Bioprinting Of Vascularized Tissue Using Volumetric Additive Manufacturing
SEP 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioprinting Evolution and Vascularization Goals
Bioprinting has evolved significantly since its inception in the early 2000s, transitioning from simple cell deposition techniques to sophisticated 3D tissue construction methodologies. The initial bioprinting approaches focused primarily on creating basic tissue structures without integrated vascular networks, severely limiting their clinical viability and functionality. As the field progressed, researchers recognized that vascularization—the development of blood vessel networks—represents the critical bottleneck in engineering functional tissues and organs for transplantation and disease modeling.
Traditional bioprinting methods, including extrusion-based, inkjet, and laser-assisted techniques, have demonstrated limited success in creating complex vascular architectures. These conventional approaches typically build structures layer-by-layer, resulting in resolution constraints and extended fabrication times that compromise cell viability. The emergence of volumetric additive manufacturing (VAM) represents a paradigm shift in bioprinting technology, offering simultaneous solidification throughout an entire volume rather than sequential layer deposition.
The integration of volumetric bioprinting with vascularization strategies aims to address several critical challenges in tissue engineering. Primary objectives include developing perfusable vascular networks that can support nutrient and oxygen delivery to cells located more than 200 micrometers from the nearest capillary—the diffusion limit in living tissues. Additionally, researchers seek to recapitulate the hierarchical organization of native vasculature, from large vessels to intricate capillary beds, within engineered constructs.
Current vascularization goals focus on achieving multi-scale vessel networks with appropriate mechanical properties and biological functionality. This includes developing bioinks that can simultaneously support structural integrity and cellular viability while enabling the formation of hollow, perfusable channels. Furthermore, researchers aim to incorporate endothelial cells that can self-organize and mature into functional blood vessels capable of responding to physiological stimuli.
The convergence of volumetric bioprinting with advanced biomaterials science presents unprecedented opportunities for creating vascularized tissues with clinically relevant dimensions. By leveraging computed tomography of light, holographic stereolithography, and tomographic volumetric techniques, researchers can now fabricate complex structures in seconds rather than hours, dramatically improving cell survival rates during the manufacturing process.
Looking forward, the field is moving toward the development of dynamic vascularized tissue models that can simulate blood flow, facilitate drug screening, and potentially serve as functional tissue replacements. The ultimate goal remains the creation of transplantable tissues and organs with integrated vascular networks capable of immediate anastomosis with host vasculature upon implantation, thereby addressing the global organ shortage crisis.
Traditional bioprinting methods, including extrusion-based, inkjet, and laser-assisted techniques, have demonstrated limited success in creating complex vascular architectures. These conventional approaches typically build structures layer-by-layer, resulting in resolution constraints and extended fabrication times that compromise cell viability. The emergence of volumetric additive manufacturing (VAM) represents a paradigm shift in bioprinting technology, offering simultaneous solidification throughout an entire volume rather than sequential layer deposition.
The integration of volumetric bioprinting with vascularization strategies aims to address several critical challenges in tissue engineering. Primary objectives include developing perfusable vascular networks that can support nutrient and oxygen delivery to cells located more than 200 micrometers from the nearest capillary—the diffusion limit in living tissues. Additionally, researchers seek to recapitulate the hierarchical organization of native vasculature, from large vessels to intricate capillary beds, within engineered constructs.
Current vascularization goals focus on achieving multi-scale vessel networks with appropriate mechanical properties and biological functionality. This includes developing bioinks that can simultaneously support structural integrity and cellular viability while enabling the formation of hollow, perfusable channels. Furthermore, researchers aim to incorporate endothelial cells that can self-organize and mature into functional blood vessels capable of responding to physiological stimuli.
The convergence of volumetric bioprinting with advanced biomaterials science presents unprecedented opportunities for creating vascularized tissues with clinically relevant dimensions. By leveraging computed tomography of light, holographic stereolithography, and tomographic volumetric techniques, researchers can now fabricate complex structures in seconds rather than hours, dramatically improving cell survival rates during the manufacturing process.
