Future Directions Of Volumetric Additive Manufacturing In Medical Applications
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Volumetric Bioprinting Evolution and Objectives
Volumetric additive manufacturing (VAM) represents a paradigm shift in bioprinting technology, evolving from traditional layer-by-layer approaches to simultaneous whole-volume fabrication. This evolution began in the early 2000s with the development of stereolithography techniques, which laid the groundwork for volumetric printing concepts. By 2015, researchers had demonstrated the first proof-of-concept for volumetric bioprinting using photosensitive hydrogels, marking a significant milestone in the field.
The technological trajectory accelerated dramatically between 2018 and 2021, with breakthrough publications demonstrating computed axial lithography (CAL) and tomographic volumetric bioprinting. These innovations enabled the creation of complex biological structures in seconds rather than hours, fundamentally changing the speed-resolution paradigm that had previously limited bioprinting applications.
Recent advancements have focused on expanding material compatibility and improving cellular viability within volumetrically printed constructs. The integration of multiple wavelength light sources and specialized photoinitiators has enabled selective polymerization of different biomaterials within a single printing process, creating heterogeneous tissue-like structures with unprecedented complexity.
The primary objective of volumetric bioprinting research is to develop platforms capable of fabricating clinically relevant tissue constructs with appropriate biological functionality. This includes achieving sufficient resolution (below 10 μm) to recreate microvascular networks, maintaining high cell viability (>90%) throughout the printing process, and ensuring mechanical properties that match native tissues.
Secondary objectives include reducing production time to enable point-of-care applications, expanding the range of compatible biomaterials to include ECM components like collagen and fibrin, and developing standardized protocols for quality control and regulatory approval pathways.
Long-term goals for volumetric bioprinting encompass the creation of vascularized organ-scale constructs suitable for transplantation, in situ printing capabilities for direct therapeutic applications, and integration with complementary technologies such as microfluidics and biosensing for functional tissue models.
The field is trending toward convergence with computational design approaches, including AI-driven optimization of light delivery patterns and predictive modeling of material responses. This computational-experimental synergy aims to overcome current limitations in structural complexity and functional integration, pushing volumetric bioprinting toward its ultimate goal of fabricating fully functional replacement tissues and organs.
The technological trajectory accelerated dramatically between 2018 and 2021, with breakthrough publications demonstrating computed axial lithography (CAL) and tomographic volumetric bioprinting. These innovations enabled the creation of complex biological structures in seconds rather than hours, fundamentally changing the speed-resolution paradigm that had previously limited bioprinting applications.
Recent advancements have focused on expanding material compatibility and improving cellular viability within volumetrically printed constructs. The integration of multiple wavelength light sources and specialized photoinitiators has enabled selective polymerization of different biomaterials within a single printing process, creating heterogeneous tissue-like structures with unprecedented complexity.
The primary objective of volumetric bioprinting research is to develop platforms capable of fabricating clinically relevant tissue constructs with appropriate biological functionality. This includes achieving sufficient resolution (below 10 μm) to recreate microvascular networks, maintaining high cell viability (>90%) throughout the printing process, and ensuring mechanical properties that match native tissues.
Secondary objectives include reducing production time to enable point-of-care applications, expanding the range of compatible biomaterials to include ECM components like collagen and fibrin, and developing standardized protocols for quality control and regulatory approval pathways.
Long-term goals for volumetric bioprinting encompass the creation of vascularized organ-scale constructs suitable for transplantation, in situ printing capabilities for direct therapeutic applications, and integration with complementary technologies such as microfluidics and biosensing for functional tissue models.
The field is trending toward convergence with computational design approaches, including AI-driven optimization of light delivery patterns and predictive modeling of material responses. This computational-experimental synergy aims to overcome current limitations in structural complexity and functional integration, pushing volumetric bioprinting toward its ultimate goal of fabricating fully functional replacement tissues and organs.
Medical Market Demand Analysis for 3D Bioprinting
The global market for 3D bioprinting is experiencing unprecedented growth, driven by increasing demand for organ transplants and personalized medicine. Current estimates value the 3D bioprinting market at approximately $1.9 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 15.8% through 2030. This remarkable expansion reflects the technology's potential to address critical healthcare challenges, particularly in tissue engineering and regenerative medicine.
