CO₂ Capture Sorbent Efficacy in Reducing Industrial Waste
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO₂ Capture Technology Background and Objectives
Carbon dioxide capture technology has evolved significantly over the past several decades, transitioning from theoretical concepts to practical applications in industrial settings. The fundamental principle behind CO₂ capture involves the separation of carbon dioxide from flue gases or ambient air through various physical, chemical, or biological processes. Initially developed in the 1970s for enhanced oil recovery applications, CO₂ capture technologies have since expanded to address growing environmental concerns related to greenhouse gas emissions and climate change.
The evolution of these technologies has followed three distinct generations. First-generation technologies primarily utilized amine-based chemical absorption processes, which while effective, suffered from high energy requirements and solvent degradation issues. Second-generation approaches introduced advanced materials such as metal-organic frameworks (MOFs), zeolites, and specialized polymeric membranes, offering improved selectivity and reduced energy penalties. Current third-generation technologies focus on direct air capture and integrated systems that combine capture with utilization or storage pathways.
Recent technological advancements have significantly improved sorbent materials, which are central to efficient CO₂ capture. These materials have evolved from simple activated carbons to sophisticated engineered structures with tailored surface chemistries and optimized pore architectures. The development trajectory indicates a clear trend toward materials that exhibit higher CO₂ selectivity, faster adsorption kinetics, and greater stability under industrial operating conditions.
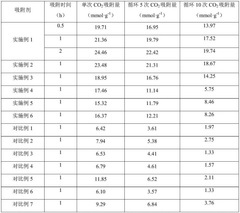
The primary objective of modern CO₂ capture sorbent technology is to achieve substantial reductions in industrial carbon emissions while maintaining economic viability. Specific technical goals include developing sorbents with CO₂ capture capacities exceeding 3 mmol/g, selectivity factors above 100 for CO₂ over N₂, and regeneration energy requirements below 2 GJ/tonne CO₂. Additionally, these materials must demonstrate mechanical and chemical stability over thousands of adsorption-desorption cycles in the presence of contaminants commonly found in industrial waste streams.
Looking forward, the field is moving toward multifunctional sorbents that not only capture CO₂ but also facilitate its conversion into valuable products, effectively transforming industrial waste into resources. This approach aligns with circular economy principles and offers potential economic incentives for widespread adoption. The integration of artificial intelligence and machine learning techniques is accelerating materials discovery and optimization, potentially reducing development timelines from decades to years.
The ultimate technological objective remains the development of cost-effective, scalable, and sustainable CO₂ capture solutions that can be deployed across diverse industrial sectors, from power generation to cement production, thereby significantly reducing global carbon emissions while supporting continued industrial development and economic growth.
The evolution of these technologies has followed three distinct generations. First-generation technologies primarily utilized amine-based chemical absorption processes, which while effective, suffered from high energy requirements and solvent degradation issues. Second-generation approaches introduced advanced materials such as metal-organic frameworks (MOFs), zeolites, and specialized polymeric membranes, offering improved selectivity and reduced energy penalties. Current third-generation technologies focus on direct air capture and integrated systems that combine capture with utilization or storage pathways.
Recent technological advancements have significantly improved sorbent materials, which are central to efficient CO₂ capture. These materials have evolved from simple activated carbons to sophisticated engineered structures with tailored surface chemistries and optimized pore architectures. The development trajectory indicates a clear trend toward materials that exhibit higher CO₂ selectivity, faster adsorption kinetics, and greater stability under industrial operating conditions.
The primary objective of modern CO₂ capture sorbent technology is to achieve substantial reductions in industrial carbon emissions while maintaining economic viability. Specific technical goals include developing sorbents with CO₂ capture capacities exceeding 3 mmol/g, selectivity factors above 100 for CO₂ over N₂, and regeneration energy requirements below 2 GJ/tonne CO₂. Additionally, these materials must demonstrate mechanical and chemical stability over thousands of adsorption-desorption cycles in the presence of contaminants commonly found in industrial waste streams.
Looking forward, the field is moving toward multifunctional sorbents that not only capture CO₂ but also facilitate its conversion into valuable products, effectively transforming industrial waste into resources. This approach aligns with circular economy principles and offers potential economic incentives for widespread adoption. The integration of artificial intelligence and machine learning techniques is accelerating materials discovery and optimization, potentially reducing development timelines from decades to years.
