Thermal and Chemical Properties of CO₂ Capture Sorbents
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO₂ Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past several decades, driven by increasing global concerns about climate change and greenhouse gas emissions. The journey began in the 1930s with the first industrial applications of CO₂ capture for natural gas purification, utilizing basic amine scrubbing techniques. By the 1970s, environmental awareness prompted research into more efficient capture methods, leading to the development of improved solvent-based systems.
The 1990s marked a turning point with the Kyoto Protocol highlighting the urgency of reducing carbon emissions, catalyzing substantial investment in carbon capture research. This period saw the emergence of various technological approaches including post-combustion, pre-combustion, and oxy-fuel combustion capture methods, each with distinct advantages for different industrial applications.
Since 2000, research has increasingly focused on sorbent materials with enhanced thermal and chemical properties. Traditional amine-based liquid sorbents have been complemented by solid sorbents including activated carbons, zeolites, metal-organic frameworks (MOFs), and various amine-functionalized materials. These developments have addressed key limitations in earlier technologies, particularly regarding energy penalties and operational stability.
The evolution of CO₂ capture sorbents has been characterized by progressive improvements in several critical parameters: adsorption capacity, selectivity for CO₂ over other gases, stability during multiple adsorption-desorption cycles, and energy requirements for regeneration. Each generation of materials has sought to optimize the balance between these sometimes competing properties.
Current technological objectives center on developing sorbents with superior thermal stability to withstand the high temperatures often encountered in industrial settings, while maintaining chemical reactivity for efficient CO₂ binding. Researchers aim to create materials that can operate effectively across wider temperature ranges (20-700°C) to accommodate various industrial processes, from power generation to cement production.
Another critical objective is enhancing the chemical durability of sorbents to resist degradation from contaminants like SOx, NOx, and water vapor, which are commonly present in flue gases. This includes developing materials with improved resistance to oxidative degradation and hydrolysis, thereby extending operational lifetimes and reducing replacement costs.
The field is now moving toward multifunctional sorbents that can simultaneously capture CO₂ and convert it into valuable products, representing a paradigm shift from mere carbon capture to carbon utilization. This aligns with broader sustainability goals and potentially improves the economic viability of carbon capture technologies by creating value-added products from what was previously considered waste.
The 1990s marked a turning point with the Kyoto Protocol highlighting the urgency of reducing carbon emissions, catalyzing substantial investment in carbon capture research. This period saw the emergence of various technological approaches including post-combustion, pre-combustion, and oxy-fuel combustion capture methods, each with distinct advantages for different industrial applications.
Since 2000, research has increasingly focused on sorbent materials with enhanced thermal and chemical properties. Traditional amine-based liquid sorbents have been complemented by solid sorbents including activated carbons, zeolites, metal-organic frameworks (MOFs), and various amine-functionalized materials. These developments have addressed key limitations in earlier technologies, particularly regarding energy penalties and operational stability.
The evolution of CO₂ capture sorbents has been characterized by progressive improvements in several critical parameters: adsorption capacity, selectivity for CO₂ over other gases, stability during multiple adsorption-desorption cycles, and energy requirements for regeneration. Each generation of materials has sought to optimize the balance between these sometimes competing properties.
Current technological objectives center on developing sorbents with superior thermal stability to withstand the high temperatures often encountered in industrial settings, while maintaining chemical reactivity for efficient CO₂ binding. Researchers aim to create materials that can operate effectively across wider temperature ranges (20-700°C) to accommodate various industrial processes, from power generation to cement production.
Another critical objective is enhancing the chemical durability of sorbents to resist degradation from contaminants like SOx, NOx, and water vapor, which are commonly present in flue gases. This includes developing materials with improved resistance to oxidative degradation and hydrolysis, thereby extending operational lifetimes and reducing replacement costs.
The field is now moving toward multifunctional sorbents that can simultaneously capture CO₂ and convert it into valuable products, representing a paradigm shift from mere carbon capture to carbon utilization. This aligns with broader sustainability goals and potentially improves the economic viability of carbon capture technologies by creating value-added products from what was previously considered waste.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market size has reached approximately $7 billion, with projections indicating expansion to $30 billion by 2030, representing a compound annual growth rate of over 20%. This growth trajectory is particularly evident in regions with stringent carbon pricing mechanisms, such as the European Union, Canada, and increasingly, parts of Asia.
The demand for CO₂ capture sorbents is segmented across multiple industries, with power generation, cement production, and chemical manufacturing representing the largest market segments. Power generation alone accounts for nearly 40% of the current market share, as coal and natural gas plants seek solutions to reduce their carbon footprint while maintaining operational efficiency.