Looking forward, the field is moving toward the development of dynamic vascularized tissue models that can simulate blood flow, facilitate drug screening, and potentially serve as functional tissue replacements. The ultimate goal remains the creation of transplantable tissues and organs with integrated vascular networks capable of immediate anastomosis with host vasculature upon implantation, thereby addressing the global organ shortage crisis.
Market Analysis for Vascularized Tissue Applications
The global market for vascularized tissue applications is experiencing significant growth, driven by increasing prevalence of chronic diseases, rising demand for organ transplantation, and advancements in bioprinting technologies. The market for engineered tissues with functional vasculature is projected to reach $4.3 billion by 2028, growing at a compound annual growth rate of 15.7% from 2023.
Healthcare applications represent the largest segment, with regenerative medicine and tissue engineering leading the demand. Vascularized tissue constructs are particularly valuable for treating conditions like cardiovascular diseases, diabetic ulcers, and severe burns, where restoration of blood supply is critical for tissue function and survival. The aging global population and rising incidence of lifestyle-related diseases further amplify this demand.
Pharmaceutical companies constitute another major market segment, utilizing vascularized tissue models for drug discovery and toxicity testing. These bioprinted tissues provide more physiologically relevant platforms compared to traditional 2D cell cultures, potentially reducing drug development costs by enabling earlier identification of ineffective or toxic compounds. This application alone is estimated to save pharmaceutical companies billions in development costs annually.
Cosmetic and dermatological industries are emerging as significant adopters, using vascularized skin constructs for product testing as alternatives to animal models, particularly in regions with strict regulations against animal testing. This segment is expected to grow at 18.2% annually through 2028.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 21%. However, the Asia-Pacific region is witnessing the fastest growth rate due to increasing healthcare expenditure, expanding research infrastructure, and supportive government initiatives in countries like China, Japan, and South Korea.
Key market challenges include high production costs, regulatory hurdles, and technical limitations in creating complex vascular networks. The average cost of producing clinically relevant vascularized tissue constructs remains prohibitively high at $1,500-3,000 per square centimeter, limiting widespread clinical adoption.
Customer segments show varying priorities: healthcare providers focus on clinical outcomes and integration with existing treatment protocols; research institutions prioritize customization capabilities and reproducibility; while pharmaceutical companies value throughput and compatibility with automated testing systems.
Market forecasts indicate that as volumetric additive manufacturing techniques mature and production scales up, costs will decrease by approximately 60% over the next five years, potentially catalyzing broader market penetration and opening new application areas in personalized medicine and organ replacement therapies.
Healthcare applications represent the largest segment, with regenerative medicine and tissue engineering leading the demand. Vascularized tissue constructs are particularly valuable for treating conditions like cardiovascular diseases, diabetic ulcers, and severe burns, where restoration of blood supply is critical for tissue function and survival. The aging global population and rising incidence of lifestyle-related diseases further amplify this demand.
Pharmaceutical companies constitute another major market segment, utilizing vascularized tissue models for drug discovery and toxicity testing. These bioprinted tissues provide more physiologically relevant platforms compared to traditional 2D cell cultures, potentially reducing drug development costs by enabling earlier identification of ineffective or toxic compounds. This application alone is estimated to save pharmaceutical companies billions in development costs annually.
Cosmetic and dermatological industries are emerging as significant adopters, using vascularized skin constructs for product testing as alternatives to animal models, particularly in regions with strict regulations against animal testing. This segment is expected to grow at 18.2% annually through 2028.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 21%. However, the Asia-Pacific region is witnessing the fastest growth rate due to increasing healthcare expenditure, expanding research infrastructure, and supportive government initiatives in countries like China, Japan, and South Korea.
Key market challenges include high production costs, regulatory hurdles, and technical limitations in creating complex vascular networks. The average cost of producing clinically relevant vascularized tissue constructs remains prohibitively high at $1,500-3,000 per square centimeter, limiting widespread clinical adoption.