The ultimate technological objective remains the development of cost-effective, scalable, and sustainable CO₂ capture solutions that can be deployed across diverse industrial sectors, from power generation to cement production, thereby significantly reducing global carbon emissions while supporting continued industrial development and economic growth.
Market Analysis for Industrial Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the industrial carbon capture solutions market is valued at approximately $2.1 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030. This rapid expansion reflects the urgent need for effective CO₂ reduction technologies across various industrial sectors, particularly in power generation, cement production, and chemical manufacturing.
Regional analysis reveals that North America currently dominates the market with a 38% share, followed by Europe at 31% and Asia-Pacific at 24%. The remaining 7% is distributed across other regions. This distribution correlates strongly with regulatory frameworks and carbon pricing mechanisms in these regions. The European Union's Emissions Trading System and the United States' 45Q tax credits have been particularly influential in driving market adoption.
Customer segmentation within the industrial carbon capture market shows distinct patterns. Large-scale emitters (>500,000 tons CO₂/year) constitute approximately 65% of the current market, while medium-sized facilities (100,000-500,000 tons CO₂/year) represent 28%. The remaining 7% comes from smaller industrial operations seeking to reduce their carbon footprint. This segmentation highlights the concentration of market potential among major industrial players.
Demand drivers for CO₂ capture sorbent technologies include increasingly stringent emissions regulations, rising carbon prices (averaging $40-85 per ton in developed markets), and growing pressure from investors and consumers for decarbonization efforts. Additionally, the potential for revenue generation through carbon utilization pathways is emerging as a significant market incentive.
Price sensitivity analysis indicates that industrial adopters typically require a return on investment period of 3-5 years, with cost per ton of CO₂ captured being the critical metric. Current market solutions range from $40-120 per ton, with sorbent-based technologies generally positioned in the $50-80 range. This pricing structure creates a competitive landscape where efficacy, durability, and operational costs of sorbents become key differentiators.
Market barriers include high initial capital expenditure requirements, technological uncertainties regarding long-term sorbent performance, and infrastructure limitations for CO₂ transport and storage. Additionally, policy uncertainty in some regions creates hesitation among potential adopters, despite the overall positive regulatory trend toward carbon reduction incentives.
The competitive landscape features established industrial gas companies, specialized carbon capture technology providers, and emerging startups focused on novel sorbent materials. Recent market consolidation through strategic acquisitions indicates growing interest from major industrial players in securing advanced carbon capture capabilities.
Regional analysis reveals that North America currently dominates the market with a 38% share, followed by Europe at 31% and Asia-Pacific at 24%. The remaining 7% is distributed across other regions. This distribution correlates strongly with regulatory frameworks and carbon pricing mechanisms in these regions. The European Union's Emissions Trading System and the United States' 45Q tax credits have been particularly influential in driving market adoption.
Customer segmentation within the industrial carbon capture market shows distinct patterns. Large-scale emitters (>500,000 tons CO₂/year) constitute approximately 65% of the current market, while medium-sized facilities (100,000-500,000 tons CO₂/year) represent 28%. The remaining 7% comes from smaller industrial operations seeking to reduce their carbon footprint. This segmentation highlights the concentration of market potential among major industrial players.
Demand drivers for CO₂ capture sorbent technologies include increasingly stringent emissions regulations, rising carbon prices (averaging $40-85 per ton in developed markets), and growing pressure from investors and consumers for decarbonization efforts. Additionally, the potential for revenue generation through carbon utilization pathways is emerging as a significant market incentive.
Price sensitivity analysis indicates that industrial adopters typically require a return on investment period of 3-5 years, with cost per ton of CO₂ captured being the critical metric. Current market solutions range from $40-120 per ton, with sorbent-based technologies generally positioned in the $50-80 range. This pricing structure creates a competitive landscape where efficacy, durability, and operational costs of sorbents become key differentiators.
Market barriers include high initial capital expenditure requirements, technological uncertainties regarding long-term sorbent performance, and infrastructure limitations for CO₂ transport and storage. Additionally, policy uncertainty in some regions creates hesitation among potential adopters, despite the overall positive regulatory trend toward carbon reduction incentives.
The competitive landscape features established industrial gas companies, specialized carbon capture technology providers, and emerging startups focused on novel sorbent materials. Recent market consolidation through strategic acquisitions indicates growing interest from major industrial players in securing advanced carbon capture capabilities.