Geographically, North America currently leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to demonstrate the fastest growth rate over the next decade, driven by China's ambitious carbon neutrality goals and increasing industrial activity in India and Southeast Asia.
The market for thermal-swing adsorption (TSA) sorbents is growing at a faster rate than pressure-swing alternatives, due to their lower energy requirements and operational costs. Amine-functionalized sorbents dominate the chemical sorbent segment with approximately 45% market share, valued for their high CO₂ selectivity and capacity, despite challenges related to thermal degradation.
Customer segments are increasingly diversifying, with early adopters primarily being large industrial emitters subject to carbon pricing. However, medium-sized enterprises are now entering the market as technology costs decrease and regulatory pressures expand. The willingness to pay varies significantly by region and industry, with European customers demonstrating higher price tolerance due to carbon prices exceeding €80 per ton.
Market barriers include high initial capital costs, uncertain regulatory landscapes in developing markets, and competition from alternative carbon reduction strategies. The levelized cost of carbon capture currently ranges from $50-150 per ton of CO₂, depending on the technology and application, with chemical sorbents typically positioned in the middle of this range.
Emerging market opportunities include direct air capture applications, which though currently small (less than 5% of the total market), are projected to grow substantially as net-zero commitments drive demand for negative emissions technologies. Additionally, the integration of captured CO₂ into value-added products represents a growing market segment, potentially improving the economic case for carbon capture technologies.
The demand for CO₂ capture sorbents is segmented across multiple industries, with power generation, cement production, and chemical manufacturing representing the largest market segments. Power generation alone accounts for nearly 40% of the current market share, as coal and natural gas plants seek solutions to reduce their carbon footprint while maintaining operational efficiency.
Geographically, North America currently leads the market with approximately 35% share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to demonstrate the fastest growth rate over the next decade, driven by China's ambitious carbon neutrality goals and increasing industrial activity in India and Southeast Asia.
The market for thermal-swing adsorption (TSA) sorbents is growing at a faster rate than pressure-swing alternatives, due to their lower energy requirements and operational costs. Amine-functionalized sorbents dominate the chemical sorbent segment with approximately 45% market share, valued for their high CO₂ selectivity and capacity, despite challenges related to thermal degradation.
Customer segments are increasingly diversifying, with early adopters primarily being large industrial emitters subject to carbon pricing. However, medium-sized enterprises are now entering the market as technology costs decrease and regulatory pressures expand. The willingness to pay varies significantly by region and industry, with European customers demonstrating higher price tolerance due to carbon prices exceeding €80 per ton.
Market barriers include high initial capital costs, uncertain regulatory landscapes in developing markets, and competition from alternative carbon reduction strategies. The levelized cost of carbon capture currently ranges from $50-150 per ton of CO₂, depending on the technology and application, with chemical sorbents typically positioned in the middle of this range.
Emerging market opportunities include direct air capture applications, which though currently small (less than 5% of the total market), are projected to grow substantially as net-zero commitments drive demand for negative emissions technologies. Additionally, the integration of captured CO₂ into value-added products represents a growing market segment, potentially improving the economic case for carbon capture technologies.
Current Sorbent Technologies and Challenges
Carbon dioxide capture technologies have evolved significantly over the past decades, with various sorbent materials being developed to address the growing need for efficient CO₂ removal. Currently, the market is dominated by several key sorbent technologies, each with distinct thermal and chemical properties that determine their effectiveness in different applications.
Amine-based sorbents remain the most widely deployed technology, particularly monoethanolamine (MEA) solutions which typically operate at 40-60°C during absorption and 100-120°C during regeneration. These sorbents offer high CO₂ selectivity and relatively fast kinetics but suffer from significant energy penalties during regeneration, with heat requirements of 3.5-4.0 GJ/ton CO₂. Additionally, amine degradation through oxidation and thermal processes presents a major operational challenge, reducing sorbent lifetime to approximately 1-2 years in industrial settings.
Solid sorbents have gained considerable attention as alternatives, with metal-organic frameworks (MOFs) demonstrating exceptional theoretical CO₂ capacities exceeding 1.5 g CO₂/g sorbent under ideal conditions. However, their practical implementation faces challenges related to hydrothermal stability, with many promising MOFs losing 30-50% of their capacity after exposure to moisture at operating temperatures.