Customer segments show varying priorities: healthcare providers focus on clinical outcomes and integration with existing treatment protocols; research institutions prioritize customization capabilities and reproducibility; while pharmaceutical companies value throughput and compatibility with automated testing systems.
Market forecasts indicate that as volumetric additive manufacturing techniques mature and production scales up, costs will decrease by approximately 60% over the next five years, potentially catalyzing broader market penetration and opening new application areas in personalized medicine and organ replacement therapies.
Current Bioprinting Technologies and Vascularization Challenges
Bioprinting technologies have evolved significantly over the past decade, with several approaches emerging to address the complex challenge of creating functional vascularized tissues. Current mainstream bioprinting technologies include extrusion-based bioprinting, inkjet bioprinting, laser-assisted bioprinting, and stereolithography-based bioprinting. Each of these methods offers distinct advantages and limitations when applied to tissue engineering applications.
Extrusion-based bioprinting remains the most widely adopted technique due to its versatility and relatively low cost. This approach utilizes mechanical or pneumatic forces to dispense continuous filaments of bioink containing cells and supporting materials. While effective for creating larger tissue constructs, extrusion-based methods typically operate at lower resolutions (100-500 μm) and subject cells to significant shear stresses during the printing process, potentially compromising cell viability.
Inkjet bioprinting offers improved resolution (20-100 μm) through the precise deposition of bioink droplets. This technology enables higher printing speeds and better cell viability compared to extrusion methods. However, it faces limitations in terms of viscosity constraints and difficulties in producing complex 3D structures with adequate mechanical integrity.
Laser-assisted bioprinting, including laser-induced forward transfer (LIFT), provides exceptional resolution (below 10 μm) and cell viability. This technique uses laser energy to propel small volumes of cell-laden materials onto a substrate with high precision. Despite these advantages, the technology suffers from low throughput, high equipment costs, and challenges in scaling to clinically relevant tissue dimensions.
Stereolithography-based bioprinting utilizes light to selectively crosslink photosensitive bioinks, offering excellent resolution (25-50 μm) and the ability to create complex geometries. However, the limited availability of biocompatible photocurable materials and potential cytotoxicity from photoinitiators present ongoing challenges.
The central challenge across all bioprinting technologies remains effective vascularization. Natural tissues rely on hierarchical vascular networks ranging from large vessels (>100 μm) to capillaries (5-10 μm) to ensure adequate nutrient and oxygen delivery. Current bioprinting approaches struggle to recreate this multi-scale vasculature, particularly at the capillary level where diffusion limitations become critical beyond 200 μm from the nearest blood vessel.
Several strategies have emerged to address vascularization challenges, including sacrificial bioprinting, where temporary structures are printed and later removed to create vessel-like channels; embedded bioprinting within supportive matrices; and pre-vascularization approaches utilizing endothelial cells to form vessel networks prior to tissue maturation. Despite these advances, creating functional, perfusable vascular networks that seamlessly integrate across different scales remains an unresolved challenge in the field.
Volumetric additive manufacturing represents a promising new direction, potentially overcoming resolution and speed limitations of traditional layer-by-layer approaches. However, adapting this technology to incorporate living cells while maintaining biocompatibility presents significant technical hurdles that must be addressed to realize its potential for vascularized tissue engineering.
Extrusion-based bioprinting remains the most widely adopted technique due to its versatility and relatively low cost. This approach utilizes mechanical or pneumatic forces to dispense continuous filaments of bioink containing cells and supporting materials. While effective for creating larger tissue constructs, extrusion-based methods typically operate at lower resolutions (100-500 μm) and subject cells to significant shear stresses during the printing process, potentially compromising cell viability.
Inkjet bioprinting offers improved resolution (20-100 μm) through the precise deposition of bioink droplets. This technology enables higher printing speeds and better cell viability compared to extrusion methods. However, it faces limitations in terms of viscosity constraints and difficulties in producing complex 3D structures with adequate mechanical integrity.