Volumetric additive manufacturing, as an advanced form of 3D bioprinting, is positioned to capture significant market share due to its superior speed and resolution capabilities. The medical sector represents the largest application area, accounting for nearly 35% of the total bioprinting market, followed by pharmaceutical testing and research applications.
Organ transplantation remains a critical medical challenge globally, with over 100,000 patients in the United States alone waiting for donor organs. The severe shortage of viable donor organs has created an urgent market need that volumetric bioprinting aims to address. The ability to produce functional tissues and organs could potentially save countless lives and reduce healthcare costs associated with long-term patient care.
Personalized medicine represents another substantial market driver, with patients and healthcare providers increasingly seeking customized treatment options. The global personalized medicine market is expected to reach $3.2 trillion by 2030, with bioprinting technologies playing a crucial role in this transformation. Volumetric manufacturing enables the production of patient-specific implants, prosthetics, and tissues, offering improved outcomes and reduced rejection rates.
Pharmaceutical companies are also emerging as key stakeholders in the bioprinting market. The drug development process currently costs an average of $2.6 billion per approved drug, with a significant portion allocated to clinical trials. Bioprinted tissue models offer a cost-effective alternative for drug testing, potentially reducing development costs by 30% while accelerating time-to-market.
Geographical analysis reveals North America as the dominant market for bioprinting technologies, holding approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade, driven by increasing healthcare expenditure and government initiatives supporting advanced medical technologies.
Regulatory considerations remain a significant factor influencing market adoption. The FDA and other regulatory bodies are actively developing frameworks for bioprinted products, with several milestone approvals expected in the coming years that could substantially accelerate market growth for volumetric manufacturing technologies in medical applications.
Volumetric additive manufacturing, as an advanced form of 3D bioprinting, is positioned to capture significant market share due to its superior speed and resolution capabilities. The medical sector represents the largest application area, accounting for nearly 35% of the total bioprinting market, followed by pharmaceutical testing and research applications.
Organ transplantation remains a critical medical challenge globally, with over 100,000 patients in the United States alone waiting for donor organs. The severe shortage of viable donor organs has created an urgent market need that volumetric bioprinting aims to address. The ability to produce functional tissues and organs could potentially save countless lives and reduce healthcare costs associated with long-term patient care.
Personalized medicine represents another substantial market driver, with patients and healthcare providers increasingly seeking customized treatment options. The global personalized medicine market is expected to reach $3.2 trillion by 2030, with bioprinting technologies playing a crucial role in this transformation. Volumetric manufacturing enables the production of patient-specific implants, prosthetics, and tissues, offering improved outcomes and reduced rejection rates.
Pharmaceutical companies are also emerging as key stakeholders in the bioprinting market. The drug development process currently costs an average of $2.6 billion per approved drug, with a significant portion allocated to clinical trials. Bioprinted tissue models offer a cost-effective alternative for drug testing, potentially reducing development costs by 30% while accelerating time-to-market.
Geographical analysis reveals North America as the dominant market for bioprinting technologies, holding approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next decade, driven by increasing healthcare expenditure and government initiatives supporting advanced medical technologies.
Regulatory considerations remain a significant factor influencing market adoption. The FDA and other regulatory bodies are actively developing frameworks for bioprinted products, with several milestone approvals expected in the coming years that could substantially accelerate market growth for volumetric manufacturing technologies in medical applications.
Current Capabilities and Barriers in Volumetric Additive Manufacturing
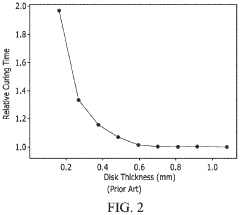
Volumetric Additive Manufacturing (VAM) represents a paradigm shift in 3D printing technology, offering unprecedented speed and resolution capabilities compared to traditional layer-by-layer approaches. Currently, VAM systems can produce complex structures in seconds to minutes rather than hours to days, with resolution capabilities reaching the microscale (1-100 μm) in commercial systems and even nanoscale in research settings.