Alkaline earth metal-based sorbents, particularly calcium looping systems, operate at significantly higher temperatures (600-700°C for carbonation, 850-950°C for calcination). While these systems offer high theoretical capacities and utilize abundant, low-cost materials, they suffer from rapid capacity decay, typically losing 30-40% of their initial capacity within the first 20 cycles due to sintering and morphological changes.
Emerging technologies include ionic liquids and porous polymer networks, which demonstrate promising CO₂ selectivity but currently face scalability challenges. The viscosity of ionic liquids (50-500 cP at room temperature) significantly impedes mass transfer rates, while polymer networks often require complex, multi-step syntheses that limit industrial-scale production.
A critical challenge across all sorbent technologies is the trade-off between CO₂ affinity and regeneration energy. Materials with strong CO₂ binding typically require more energy for regeneration, creating an efficiency paradox that researchers continue to address through molecular engineering and process optimization.
Water stability represents another universal challenge, as most deployment scenarios involve humid conditions that can dramatically reduce sorbent performance. Hydrophobic modifications have shown promise but often come at the cost of reduced CO₂ capacity or increased production complexity.
The development of next-generation sorbents increasingly focuses on multifunctional materials that can maintain stability across wider temperature and humidity ranges while offering improved regeneration characteristics and longer operational lifetimes.
Amine-based sorbents remain the most widely deployed technology, particularly monoethanolamine (MEA) solutions which typically operate at 40-60°C during absorption and 100-120°C during regeneration. These sorbents offer high CO₂ selectivity and relatively fast kinetics but suffer from significant energy penalties during regeneration, with heat requirements of 3.5-4.0 GJ/ton CO₂. Additionally, amine degradation through oxidation and thermal processes presents a major operational challenge, reducing sorbent lifetime to approximately 1-2 years in industrial settings.
Solid sorbents have gained considerable attention as alternatives, with metal-organic frameworks (MOFs) demonstrating exceptional theoretical CO₂ capacities exceeding 1.5 g CO₂/g sorbent under ideal conditions. However, their practical implementation faces challenges related to hydrothermal stability, with many promising MOFs losing 30-50% of their capacity after exposure to moisture at operating temperatures.
Alkaline earth metal-based sorbents, particularly calcium looping systems, operate at significantly higher temperatures (600-700°C for carbonation, 850-950°C for calcination). While these systems offer high theoretical capacities and utilize abundant, low-cost materials, they suffer from rapid capacity decay, typically losing 30-40% of their initial capacity within the first 20 cycles due to sintering and morphological changes.
Emerging technologies include ionic liquids and porous polymer networks, which demonstrate promising CO₂ selectivity but currently face scalability challenges. The viscosity of ionic liquids (50-500 cP at room temperature) significantly impedes mass transfer rates, while polymer networks often require complex, multi-step syntheses that limit industrial-scale production.
A critical challenge across all sorbent technologies is the trade-off between CO₂ affinity and regeneration energy. Materials with strong CO₂ binding typically require more energy for regeneration, creating an efficiency paradox that researchers continue to address through molecular engineering and process optimization.
Water stability represents another universal challenge, as most deployment scenarios involve humid conditions that can dramatically reduce sorbent performance. Hydrophobic modifications have shown promise but often come at the cost of reduced CO₂ capacity or increased production complexity.
The development of next-generation sorbents increasingly focuses on multifunctional materials that can maintain stability across wider temperature and humidity ranges while offering improved regeneration characteristics and longer operational lifetimes.
Mainstream Sorbent Formulations and Performance
01 Metal-organic frameworks (MOFs) for CO₂ capture
Metal-organic frameworks are crystalline porous materials with high surface area and tunable pore sizes that demonstrate excellent CO₂ adsorption capacity. These materials exhibit favorable thermal stability at various operating temperatures and can be designed with specific chemical functionalities to enhance CO₂ selectivity. Their regeneration properties allow for multiple adsorption-desorption cycles with minimal degradation, making them promising candidates for carbon capture applications.- Metal-organic frameworks (MOFs) for CO₂ capture: Metal-organic frameworks are crystalline porous materials with high surface area and tunable pore sizes that demonstrate excellent CO₂ adsorption capacity. These materials exhibit thermal stability at various temperatures and can be designed with specific chemical functionalities to enhance CO₂ selectivity. Their regeneration properties allow for multiple adsorption-desorption cycles while maintaining structural integrity, making them promising candidates for carbon capture applications.
- Amine-functionalized sorbents for enhanced CO₂ capture: Amine-functionalized materials leverage the chemical affinity between amine groups and CO₂ molecules to achieve high capture efficiency. These sorbents can be designed with various amine types (primary, secondary, tertiary) to optimize adsorption kinetics and capacity. The thermal properties of these materials are crucial for regeneration processes, as they must withstand temperature swings during CO₂ desorption while maintaining their chemical structure and functionality over multiple cycles.