Laser-assisted bioprinting, including laser-induced forward transfer (LIFT), provides exceptional resolution (below 10 μm) and cell viability. This technique uses laser energy to propel small volumes of cell-laden materials onto a substrate with high precision. Despite these advantages, the technology suffers from low throughput, high equipment costs, and challenges in scaling to clinically relevant tissue dimensions.
Stereolithography-based bioprinting utilizes light to selectively crosslink photosensitive bioinks, offering excellent resolution (25-50 μm) and the ability to create complex geometries. However, the limited availability of biocompatible photocurable materials and potential cytotoxicity from photoinitiators present ongoing challenges.
The central challenge across all bioprinting technologies remains effective vascularization. Natural tissues rely on hierarchical vascular networks ranging from large vessels (>100 μm) to capillaries (5-10 μm) to ensure adequate nutrient and oxygen delivery. Current bioprinting approaches struggle to recreate this multi-scale vasculature, particularly at the capillary level where diffusion limitations become critical beyond 200 μm from the nearest blood vessel.
Several strategies have emerged to address vascularization challenges, including sacrificial bioprinting, where temporary structures are printed and later removed to create vessel-like channels; embedded bioprinting within supportive matrices; and pre-vascularization approaches utilizing endothelial cells to form vessel networks prior to tissue maturation. Despite these advances, creating functional, perfusable vascular networks that seamlessly integrate across different scales remains an unresolved challenge in the field.
Volumetric additive manufacturing represents a promising new direction, potentially overcoming resolution and speed limitations of traditional layer-by-layer approaches. However, adapting this technology to incorporate living cells while maintaining biocompatibility presents significant technical hurdles that must be addressed to realize its potential for vascularized tissue engineering.
Current Volumetric Additive Manufacturing Approaches
01 3D bioprinting techniques for vascularized tissue construction
Various 3D bioprinting techniques can be employed to create vascularized tissue constructs. These methods include extrusion-based bioprinting, inkjet bioprinting, laser-assisted bioprinting, and stereolithography. Each technique offers unique advantages for creating complex vascular networks within engineered tissues. The bioprinting process typically involves layer-by-layer deposition of bioinks containing cells and supporting materials to form three-dimensional structures with integrated vascular channels.- 3D bioprinting techniques for vascularized tissue: Various 3D bioprinting techniques can be used to create vascularized tissue constructs. These techniques include extrusion-based bioprinting, inkjet bioprinting, laser-assisted bioprinting, and stereolithography. Each method offers different advantages for creating complex vascular networks within engineered tissues. These approaches allow for precise positioning of cells and biomaterials to mimic the natural architecture of blood vessels in native tissues.
- Biomaterials and scaffolds for vascular network formation: Specialized biomaterials and scaffolds are essential for supporting the formation of vascular networks in bioprinted tissues. These materials include natural polymers (collagen, fibrin, alginate), synthetic polymers, and hybrid materials that provide structural support while allowing for cell migration and vessel formation. The physical and chemical properties of these scaffolds can be tailored to promote angiogenesis and vasculogenesis within the engineered tissue constructs.
- Cell sources and co-culture systems for vascularization: Various cell types and co-culture systems are utilized to create functional vascular networks in bioprinted tissues. Endothelial cells, smooth muscle cells, pericytes, and stem cells can be combined in specific ratios to promote vessel formation. Co-culture systems that include parenchymal cells alongside vascular cells help to create more physiologically relevant tissue constructs with integrated vascular networks that can support nutrient and oxygen delivery.
- Growth factors and signaling molecules for vascular development: Growth factors and signaling molecules play crucial roles in promoting vascularization in bioprinted tissues. Factors such as VEGF, bFGF, PDGF, and angiopoietins can be incorporated into bioinks or scaffolds to stimulate angiogenesis and vessel maturation. Controlled release systems can be designed to deliver these factors in a spatiotemporally regulated manner, mimicking the natural process of blood vessel formation and maturation in developing tissues.