Current Sorbent Technologies and Implementation Challenges
Current carbon dioxide capture technologies primarily rely on various sorbent materials that can selectively adsorb CO₂ from industrial emissions. Amine-based sorbents remain the most widely deployed solution, with monoethanolamine (MEA) being the industry standard for decades. These liquid absorbents typically achieve 85-95% capture efficiency under optimal conditions but suffer from high regeneration energy requirements (3.5-4.2 GJ/tonne CO₂) and degradation issues when exposed to oxygen and impurities in flue gas.
Solid sorbents have emerged as promising alternatives, with metal-organic frameworks (MOFs) demonstrating exceptional CO₂ selectivity and capacity. Notable examples include Mg-MOF-74 and HKUST-1, which exhibit CO₂ uptake capacities of 5-8 mmol/g at ambient conditions. However, their implementation faces challenges related to moisture sensitivity, mechanical stability during pressure-swing operations, and high synthesis costs that currently limit industrial-scale deployment.
Calcium looping technology utilizing limestone (CaCO₃) as a sorbent offers a cost-effective approach with abundant raw material availability. This process operates at high temperatures (600-700°C) and can achieve 90% capture efficiency. The primary implementation challenge stems from sorbent deactivation after multiple carbonation-calcination cycles, requiring continuous makeup of fresh material that increases operational costs.
Zeolites and activated carbons represent another category of commercially available sorbents with moderate CO₂ capacities (2-4 mmol/g). Their advantages include excellent thermal stability and lower regeneration energy requirements compared to amine systems. However, their performance deteriorates significantly in humid conditions, necessitating additional flue gas dehydration steps that increase system complexity and energy consumption.
Novel hybrid materials combining the advantages of different sorbent types have shown promising results in laboratory settings. Amine-functionalized silica and polymer-supported amine sorbents demonstrate improved stability while maintaining high CO₂ selectivity. Nevertheless, scaling these materials to industrial quantities presents significant engineering challenges related to manufacturing consistency and long-term performance verification.
Implementation challenges extend beyond material properties to system integration concerns. Retrofitting existing industrial facilities with carbon capture systems requires substantial capital investment and physical space that may not be readily available. The energy penalty associated with sorbent regeneration typically reduces plant efficiency by 20-30%, creating economic barriers to widespread adoption without supportive carbon pricing mechanisms or regulatory frameworks.
Waste management issues also present significant challenges, particularly for amine-based systems that produce hazardous degradation products requiring specialized disposal procedures. Additionally, the environmental footprint of sorbent manufacturing and replacement must be considered in lifecycle assessments to ensure net carbon reduction benefits from implementation.
Solid sorbents have emerged as promising alternatives, with metal-organic frameworks (MOFs) demonstrating exceptional CO₂ selectivity and capacity. Notable examples include Mg-MOF-74 and HKUST-1, which exhibit CO₂ uptake capacities of 5-8 mmol/g at ambient conditions. However, their implementation faces challenges related to moisture sensitivity, mechanical stability during pressure-swing operations, and high synthesis costs that currently limit industrial-scale deployment.
Calcium looping technology utilizing limestone (CaCO₃) as a sorbent offers a cost-effective approach with abundant raw material availability. This process operates at high temperatures (600-700°C) and can achieve 90% capture efficiency. The primary implementation challenge stems from sorbent deactivation after multiple carbonation-calcination cycles, requiring continuous makeup of fresh material that increases operational costs.
Zeolites and activated carbons represent another category of commercially available sorbents with moderate CO₂ capacities (2-4 mmol/g). Their advantages include excellent thermal stability and lower regeneration energy requirements compared to amine systems. However, their performance deteriorates significantly in humid conditions, necessitating additional flue gas dehydration steps that increase system complexity and energy consumption.
Novel hybrid materials combining the advantages of different sorbent types have shown promising results in laboratory settings. Amine-functionalized silica and polymer-supported amine sorbents demonstrate improved stability while maintaining high CO₂ selectivity. Nevertheless, scaling these materials to industrial quantities presents significant engineering challenges related to manufacturing consistency and long-term performance verification.
Implementation challenges extend beyond material properties to system integration concerns. Retrofitting existing industrial facilities with carbon capture systems requires substantial capital investment and physical space that may not be readily available. The energy penalty associated with sorbent regeneration typically reduces plant efficiency by 20-30%, creating economic barriers to widespread adoption without supportive carbon pricing mechanisms or regulatory frameworks.