- Zeolite-based CO₂ capture materials: Zeolites are aluminosilicate materials with well-defined microporous structures that demonstrate good thermal stability and CO₂ adsorption properties. Their chemical composition can be modified to enhance selectivity toward CO₂ over other gases. The thermal properties of zeolites allow them to maintain structural integrity at elevated temperatures, which is beneficial for regeneration processes. Their hydrophilic/hydrophobic characteristics can be tuned to optimize performance in humid conditions typical of industrial gas streams.
- Novel composite sorbents with enhanced thermal stability: Composite sorbents combine multiple materials to achieve synergistic effects in CO₂ capture performance. These materials often incorporate a high-surface-area support with active capture components to maximize adsorption capacity while maintaining good heat transfer properties. The thermal stability of these composites is engineered to withstand temperature fluctuations during capture and regeneration cycles. Chemical modifications can be introduced to improve resistance to degradation from contaminants in flue gas streams, extending operational lifetime in industrial settings.
- Temperature-responsive CO₂ capture materials: Temperature-responsive sorbents exhibit different CO₂ capture behaviors at various temperature ranges, allowing for efficient capture and release cycles. These materials are designed with specific phase transition properties that can be leveraged for energy-efficient carbon capture processes. Their chemical structure enables strong CO₂ binding at capture temperatures while facilitating release at moderately elevated temperatures, reducing the energy penalty associated with sorbent regeneration. Advanced formulations incorporate stabilizers to prevent thermal degradation during repeated temperature cycling.
02 Amine-functionalized sorbents for enhanced CO₂ capture
Amine-functionalized materials leverage the chemical affinity between amine groups and CO₂ molecules to achieve high capture efficiency. These sorbents can be designed with various amine types (primary, secondary, tertiary) to optimize adsorption capacity and kinetics. The thermal properties of these materials are crucial for regeneration processes, with working temperature ranges typically between 40-120°C. Chemical stability during multiple temperature swing cycles is a key consideration for long-term performance.Expand Specific Solutions03 Zeolite-based CO₂ capture materials
Zeolites are aluminosilicate materials with well-defined microporous structures that demonstrate good thermal stability and CO₂ adsorption properties. Their chemical composition can be modified to enhance selectivity toward CO₂ over other gases. These materials typically operate effectively at moderate temperatures and can withstand multiple thermal cycling processes. The adsorption mechanism is primarily based on physical interactions, with regeneration possible through temperature or pressure swing approaches.Expand Specific Solutions04 Thermally stable alkaline earth metal-based CO₂ sorbents
Alkaline earth metal-based sorbents, particularly those containing calcium and magnesium oxides, demonstrate high CO₂ capture capacity through carbonation reactions. These materials exhibit good thermal stability at elevated temperatures (500-900°C), making them suitable for high-temperature carbon capture applications. Their chemical properties can be enhanced through various synthesis methods and the addition of stabilizing agents to prevent sintering during multiple carbonation-calcination cycles, thereby extending operational lifespan.Expand Specific Solutions05 Novel composite and hybrid CO₂ capture materials
Composite and hybrid materials combine the advantages of different sorbent types to achieve enhanced CO₂ capture performance. These materials often integrate organic and inorganic components to optimize both chemical affinity and physical adsorption mechanisms. Their thermal properties can be tailored for specific operating conditions, with some designed for low-temperature applications and others for high-temperature environments. The chemical stability of these hybrid materials is typically superior to single-component sorbents, resulting in improved cycling performance and regenerability.Expand Specific Solutions
Leading Organizations in Carbon Capture Research
The CO₂ capture sorbent technology market is currently in a growth phase, with increasing global focus on carbon reduction strategies driving market expansion. The global market for carbon capture technologies is projected to reach approximately $7-10 billion by 2025, with sorbent technologies representing a significant segment. Technical maturity varies across different sorbent types, with companies like ExxonMobil, Shell, and China Petroleum & Chemical Corp leading commercial deployment of conventional amine-based sorbents. Research institutions such as East China University of Science & Technology, University of Southern California, and National Institute for Materials Science are advancing next-generation solid sorbents with improved thermal and chemical properties. Specialized firms like Susteon, TDA Research, and Global Thermostat are developing proprietary sorbent technologies with enhanced CO₂ selectivity and regeneration characteristics, positioning themselves as emerging players in this competitive landscape.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed advanced carbonate-based sorbents for CO₂ capture that operate through a pressure swing adsorption (PSA) process. Their proprietary Metal-Organic Framework (MOF) materials demonstrate exceptional CO₂ selectivity and capacity under various temperature conditions. The company's approach involves specially engineered porous materials with tailored surface chemistry that can selectively adsorb CO₂ from flue gas streams. Their research has shown that these materials maintain structural stability after multiple adsorption-desorption cycles, with minimal degradation in capture efficiency. ExxonMobil's sorbents are designed to operate at moderate temperatures (40-80°C) with significantly reduced regeneration energy requirements compared to conventional amine scrubbing technologies. The company has also developed hybrid materials combining the benefits of physical adsorption and chemical absorption mechanisms to optimize both capture capacity and regeneration energy. Recent developments include incorporating catalytic functionalities that can convert captured CO₂ into valuable products, creating potential economic incentives for carbon capture implementation.