- Perfusion systems and bioreactors for vascularized tissue maturation: Perfusion systems and bioreactors are essential for the maturation and maintenance of bioprinted vascularized tissues. These systems provide dynamic culture conditions that simulate physiological fluid flow, pressure, and shear stress, which are critical for proper vessel formation and function. Advanced bioreactors can incorporate sensors to monitor tissue development and adjust culture conditions accordingly, enabling the creation of more functional and clinically relevant vascularized tissue constructs.
02 Bioinks and biomaterials for vascular network formation
Specialized bioinks and biomaterials play a crucial role in bioprinting vascularized tissues. These materials must support cell viability, promote vascular network formation, and provide appropriate mechanical properties. Common biomaterials include natural hydrogels (collagen, fibrin, gelatin), synthetic polymers, and decellularized extracellular matrix. These materials can be modified with growth factors and adhesion molecules to enhance vascular development and integration within the printed tissue constructs.Expand Specific Solutions03 Cell sources and co-culture systems for vascularization
Various cell types are utilized in bioprinting vascularized tissues, including endothelial cells, smooth muscle cells, pericytes, and stem cells. Co-culture systems combining multiple cell types can enhance vascular network formation and stability. Endothelial cells form the inner lining of blood vessels, while supporting cells provide structural integrity and promote vessel maturation. Stem cells can differentiate into vascular cell types and contribute to the formation of functional blood vessels within the bioprinted constructs.Expand Specific Solutions04 Growth factors and signaling molecules for vascular development
Growth factors and signaling molecules are essential for promoting vascularization in bioprinted tissues. Factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), and angiopoietins can be incorporated into bioinks or delivered through controlled release systems. These bioactive molecules stimulate endothelial cell proliferation, migration, and tube formation, leading to the development of functional vascular networks within the engineered tissues.Expand Specific Solutions05 Perfusion systems and bioreactors for vascularized tissue maturation
Perfusion systems and bioreactors are crucial for the maturation and maintenance of bioprinted vascularized tissues. These systems provide continuous flow of culture medium, nutrients, and oxygen through the vascular channels, mimicking physiological conditions. Dynamic culture conditions created by bioreactors can enhance cell viability, promote vascular network formation, and improve the functional properties of the engineered tissues. Advanced perfusion systems may incorporate pulsatile flow to better simulate in vivo hemodynamic conditions.Expand Specific Solutions
Leading Organizations in Vascularized Tissue Bioprinting
The bioprinting of vascularized tissue using volumetric additive manufacturing is currently in an early growth phase, with significant research momentum but limited commercial applications. The global market for this technology is expanding rapidly, projected to reach several billion dollars by 2030, driven by increasing demand for organ transplantation alternatives and drug testing platforms. Technologically, the field shows varying maturity levels across players: academic institutions like Tsinghua University, EPFL, and Harvard are pioneering fundamental research, while companies such as CollPlant, Readily3D, and Sichuan Revotek are developing specialized bioprinting platforms. Advanced Solutions Life Sciences and Stratasys represent established players adapting their expertise to this emerging field, with Fluicell and Brinter focusing on single-cell precision and specialized bioprinting solutions respectively.
President & Fellows of Harvard College
Technical Solution: Harvard's approach to bioprinting vascularized tissue employs SWIFT (Sacrificial Writing Into Functional Tissue) technology, which combines organ-specific cell spheroids with a sacrificial gelatin ink. The process creates a perfusable vascular network within dense tissues by extruding the sacrificial ink into a compacted organoid matrix, which is later removed to form channels. Their volumetric manufacturing technique enables the creation of complex, hierarchical vascular architectures that mimic native tissue organization. Harvard researchers have demonstrated successful perfusion of these engineered tissues with oxygenated blood, maintaining cell viability in constructs exceeding 1cm thickness. The technology incorporates multiple cell types including endothelial cells that line the vascular channels, promoting proper barrier function and physiological responses. Recent advancements have integrated this approach with computational modeling to optimize flow dynamics and nutrient distribution throughout the printed constructs.