The primary technical advantage of VAM lies in its ability to simultaneously cure entire volumes of photosensitive resins using computed tomography principles. This approach eliminates the mechanical constraints of layer-by-layer printing, allowing for the creation of structures with overhangs and internal features without support materials. Commercial systems from companies like Readily3D and Xolo have demonstrated the ability to produce centimeter-scale objects with microscale features in minutes.
Despite these advances, significant barriers limit VAM's widespread adoption in medical applications. Material limitations represent the foremost challenge, as only a narrow range of photopolymer resins currently meet both the optical requirements for volumetric printing and biocompatibility standards necessary for medical use. Most commercially available VAM-compatible materials lack the mechanical properties and long-term stability required for implantable devices.
Resolution and scale present another critical barrier. While VAM excels at producing small, intricate structures, scaling to clinically relevant sizes (>5 cm) while maintaining microscale resolution remains challenging due to light scattering and absorption effects in larger resin volumes. This limitation particularly affects applications like patient-specific implants or anatomical models.
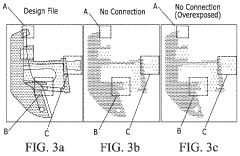
Process control and repeatability issues also hinder medical implementation. Current VAM systems struggle with consistent material properties throughout printed structures, resulting in anisotropic mechanical behavior that complicates regulatory approval processes. The complex relationship between light exposure patterns, resin chemistry, and final material properties requires sophisticated modeling and control systems not yet fully developed.
Regulatory hurdles present perhaps the most formidable barrier to clinical adoption. The novel nature of VAM processes means limited precedent exists for FDA or equivalent regulatory approval pathways. Questions regarding long-term stability, degradation profiles, and potential leaching of uncured resin components remain inadequately addressed in the current literature.
Cost considerations further limit accessibility, with high-precision VAM systems requiring significant capital investment ($100,000-500,000) and specialized expertise to operate effectively. This economic barrier restricts research and development primarily to well-funded academic institutions and large medical device manufacturers, limiting innovation from smaller entities.
The primary technical advantage of VAM lies in its ability to simultaneously cure entire volumes of photosensitive resins using computed tomography principles. This approach eliminates the mechanical constraints of layer-by-layer printing, allowing for the creation of structures with overhangs and internal features without support materials. Commercial systems from companies like Readily3D and Xolo have demonstrated the ability to produce centimeter-scale objects with microscale features in minutes.
Despite these advances, significant barriers limit VAM's widespread adoption in medical applications. Material limitations represent the foremost challenge, as only a narrow range of photopolymer resins currently meet both the optical requirements for volumetric printing and biocompatibility standards necessary for medical use. Most commercially available VAM-compatible materials lack the mechanical properties and long-term stability required for implantable devices.
Resolution and scale present another critical barrier. While VAM excels at producing small, intricate structures, scaling to clinically relevant sizes (>5 cm) while maintaining microscale resolution remains challenging due to light scattering and absorption effects in larger resin volumes. This limitation particularly affects applications like patient-specific implants or anatomical models.
Process control and repeatability issues also hinder medical implementation. Current VAM systems struggle with consistent material properties throughout printed structures, resulting in anisotropic mechanical behavior that complicates regulatory approval processes. The complex relationship between light exposure patterns, resin chemistry, and final material properties requires sophisticated modeling and control systems not yet fully developed.
Regulatory hurdles present perhaps the most formidable barrier to clinical adoption. The novel nature of VAM processes means limited precedent exists for FDA or equivalent regulatory approval pathways. Questions regarding long-term stability, degradation profiles, and potential leaching of uncured resin components remain inadequately addressed in the current literature.
Cost considerations further limit accessibility, with high-precision VAM systems requiring significant capital investment ($100,000-500,000) and specialized expertise to operate effectively. This economic barrier restricts research and development primarily to well-funded academic institutions and large medical device manufacturers, limiting innovation from smaller entities.