Waste management issues also present significant challenges, particularly for amine-based systems that produce hazardous degradation products requiring specialized disposal procedures. Additionally, the environmental footprint of sorbent manufacturing and replacement must be considered in lifecycle assessments to ensure net carbon reduction benefits from implementation.
Existing Sorbent Solutions for Industrial CO₂ Reduction
01 Metal-organic frameworks (MOFs) for CO₂ capture
Metal-organic frameworks are porous materials with high surface area that can effectively capture CO₂. These materials can be tailored with specific metal centers and organic linkers to enhance CO₂ adsorption capacity and selectivity. MOFs offer advantages such as tunable pore sizes, high thermal stability, and regeneration capabilities, making them promising sorbents for carbon capture applications.- Metal-organic frameworks for CO₂ capture: Metal-organic frameworks (MOFs) are highly porous materials with large surface areas that can effectively capture CO₂. These materials can be designed with specific metal centers and organic linkers to enhance CO₂ adsorption capacity and selectivity. MOFs can be modified with functional groups to increase their affinity for CO₂, making them efficient sorbents for carbon capture applications.
- Amine-functionalized sorbents: Amine-functionalized materials are effective CO₂ sorbents due to the strong chemical interaction between amine groups and CO₂ molecules. These sorbents can be prepared by incorporating amine compounds onto various supports such as silica, polymers, or porous carbon. The amine groups react with CO₂ to form carbamates or bicarbonates, enabling high CO₂ capture capacity even at low concentrations. These materials often demonstrate good regenerability and stability over multiple adsorption-desorption cycles.
- Zeolite-based CO₂ capture materials: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that can selectively adsorb CO₂. Their high thermal stability and tunable pore size make them suitable for various carbon capture applications. Zeolites can be modified by ion exchange or impregnation with alkali metals to enhance their CO₂ adsorption capacity. These materials are particularly effective for CO₂ capture from flue gas streams and can be regenerated through temperature or pressure swing processes.
- Carbon-based sorbents for CO₂ capture: Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, are effective CO₂ sorbents due to their high surface area and porous structure. These materials can be functionalized with nitrogen-containing groups or metal particles to enhance their CO₂ capture performance. Carbon-based sorbents offer advantages such as low cost, high stability, and ease of regeneration. They can be produced from various precursors, including biomass, making them environmentally friendly options for carbon capture applications.
- Novel composite materials for enhanced CO₂ adsorption: Composite materials combining different types of sorbents can achieve superior CO₂ capture performance by synergistically leveraging the advantages of each component. These materials may integrate organic and inorganic components, such as polymer-inorganic hybrids or mixed matrix membranes. The composite structure can provide improved mechanical stability, enhanced adsorption capacity, and better selectivity for CO₂ over other gases. These advanced materials often demonstrate improved heat management during adsorption-desorption cycles and can be tailored for specific operating conditions.
02 Amine-functionalized sorbents
Amine-functionalized materials are effective CO₂ sorbents that operate through chemical adsorption mechanisms. These materials include amine-grafted silicas, polymers, and porous supports that form carbamates when reacting with CO₂. The incorporation of amine groups significantly enhances CO₂ capture capacity and selectivity, particularly at lower temperatures and in humid conditions, while allowing for relatively easy regeneration through temperature or pressure swing processes.Expand Specific Solutions03 Zeolite and activated carbon-based sorbents
Zeolites and activated carbons are traditional CO₂ capture materials with well-established efficacy. These materials rely on physical adsorption mechanisms and offer benefits such as high thermal stability, low cost, and resistance to contaminants. Modified zeolites with tailored pore structures and surface chemistry can achieve enhanced CO₂ selectivity, while activated carbons can be produced from various precursors including biomass to create sustainable carbon capture solutions.Expand Specific Solutions04 Novel composite and hybrid sorbent materials
Composite and hybrid materials combine the advantages of multiple sorbent types to achieve superior CO₂ capture performance. These include MOF-polymer composites, amine-silica hybrids, and layered double hydroxide materials. By integrating different capture mechanisms and material properties, these composites can overcome limitations of individual sorbent types, offering improved capacity, selectivity, stability, and regeneration characteristics under various operating conditions.Expand Specific Solutions05 Sorbent regeneration and cyclic performance enhancement
Improving sorbent regeneration efficiency and maintaining performance over multiple adsorption-desorption cycles is critical for practical CO₂ capture applications. Innovations in this area include optimized temperature and pressure swing processes, novel desorption techniques using microwave or electrical heating, and structural modifications to prevent sorbent degradation. These approaches reduce energy requirements for regeneration while extending sorbent lifetime, thereby improving the overall economics and sustainability of carbon capture systems.Expand Specific Solutions
Leading Companies and Research Institutions in Carbon Capture
The CO₂ capture sorbent market is in a growth phase, driven by increasing industrial decarbonization demands, with the global carbon capture market expected to reach $7-10 billion by 2030. Technology maturity varies significantly across players, with established energy corporations like Korea Electric Power Corp. and its subsidiaries leading commercial deployment in Asia, while Climeworks AG and Mantel Capture represent innovative startups advancing direct air capture technologies. Research institutions including California Institute of Technology and University of Queensland are developing next-generation sorbent materials, while chemical industry giants such as UOP LLC, Rhodia Operations, and PetroChina are leveraging their expertise to scale industrial applications. The competitive landscape shows regional clusters forming in East Asia, North America, and Europe, with cross-sector collaborations emerging between energy producers and technology developers.