Strengths: Superior capability to create complex, hierarchical vascular networks that closely mimic natural tissue architecture; demonstrated success in maintaining cell viability in thick tissue constructs; strong integration of multiple cell types. Weaknesses: Requires sophisticated equipment and expertise; scaling up to clinically relevant tissue sizes remains challenging; potential limitations in mechanical strength of the resulting constructs.
Sichuan Revotek
Technical Solution: Sichuan Revotek has developed a comprehensive bioprinting platform specifically targeting vascularized tissue engineering using their proprietary "Biosynsphere" technology. Their approach combines stem cell-derived bioinks with a specialized extrusion-based volumetric printing system that creates vascular structures with precise spatial control. The company's technology incorporates a temperature-controlled printing environment that maintains optimal conditions for cell viability throughout the fabrication process. Revotek's system utilizes a dual-nozzle printing mechanism that simultaneously deposits structural and sacrificial materials to create hollow vascular channels within tissue constructs. Their bioinks contain growth factors and ECM components that promote endothelialization of the printed vessels, enhancing their functionality. Notably, Revotek has demonstrated successful implantation of 3D-printed blood vessels in animal models, with evidence of integration with host vasculature and maintenance of patency. Their recent advancements include the development of patient-specific vascular grafts using autologous cells, moving toward personalized tissue engineering solutions.
Strengths: Demonstrated in vivo success with functional vascular implants; comprehensive platform combining proprietary bioinks and specialized printing hardware; strong focus on clinical translation with animal validation studies. Weaknesses: Potential limitations in creating complex multi-branched vascular networks; challenges in scaling production for clinical applications; proprietary nature of technology may limit broader research applications.
Key Patents in Vascularized Tissue Bioprinting
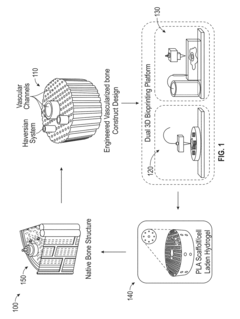
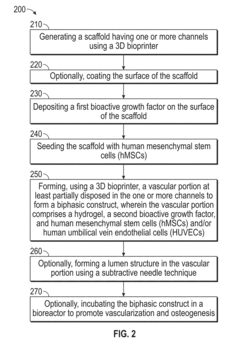
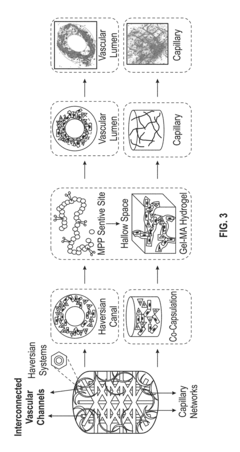
Vascularized biphasic tissue constructs
PatentActiveUS20190030212A1
Innovation
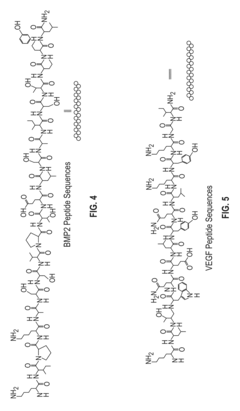
- A dual 3D bioprinting approach combining FDM and SLA bioprinters with regional bioactive peptide immobilization using mussel-inspired chemistry and thiol-ene click reactions to create vascularized biphasic tissue constructs that mimic native bone, incorporating BMP2 and VEGF peptides to promote osteogenesis and angiogenesis.
Biomaterials for Volumetric Tissue Engineering
The selection of appropriate biomaterials is critical for successful volumetric tissue engineering, particularly in the context of vascularized tissue bioprinting. These materials must satisfy multiple requirements including biocompatibility, photocrosslinking capabilities, and mechanical properties that support both the printing process and subsequent tissue function.