Contemporary Volumetric Bioprinting Methodologies
01 Volumetric additive manufacturing techniques and processes
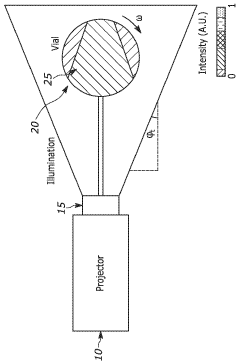
Volumetric additive manufacturing involves creating three-dimensional objects by solidifying material throughout a volume simultaneously, rather than layer by layer. This approach enables faster production times and can create complex geometries without the need for support structures. Various techniques such as computed axial lithography, holographic stereolithography, and tomographic volumetric printing are used to achieve volumetric fabrication by projecting patterned light into photosensitive resins.- Volumetric additive manufacturing techniques: Volumetric additive manufacturing involves creating 3D objects by solidifying material throughout a volume simultaneously, rather than layer by layer. This approach uses techniques such as computed axial lithography, tomographic volumetric printing, or holographic lithography to project patterns of light into photosensitive resins. These techniques enable faster production times compared to traditional layer-by-layer methods and can create complex geometries without the need for support structures.
- Materials for volumetric additive manufacturing: Various materials are used in volumetric additive manufacturing, including photopolymer resins, hydrogels, and composite materials. These materials are formulated with specific photoinitiators and absorbers to control light penetration and curing characteristics. Advanced formulations enable the creation of objects with gradient properties, functional components, or biocompatible structures. The development of new materials continues to expand the applications of volumetric manufacturing in fields such as medicine, electronics, and engineering.
- Equipment and systems for volumetric printing: Specialized equipment for volumetric additive manufacturing includes projection systems, rotating resin containers, and synchronized light sources. These systems often incorporate digital light processing (DLP) technology, spatial light modulators, or multiple synchronized projectors to deliver patterned light into the printing volume. Advanced systems may include real-time monitoring capabilities, feedback control mechanisms, and integrated post-processing features to optimize print quality and efficiency.
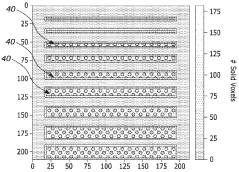
- Software and computational methods: Volumetric additive manufacturing relies on sophisticated software algorithms and computational methods to generate and optimize projection patterns. These include tomographic reconstruction techniques, computational optimization of light dose distribution, and simulation of photopolymerization kinetics. Advanced software solutions enable the prediction of material behavior during curing, compensation for optical distortions, and optimization of energy delivery to achieve desired material properties and geometric accuracy.
- Applications and industry implementations: Volumetric additive manufacturing is being applied across various industries including medical devices, microfluidics, optics, and consumer products. The technology enables the rapid production of complex parts with features that would be difficult to achieve using conventional manufacturing methods. Specific applications include the creation of personalized medical implants, microstructured optical components, functional prototypes, and parts with embedded functionality. The technology's ability to produce parts quickly and with minimal post-processing makes it particularly valuable for time-sensitive applications.
02 Materials for volumetric additive manufacturing
Specialized materials are essential for successful volumetric additive manufacturing. These include photosensitive resins with specific curing properties, dual-initiator systems that enable selective volumetric curing, and materials with tuned optical properties to control light penetration depth. Advanced formulations may incorporate nanoparticles, functional additives, or biomaterials to enhance mechanical properties or add functionality to the printed structures.Expand Specific Solutions03 Light projection and control systems
Sophisticated light projection and control systems are critical components of volumetric additive manufacturing. These systems utilize digital light processing (DLP), spatial light modulators, or multiple synchronized light sources to project patterned light into the printing volume. Advanced optical setups may include beam shaping elements, rotating stages, or holographic projectors to achieve precise control over the light distribution within the resin volume, enabling complex internal structures and high-resolution features.Expand Specific Solutions04 Computational methods for volumetric printing
Computational algorithms play a crucial role in volumetric additive manufacturing by calculating optimal light patterns for creating desired 3D structures. These methods include tomographic reconstruction techniques, computational optimization of light dose distribution, and simulation of photopolymerization kinetics. Advanced software solutions integrate CAD model processing with physics-based simulations to predict and compensate for optical effects like scattering and absorption, ensuring accurate reproduction of intended geometries.Expand Specific Solutions05 Applications and industry-specific implementations
Volumetric additive manufacturing is finding applications across various industries due to its unique capabilities. In the medical field, it enables the rapid production of patient-specific implants, tissue engineering scaffolds, and anatomical models. For industrial applications, it offers advantages in producing complex components with internal features, embedded functionality, or gradient properties. Other implementations include microfluidic devices, optical components, and customized consumer products that benefit from the speed and geometric freedom of volumetric fabrication.Expand Specific Solutions
Leading Organizations in Medical Volumetric Manufacturing
Volumetric Additive Manufacturing (VAM) in medical applications is currently transitioning from early development to growth phase, with an estimated market value of $2-3 billion and projected annual growth of 15-20%. The technology maturity varies across applications, with dental and orthopedic solutions being more advanced. Leading players include established companies like Stratasys and Align Technology focusing on commercial applications, while research institutions such as Lawrence Livermore National Security, Cornell University, and EPFL are driving fundamental innovations. Specialized firms like Readily3D, Regenhu, and 3Dmorphic are developing niche medical solutions. The competitive landscape is characterized by strategic partnerships between academic institutions and industry players to accelerate clinical translation and regulatory approval of patient-specific medical devices and bioprinted tissues.