UOP LLC
Technical Solution: UOP LLC (a Honeywell company) has developed advanced molecular sieve adsorbents and proprietary pressure swing adsorption (PSA) technology for industrial CO₂ capture. Their Polybed™ PSA system utilizes specialized zeolite-based sorbents with high selectivity for CO₂ over other gases. The process operates through pressure cycling, where CO₂ adsorbs onto the sorbent material at high pressure and desorbs when pressure is reduced, enabling continuous capture from industrial flue gases. UOP's technology achieves capture rates of over 90% with high purity (>99%) CO₂ output suitable for utilization or sequestration. Their modular design allows for retrofitting existing industrial facilities, particularly in natural gas processing, hydrogen production, and petrochemical manufacturing. UOP has implemented this technology in over 1,000 installations worldwide, demonstrating its commercial viability and scalability for industrial applications.
Strengths: High CO₂ selectivity and capture efficiency; modular design enables retrofitting existing facilities; proven commercial technology with extensive deployment history; produces high-purity CO₂ suitable for various applications. Weaknesses: Energy requirements for pressure cycling can be significant; primarily focused on high-concentration CO₂ streams rather than dilute sources; requires regular sorbent replacement; economic viability depends on scale and local energy costs.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has pioneered advanced metal-organic framework (MOF) sorbents for selective CO₂ capture from industrial emissions. Their research team has developed novel MOF structures with unprecedented CO₂ selectivity and capacity, particularly the Mg-MOF-74 and SIFSIX series, which demonstrate CO₂ uptake exceeding 5 mmol/g under industrial conditions. These materials feature precisely engineered pore structures and functionalized binding sites that preferentially adsorb CO₂ over other flue gas components. Caltech's approach incorporates computational design and high-throughput synthesis techniques to rapidly optimize MOF compositions for specific industrial applications. Their temperature-vacuum swing adsorption (TVSA) process enables efficient sorbent regeneration with 40-50% lower energy requirements than conventional amine scrubbing. Laboratory demonstrations have achieved >95% capture efficiency from simulated flue gas streams with minimal performance degradation over hundreds of cycles. Caltech is currently scaling this technology through industrial partnerships, focusing on cement and power generation applications where traditional capture methods face significant challenges.
Strengths: Exceptional CO₂ selectivity and capacity compared to conventional sorbents; lower regeneration energy requirements; highly tunable material properties for specific industrial conditions; potential for breakthrough performance improvements through continued materials innovation. Weaknesses: MOF synthesis costs remain higher than conventional sorbents; scale-up manufacturing challenges for maintaining material performance; mechanical stability concerns in industrial environments with moisture and contaminants; technology primarily at laboratory and pilot scale rather than commercial deployment.
Key Patents and Innovations in CO₂ Capture Materials
A composite adsorbent for carbon dioxide capture and its preparation method and application
PatentPendingCN120479373A
Innovation
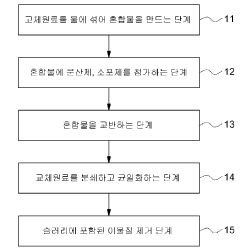
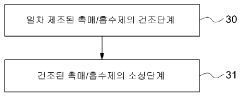
- Combined with industrial solid waste such as calcium carbide slag, steel slag and fly ash with alkaline compounds sodium hydroxide and magnesium hydroxide, composite adsorbents are prepared through high-temperature calcination treatment, and the amount of water is optimized to improve mixing uniformity and porosity.