Hydrogels represent the primary class of biomaterials employed in volumetric additive manufacturing for tissue engineering. Natural hydrogels such as gelatin methacrylate (GelMA), alginate, and collagen derivatives offer excellent biocompatibility and cell-adhesion properties. These materials closely mimic the native extracellular matrix, providing an environment conducive to cell proliferation and migration. GelMA, in particular, has emerged as a versatile option due to its tunable mechanical properties and photocrosslinking capability.
Synthetic hydrogels, including poly(ethylene glycol) diacrylate (PEGDA) and polyvinyl alcohol (PVA), offer advantages in terms of batch consistency and customizable mechanical properties. These materials can be precisely engineered to achieve specific degradation rates and mechanical stiffness, critical factors for vascular tissue development. Recent advances have focused on developing hybrid materials that combine the biocompatibility of natural hydrogels with the tunability of synthetic options.
Photoinitiators play a crucial role in volumetric bioprinting, as they facilitate rapid crosslinking when exposed to specific wavelengths of light. Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) and Irgacure series compounds are commonly used due to their efficiency at wavelengths that minimize cellular damage. The selection of appropriate photoinitiators must balance crosslinking efficiency with cytocompatibility.
For vascularized tissue specifically, sacrificial biomaterials have gained significant attention. These materials, such as pluronic F-127 and carbohydrate glass, can be incorporated during the printing process and subsequently removed to create perfusable channels. This approach enables the formation of complex vascular networks within volumetrically printed constructs.
Recent innovations include responsive biomaterials that can change properties in response to specific stimuli, allowing for dynamic remodeling of the tissue construct post-printing. Additionally, materials incorporating growth factors or bioactive molecules can promote angiogenesis and vascular maturation within the printed construct, enhancing functional integration with host tissue upon implantation.
The development of biomaterials with optimal rheological properties remains a significant challenge, as materials must flow during the printing process yet maintain structural integrity afterward. Ongoing research focuses on developing shear-thinning hydrogels that can recover their mechanical properties rapidly after extrusion while supporting cellular viability and function.
Hydrogels represent the primary class of biomaterials employed in volumetric additive manufacturing for tissue engineering. Natural hydrogels such as gelatin methacrylate (GelMA), alginate, and collagen derivatives offer excellent biocompatibility and cell-adhesion properties. These materials closely mimic the native extracellular matrix, providing an environment conducive to cell proliferation and migration. GelMA, in particular, has emerged as a versatile option due to its tunable mechanical properties and photocrosslinking capability.
Synthetic hydrogels, including poly(ethylene glycol) diacrylate (PEGDA) and polyvinyl alcohol (PVA), offer advantages in terms of batch consistency and customizable mechanical properties. These materials can be precisely engineered to achieve specific degradation rates and mechanical stiffness, critical factors for vascular tissue development. Recent advances have focused on developing hybrid materials that combine the biocompatibility of natural hydrogels with the tunability of synthetic options.
Photoinitiators play a crucial role in volumetric bioprinting, as they facilitate rapid crosslinking when exposed to specific wavelengths of light. Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) and Irgacure series compounds are commonly used due to their efficiency at wavelengths that minimize cellular damage. The selection of appropriate photoinitiators must balance crosslinking efficiency with cytocompatibility.
For vascularized tissue specifically, sacrificial biomaterials have gained significant attention. These materials, such as pluronic F-127 and carbohydrate glass, can be incorporated during the printing process and subsequently removed to create perfusable channels. This approach enables the formation of complex vascular networks within volumetrically printed constructs.
Recent innovations include responsive biomaterials that can change properties in response to specific stimuli, allowing for dynamic remodeling of the tissue construct post-printing. Additionally, materials incorporating growth factors or bioactive molecules can promote angiogenesis and vascular maturation within the printed construct, enhancing functional integration with host tissue upon implantation.
The development of biomaterials with optimal rheological properties remains a significant challenge, as materials must flow during the printing process yet maintain structural integrity afterward. Ongoing research focuses on developing shear-thinning hydrogels that can recover their mechanical properties rapidly after extrusion while supporting cellular viability and function.