Lawrence Livermore National Security LLC
Technical Solution: Lawrence Livermore National Laboratory has pioneered volumetric additive manufacturing (VAM) technology for medical applications through their development of the Computed Axial Lithography (CAL) process. This innovative approach creates complex 3D structures by projecting synchronized 2D light patterns into photosensitive resin, enabling the simultaneous solidification of an entire volume rather than layer-by-layer printing. Their technology achieves printing speeds 100-1000 times faster than conventional methods while maintaining high resolution (below 100 microns) [1]. LLNL has specifically focused on biomedical applications including patient-specific implants, tissue engineering scaffolds, and drug delivery systems. Their proprietary algorithms optimize light delivery patterns to create complex internal architectures with controlled porosity and mechanical properties suitable for bone and tissue replacement. Recent advancements include the incorporation of biocompatible and biodegradable materials that can be processed using their VAM technology while maintaining cell viability for tissue engineering applications [2].
Strengths: Superior speed (100-1000x faster than conventional methods); ability to create complex internal structures impossible with traditional layer-by-layer approaches; excellent resolution capabilities; development of biocompatible material formulations. Weaknesses: Limited material selection compared to established technologies; challenges in scaling to very large structures; higher initial equipment costs; regulatory approval pathway still being established for novel medical applications.
Align Technology, Inc.
Technical Solution: Align Technology has revolutionized dental applications of volumetric additive manufacturing through their proprietary digital workflow and manufacturing systems. While primarily known for Invisalign clear aligners, their technology platform incorporates advanced volumetric printing techniques to create patient-specific dental devices with exceptional accuracy. Their manufacturing process utilizes photopolymerization-based volumetric printing that enables the creation of dental models and orthodontic devices with accuracy to 50 microns. The company has developed specialized resins optimized for dental applications that meet biocompatibility requirements while providing the mechanical properties necessary for functional dental devices [9]. Align's technology integrates seamlessly with their digital workflow, which begins with intraoral scanning and proceeds through AI-powered treatment planning to final manufacturing. Their volumetric printing approach allows for the simultaneous production of multiple patient-specific devices, achieving throughput levels that have transformed the economics of personalized orthodontics. Recent advancements include the development of multi-material capabilities that can create devices with regionally varying properties to better simulate the biomechanical behavior of natural dentition. Their manufacturing facilities produce over 700,000 unique devices daily, demonstrating the scalability of their volumetric manufacturing approach [10].
Strengths: Unparalleled scale and efficiency in personalized medical device manufacturing; fully integrated digital workflow from patient data to final product; extensive clinical validation data; established regulatory pathways; global manufacturing and distribution network. Weaknesses: Technology primarily optimized for dental applications with limited crossover to other medical fields; proprietary closed system limiting third-party material use; higher per-unit costs for small production runs; significant capital investment requirements for implementation.
Critical Patents in Medical Volumetric Manufacturing
System and method for subzero molding, imprinting, and casting
PatentWO2023086606A1
Innovation
- The system employs Layerless Subzero Molding of Photopolymers using UV curable resins that solidify under concentrated sunlight or artificial UV light, enabling high-throughput, layer-less volumetric manufacturing through subzero imprinting and molding with a subzero pressure system, allowing for the creation of complex 3D shapes with ultra-thin cell walls and diverse materials like glass, silicon, and ceramics.
