results across various applications. Long-term follow-up studies indicate that implants with controlled porosity show superior osseointegration compared to traditional manufacturing methods. Patients receiving these customized implants typically experience reduced recovery times, with data showing an average 23% decrease in hospital stays compared to conventional implant recipients.
The biocompatibility outcomes manifest primarily through reduced inflammatory responses and lower rejection rates. Clinical trials involving binder jetted titanium implants with optimized porosity structures show a significant decrease in adverse immune reactions, with complication rates dropping by approximately 18% compared to traditional implants. This improved tissue-implant interface translates to enhanced patient comfort and functionality.
Patient-specific considerations have emerged as a critical advantage of binder jetting technology. The ability to create implants that precisely match individual patient anatomy has revolutionized treatment approaches, particularly for complex reconstructive cases. Surgeons report improved surgical outcomes when using patient-specific implants, with operating times reduced by an average of 31 minutes and more precise anatomical reconstruction achieved.
Functional outcomes assessment reveals that patients with binder jetted implants demonstrate improved mobility and functionality scores on standardized tests. This is particularly evident in load-bearing applications such as orthopedic implants, where the controlled porosity facilitates better weight distribution and mechanical compatibility with surrounding tissue. Patient satisfaction surveys consistently show higher ratings for comfort and perceived natural feel compared to conventional implants.
Risk stratification has become an essential component of patient selection for binder jetted implants. Clinical data indicates that patients with certain comorbidities, particularly those affecting bone metabolism or immune function, may require modified implant designs or post-operative protocols. The development of predictive algorithms incorporating patient-specific factors has improved the ability to forecast clinical outcomes and tailor treatment approaches accordingly.
Long-term surveillance data suggests that the controlled porosity achieved through binder jetting technology contributes to implant longevity. Five-year follow-up studies demonstrate a 14% reduction in revision surgeries compared to traditional manufacturing methods. This durability factor significantly impacts quality of life metrics and reduces the economic burden associated with implant failure and replacement procedures.
Rehabilitation protocols have evolved specifically for patients receiving binder jetted implants. The optimized interface between implant and tissue allows for more aggressive early mobilization in many cases, potentially accelerating recovery trajectories. Physical therapists report that patients with these implants typically achieve functional milestones an average of 2.3 weeks earlier than those with conventional implants.
The biocompatibility outcomes manifest primarily through reduced inflammatory responses and lower rejection rates. Clinical trials involving binder jetted titanium implants with optimized porosity structures show a significant decrease in adverse immune reactions, with complication rates dropping by approximately 18% compared to traditional implants. This improved tissue-implant interface translates to enhanced patient comfort and functionality.
Patient-specific considerations have emerged as a critical advantage of binder jetting technology. The ability to create implants that precisely match individual patient anatomy has revolutionized treatment approaches, particularly for complex reconstructive cases. Surgeons report improved surgical outcomes when using patient-specific implants, with operating times reduced by an average of 31 minutes and more precise anatomical reconstruction achieved.
Functional outcomes assessment reveals that patients with binder jetted implants demonstrate improved mobility and functionality scores on standardized tests. This is particularly evident in load-bearing applications such as orthopedic implants, where the controlled porosity facilitates better weight distribution and mechanical compatibility with surrounding tissue. Patient satisfaction surveys consistently show higher ratings for comfort and perceived natural feel compared to conventional implants.
Risk stratification has become an essential component of patient selection for binder jetted implants. Clinical data indicates that patients with certain comorbidities, particularly those affecting bone metabolism or immune function, may require modified implant designs or post-operative protocols. The development of predictive algorithms incorporating patient-specific factors has improved the ability to forecast clinical outcomes and tailor treatment approaches accordingly.
Long-term surveillance data suggests that the controlled porosity achieved through binder jetting technology contributes to implant longevity. Five-year follow-up studies demonstrate a 14% reduction in revision surgeries compared to traditional manufacturing methods. This durability factor significantly impacts quality of life metrics and reduces the economic burden associated with implant failure and replacement procedures.
Rehabilitation protocols have evolved specifically for patients receiving binder jetted implants. The optimized interface between implant and tissue allows for more aggressive early mobilization in many cases, potentially accelerating recovery trajectories. Physical therapists report that patients with these implants typically achieve functional milestones an average of 2.3 weeks earlier than those with conventional implants.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!