Regulatory Pathway for Bioprinted Tissues
The regulatory landscape for bioprinted tissues represents a complex and evolving framework that requires careful navigation by developers and manufacturers. Currently, the FDA categorizes bioprinted tissues as combination products, falling under the jurisdiction of multiple regulatory centers including the Center for Biologics Evaluation and Research (CBER), the Center for Devices and Radiological Health (CDRH), and potentially the Center for Drug Evaluation and Research (CDER), depending on the primary mode of action.
For vascularized tissues created through volumetric additive manufacturing, the regulatory pathway typically begins with preclinical testing to establish safety profiles and functional characteristics. This includes biocompatibility assessments, degradation studies, mechanical testing, and in vitro/in vivo performance evaluations. The complexity of these tissues, particularly those with integrated vascular networks, necessitates comprehensive characterization of both the biomaterials and the cellular components.
The FDA's regulatory approach for these advanced therapies follows a risk-based framework, with increasing scrutiny proportional to the perceived risk. Bioprinted tissues intended for implantation face more rigorous requirements than those developed for in vitro testing or research purposes. Developers must demonstrate consistent manufacturing processes with appropriate quality controls, addressing challenges unique to volumetric bioprinting such as resolution verification, structural integrity, and vascular patency.
International regulatory frameworks show notable variations. The European Medicines Agency (EMA) classifies most bioprinted tissues as Advanced Therapy Medicinal Products (ATMPs), requiring centralized authorization procedures. Japan has implemented an accelerated approval pathway for regenerative medicine products, potentially offering faster market access for certain bioprinted tissues. These regional differences create strategic considerations for global development programs.
Regulatory challenges specific to vascularized tissue bioprinting include establishing appropriate reference standards, defining meaningful functional endpoints, and developing validated testing methodologies. The dynamic nature of living tissues complicates traditional stability testing paradigms, requiring innovative approaches to shelf-life determination and storage validation.
Industry stakeholders are actively engaging with regulatory bodies to develop appropriate frameworks. Initiatives such as the FDA's Tissue Reference Group Rapid Inquiry Program provide mechanisms for early regulatory feedback. Several standards organizations, including ASTM International and ISO, are working to establish consensus standards for bioprinting technologies, which may eventually be recognized by regulatory authorities and streamline approval processes.
For vascularized tissues created through volumetric additive manufacturing, the regulatory pathway typically begins with preclinical testing to establish safety profiles and functional characteristics. This includes biocompatibility assessments, degradation studies, mechanical testing, and in vitro/in vivo performance evaluations. The complexity of these tissues, particularly those with integrated vascular networks, necessitates comprehensive characterization of both the biomaterials and the cellular components.
The FDA's regulatory approach for these advanced therapies follows a risk-based framework, with increasing scrutiny proportional to the perceived risk. Bioprinted tissues intended for implantation face more rigorous requirements than those developed for in vitro testing or research purposes. Developers must demonstrate consistent manufacturing processes with appropriate quality controls, addressing challenges unique to volumetric bioprinting such as resolution verification, structural integrity, and vascular patency.
International regulatory frameworks show notable variations. The European Medicines Agency (EMA) classifies most bioprinted tissues as Advanced Therapy Medicinal Products (ATMPs), requiring centralized authorization procedures. Japan has implemented an accelerated approval pathway for regenerative medicine products, potentially offering faster market access for certain bioprinted tissues. These regional differences create strategic considerations for global development programs.
Regulatory challenges specific to vascularized tissue bioprinting include establishing appropriate reference standards, defining meaningful functional endpoints, and developing validated testing methodologies. The dynamic nature of living tissues complicates traditional stability testing paradigms, requiring innovative approaches to shelf-life determination and storage validation.
Industry stakeholders are actively engaging with regulatory bodies to develop appropriate frameworks. Initiatives such as the FDA's Tissue Reference Group Rapid Inquiry Program provide mechanisms for early regulatory feedback. Several standards organizations, including ASTM International and ISO, are working to establish consensus standards for bioprinting technologies, which may eventually be recognized by regulatory authorities and streamline approval processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!