Method of volumetric additive manufacturing
PatentWO2024069272A1
Innovation
- The method involves rotating a vial of photocurable resin and projecting structured light images to cure only the shell and interior scaffolding of the object, using infilling and deconvolution to correct for diffusion effects, ensuring uniform curing of all features without over-exposure.
Regulatory Framework for Medical Additive Manufacturing
The regulatory landscape for volumetric additive manufacturing in medical applications represents a complex and evolving framework that manufacturers, healthcare providers, and researchers must navigate carefully. Currently, the FDA's Center for Devices and Radiological Health (CDRH) oversees the regulation of 3D-printed medical devices through various pathways including 510(k) clearance, De Novo classification, and Premarket Approval (PMA). The FDA's 2017 guidance document "Technical Considerations for Additive Manufactured Medical Devices" provides a foundation, but lacks specific provisions for emerging volumetric manufacturing technologies.
International regulatory bodies have developed varying approaches to additive manufacturing oversight. The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose stringent requirements for clinical evidence, post-market surveillance, and unique device identification. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specialized pathways for innovative manufacturing technologies, while China's National Medical Products Administration (NMPA) continues to develop its regulatory framework for advanced manufacturing technologies.
Harmonization efforts between regulatory agencies remain incomplete, creating challenges for global market access. The International Medical Device Regulators Forum (IMDRF) has established working groups focused on personalized medical devices and manufacturing technologies, but specific guidance for volumetric manufacturing techniques is still developing.
Key regulatory challenges specific to volumetric additive manufacturing include validation of complex internal structures, establishment of appropriate quality control metrics, and development of standardized testing protocols. The dynamic nature of volumetric printing processes, which create entire structures simultaneously rather than layer-by-layer, presents unique verification challenges that existing regulatory frameworks may not adequately address.
Looking forward, regulatory evolution will likely focus on performance-based standards rather than prescriptive manufacturing requirements. The FDA's Digital Health Software Precertification Program may serve as a model for regulating the software components of volumetric manufacturing systems. Additionally, regulatory sandboxes and innovation pathways are emerging to facilitate controlled market entry for novel manufacturing technologies while gathering real-world evidence of safety and effectiveness.
Successful navigation of this regulatory landscape requires early and frequent engagement with regulatory bodies, robust design control documentation, comprehensive validation protocols, and post-market surveillance systems capable of detecting unique failure modes associated with volumetric manufacturing processes. Companies pursuing volumetric additive manufacturing for medical applications should develop regulatory strategies that anticipate evolving requirements and demonstrate commitment to patient safety through rigorous quality systems.
International regulatory bodies have developed varying approaches to additive manufacturing oversight. The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose stringent requirements for clinical evidence, post-market surveillance, and unique device identification. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specialized pathways for innovative manufacturing technologies, while China's National Medical Products Administration (NMPA) continues to develop its regulatory framework for advanced manufacturing technologies.
Harmonization efforts between regulatory agencies remain incomplete, creating challenges for global market access. The International Medical Device Regulators Forum (IMDRF) has established working groups focused on personalized medical devices and manufacturing technologies, but specific guidance for volumetric manufacturing techniques is still developing.
Key regulatory challenges specific to volumetric additive manufacturing include validation of complex internal structures, establishment of appropriate quality control metrics, and development of standardized testing protocols. The dynamic nature of volumetric printing processes, which create entire structures simultaneously rather than layer-by-layer, presents unique verification challenges that existing regulatory frameworks may not adequately address.
Looking forward, regulatory evolution will likely focus on performance-based standards rather than prescriptive manufacturing requirements. The FDA's Digital Health Software Precertification Program may serve as a model for regulating the software components of volumetric manufacturing systems. Additionally, regulatory sandboxes and innovation pathways are emerging to facilitate controlled market entry for novel manufacturing technologies while gathering real-world evidence of safety and effectiveness.
Successful navigation of this regulatory landscape requires early and frequent engagement with regulatory bodies, robust design control documentation, comprehensive validation protocols, and post-market surveillance systems capable of detecting unique failure modes associated with volumetric manufacturing processes. Companies pursuing volumetric additive manufacturing for medical applications should develop regulatory strategies that anticipate evolving requirements and demonstrate commitment to patient safety through rigorous quality systems.