Carbon dioxide absorbent and preparation method thereof
PatentWO2012033250A1
Innovation
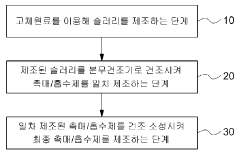
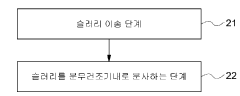
- A carbon dioxide absorbent composition comprising a metal oxide containing a transition metal, combined with an active ingredient and an inorganic binder, is developed to effectively capture and regenerate CO2, minimizing sulfur dioxide impact and enabling long-term use, with a manufacturing method involving a slurry composition and spray drying process.
Environmental Impact Assessment of Capture Technologies
The environmental implications of CO₂ capture technologies extend far beyond their primary function of reducing greenhouse gas emissions. When evaluating sorbent efficacy in industrial waste reduction, a comprehensive environmental impact assessment reveals multiple dimensions of consideration.
Carbon capture technologies, particularly those utilizing advanced sorbents, demonstrate significant potential for reducing the carbon footprint of industrial operations. However, these technologies also generate their own environmental footprints that must be carefully evaluated. The production of sorbent materials often requires energy-intensive processes and raw materials extraction, potentially offsetting some of the environmental benefits gained through carbon capture.
Life cycle assessment (LCA) studies indicate that while most capture technologies achieve net positive environmental outcomes, the magnitude varies considerably depending on sorbent type and application context. Amine-based sorbents, for example, demonstrate high capture efficiency but may contribute to eutrophication and acidification through ammonia emissions during regeneration processes. In contrast, metal-organic frameworks (MOFs) typically show lower secondary environmental impacts but require more specialized manufacturing processes.
Water consumption represents another critical environmental consideration, particularly in water-stressed regions. Wet scrubbing technologies can consume between 0.2-0.6 gallons of water per kilowatt-hour of energy produced. Solid sorbents generally demonstrate superior performance in this regard, with significantly reduced water requirements, though their regeneration may still involve substantial energy inputs.
Land use impacts vary considerably across different capture technologies. Direct air capture installations utilizing solid sorbents typically require more physical space than point-source capture systems integrated into existing industrial facilities. This spatial requirement must be factored into environmental impact assessments, particularly when considering deployment at scale.
Waste generation and management present additional environmental challenges. Spent sorbents, particularly those containing heavy metals or other hazardous components, require proper disposal or regeneration protocols. Advanced regeneration techniques have shown promise in extending sorbent lifespans, thereby reducing waste streams and improving overall environmental performance.
Cross-media effects must also be considered, as reductions in CO₂ emissions may come at the cost of increases in other environmental impacts. For instance, some capture technologies may reduce greenhouse gas emissions while simultaneously increasing particulate matter or NOx emissions, creating complex environmental trade-offs that require careful evaluation within specific industrial contexts.
Carbon capture technologies, particularly those utilizing advanced sorbents, demonstrate significant potential for reducing the carbon footprint of industrial operations. However, these technologies also generate their own environmental footprints that must be carefully evaluated. The production of sorbent materials often requires energy-intensive processes and raw materials extraction, potentially offsetting some of the environmental benefits gained through carbon capture.
Life cycle assessment (LCA) studies indicate that while most capture technologies achieve net positive environmental outcomes, the magnitude varies considerably depending on sorbent type and application context. Amine-based sorbents, for example, demonstrate high capture efficiency but may contribute to eutrophication and acidification through ammonia emissions during regeneration processes. In contrast, metal-organic frameworks (MOFs) typically show lower secondary environmental impacts but require more specialized manufacturing processes.
Water consumption represents another critical environmental consideration, particularly in water-stressed regions. Wet scrubbing technologies can consume between 0.2-0.6 gallons of water per kilowatt-hour of energy produced. Solid sorbents generally demonstrate superior performance in this regard, with significantly reduced water requirements, though their regeneration may still involve substantial energy inputs.
Land use impacts vary considerably across different capture technologies. Direct air capture installations utilizing solid sorbents typically require more physical space than point-source capture systems integrated into existing industrial facilities. This spatial requirement must be factored into environmental impact assessments, particularly when considering deployment at scale.