Biocompatibility and Material Science Advancements
The advancement of biocompatible materials represents a critical frontier for volumetric additive manufacturing (VAM) in medical applications. Current biocompatible resins used in VAM technologies often face limitations in terms of mechanical properties, degradation rates, and cellular interactions. Recent research has focused on developing novel photopolymerizable hydrogels that better mimic native tissue environments while maintaining printability in volumetric systems.
Significant progress has been made in creating multi-functional biomaterials that combine mechanical stability with bioactive properties. These materials incorporate growth factors, cell-adhesion motifs, and enzymatically degradable crosslinks to facilitate tissue integration and regeneration. The development of gradient materials with spatially controlled properties offers promising solutions for interfaces between different tissue types, such as cartilage-bone transitions.
Material science innovations are increasingly focused on stimuli-responsive biomaterials that can change their properties in response to specific biological cues. These "smart" materials enable the creation of dynamic scaffolds that evolve with the healing process, providing appropriate support throughout tissue regeneration phases. Additionally, composite materials combining synthetic polymers with natural components like decellularized extracellular matrix show enhanced biocompatibility while maintaining the precision required for VAM processes.
The integration of antimicrobial properties into VAM-compatible materials represents another significant advancement. Incorporating silver nanoparticles, antimicrobial peptides, or photoactivated disinfection agents directly into the printing material helps prevent implant-associated infections without compromising biocompatibility or mechanical performance.
Biodegradation kinetics remains a crucial area of research, with efforts directed toward developing materials with predictable and controllable degradation rates that match tissue regeneration timelines. This includes the development of enzymatically degradable crosslinks and hydrolytically sensitive bonds that can be fine-tuned based on specific application requirements.
Regulatory considerations are increasingly shaping material development pathways. Materials scientists are working closely with regulatory bodies to establish standardized testing protocols for novel VAM biomaterials, accelerating their translation to clinical applications. This collaborative approach has led to the development of materials with well-characterized safety profiles and predictable in vivo performance.
Future directions point toward personalized biomaterials that can be tailored to individual patient needs through adjustments in composition and properties at the point of care. The convergence of material science with computational modeling is enabling the prediction of material behavior in physiological environments, further refining material selection and design for specific medical applications.
Significant progress has been made in creating multi-functional biomaterials that combine mechanical stability with bioactive properties. These materials incorporate growth factors, cell-adhesion motifs, and enzymatically degradable crosslinks to facilitate tissue integration and regeneration. The development of gradient materials with spatially controlled properties offers promising solutions for interfaces between different tissue types, such as cartilage-bone transitions.
Material science innovations are increasingly focused on stimuli-responsive biomaterials that can change their properties in response to specific biological cues. These "smart" materials enable the creation of dynamic scaffolds that evolve with the healing process, providing appropriate support throughout tissue regeneration phases. Additionally, composite materials combining synthetic polymers with natural components like decellularized extracellular matrix show enhanced biocompatibility while maintaining the precision required for VAM processes.
The integration of antimicrobial properties into VAM-compatible materials represents another significant advancement. Incorporating silver nanoparticles, antimicrobial peptides, or photoactivated disinfection agents directly into the printing material helps prevent implant-associated infections without compromising biocompatibility or mechanical performance.
Biodegradation kinetics remains a crucial area of research, with efforts directed toward developing materials with predictable and controllable degradation rates that match tissue regeneration timelines. This includes the development of enzymatically degradable crosslinks and hydrolytically sensitive bonds that can be fine-tuned based on specific application requirements.
Regulatory considerations are increasingly shaping material development pathways. Materials scientists are working closely with regulatory bodies to establish standardized testing protocols for novel VAM biomaterials, accelerating their translation to clinical applications. This collaborative approach has led to the development of materials with well-characterized safety profiles and predictable in vivo performance.
Future directions point toward personalized biomaterials that can be tailored to individual patient needs through adjustments in composition and properties at the point of care. The convergence of material science with computational modeling is enabling the prediction of material behavior in physiological environments, further refining material selection and design for specific medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!