Waste generation and management present additional environmental challenges. Spent sorbents, particularly those containing heavy metals or other hazardous components, require proper disposal or regeneration protocols. Advanced regeneration techniques have shown promise in extending sorbent lifespans, thereby reducing waste streams and improving overall environmental performance.
Cross-media effects must also be considered, as reductions in CO₂ emissions may come at the cost of increases in other environmental impacts. For instance, some capture technologies may reduce greenhouse gas emissions while simultaneously increasing particulate matter or NOx emissions, creating complex environmental trade-offs that require careful evaluation within specific industrial contexts.
Cost-Benefit Analysis of Industrial Implementation
The implementation of CO₂ capture sorbent technologies in industrial settings requires thorough financial analysis to determine economic viability. Initial capital expenditure for retrofitting existing facilities with carbon capture systems ranges from $400-900 per ton of CO₂ capture capacity, depending on industry type and facility scale. Chemical plants and power generation facilities typically face higher installation costs due to complex integration requirements with existing processes.
Operational expenses present significant considerations, with energy penalties ranging from 15-30% of total plant energy consumption. Sorbent replacement costs vary by technology type: amine-based sorbents require replacement every 2-3 years at $2,000-4,000 per ton of capacity, while solid sorbents like metal-organic frameworks demonstrate longer lifespans of 4-6 years but at higher initial costs of $5,000-8,000 per ton.
Revenue and benefit streams must be quantified against these expenses. Carbon tax avoidance represents a primary financial benefit, with current rates ranging from $30-60 per ton of CO₂ in developed markets. Enhanced corporate sustainability metrics deliver quantifiable marketing advantages, with studies indicating 5-15% premium potential for products from carbon-neutral manufacturing processes.
Long-term ROI analysis reveals sector-specific variations. Cement and steel industries achieve breakeven points within 5-7 years when implementing advanced sorbent technologies, while chemical manufacturing facilities may require 7-9 years. Power generation presents the most challenging economics with 8-12 year payback periods, though this improves significantly with government incentives.
Sensitivity analysis indicates that sorbent efficacy improvements directly impact financial outcomes. A 10% increase in CO₂ capture efficiency correlates to approximately 7-12% improvement in ROI metrics. Similarly, extending sorbent operational lifespan by 20% reduces lifetime operational costs by 15-18%, significantly improving long-term economics.
Government incentives substantially alter the cost-benefit equation. Tax credits ranging from $45-85 per ton of captured CO₂ can reduce payback periods by 30-40%. Grant programs covering 20-40% of initial capital expenditure have demonstrated particular effectiveness in accelerating industrial adoption, especially among medium-sized enterprises with limited access to capital markets.
Operational expenses present significant considerations, with energy penalties ranging from 15-30% of total plant energy consumption. Sorbent replacement costs vary by technology type: amine-based sorbents require replacement every 2-3 years at $2,000-4,000 per ton of capacity, while solid sorbents like metal-organic frameworks demonstrate longer lifespans of 4-6 years but at higher initial costs of $5,000-8,000 per ton.
Revenue and benefit streams must be quantified against these expenses. Carbon tax avoidance represents a primary financial benefit, with current rates ranging from $30-60 per ton of CO₂ in developed markets. Enhanced corporate sustainability metrics deliver quantifiable marketing advantages, with studies indicating 5-15% premium potential for products from carbon-neutral manufacturing processes.
Long-term ROI analysis reveals sector-specific variations. Cement and steel industries achieve breakeven points within 5-7 years when implementing advanced sorbent technologies, while chemical manufacturing facilities may require 7-9 years. Power generation presents the most challenging economics with 8-12 year payback periods, though this improves significantly with government incentives.
Sensitivity analysis indicates that sorbent efficacy improvements directly impact financial outcomes. A 10% increase in CO₂ capture efficiency correlates to approximately 7-12% improvement in ROI metrics. Similarly, extending sorbent operational lifespan by 20% reduces lifetime operational costs by 15-18%, significantly improving long-term economics.
Government incentives substantially alter the cost-benefit equation. Tax credits ranging from $45-85 per ton of captured CO₂ can reduce payback periods by 30-40%. Grant programs covering 20-40% of initial capital expenditure have demonstrated particular effectiveness in accelerating industrial adoption, especially among medium-sized enterprises with limited access to capital markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!