Strengths: High CO₂ selectivity in mixed gas streams; lower energy requirements for regeneration compared to liquid amine systems; excellent stability over multiple adsorption-desorption cycles. Weaknesses: Performance may degrade in the presence of SOx and NOx contaminants; higher capital costs for initial implementation; requires precise temperature and pressure control for optimal operation.
TDA Research, Inc.
Technical Solution: TDA Research has developed proprietary alkali-promoted carbon-based sorbents for CO₂ capture that operate through a unique physisorption-enhanced chemisorption mechanism. Their technology utilizes specially engineered mesoporous carbon materials impregnated with alkali metal carbonates (primarily K₂CO₃) that react with CO₂ in the presence of water vapor to form bicarbonates. These materials demonstrate CO₂ capture capacities of 3-4 mmol/g at temperatures between 60-80°C, with complete regeneration achieved at temperatures as low as 120°C. A key innovation in TDA's approach is the optimization of pore structure to facilitate rapid mass transfer while maintaining high active material loading. Their sorbents have demonstrated remarkable stability, maintaining over 90% of initial capacity after more than 1,000 adsorption-desorption cycles in the presence of realistic flue gas contaminants. TDA has also developed novel reactor configurations specifically designed for their sorbent materials, including moving bed systems that enable continuous operation with separate adsorption and regeneration zones. Recent advancements include the incorporation of catalytic promoters that enhance reaction kinetics and lower the effective operating temperature, further reducing energy requirements. The company has successfully scaled their materials from laboratory to pilot scale, demonstrating the commercial viability of their technology for industrial applications.
Strengths: Low-cost materials based on abundant carbon precursors; excellent cycling stability; moderate regeneration temperatures that enable the use of low-grade waste heat. Weaknesses: Requires the presence of water vapor for optimal performance; slower kinetics compared to some amine-based systems; potential for alkali component migration during extended operation.
Key Innovations in Thermal-Resistant Sorbents
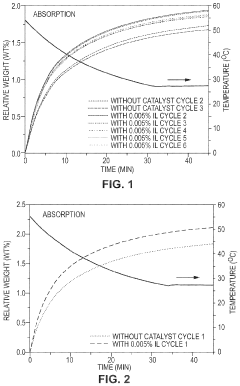
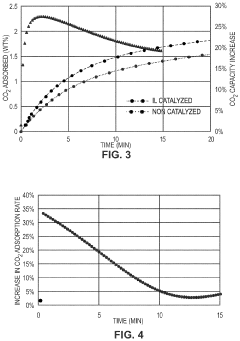
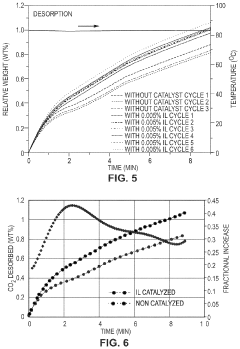
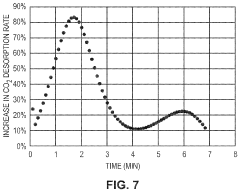
Co2 capture sorbents with low regeneration temperature and high desorption rates
PatentPendingUS20240009613A1
Innovation
- Development of CO2 capture sorbents comprising a solid support with CO2-sorbing amine and ionic liquid, which enhances CO2 sorption and desorption characteristics, allowing for regeneration at lower temperatures and maintaining high selectivity and capacity through catalytic action.
Sorbent structure applicable for carbon dioxide capture
PatentInactiveEP2401054A2
Innovation
- A sorbent structure comprising a continuous activated carbon body with a flow-through substrate and an additive, such as a weak base or transition metal oxide, enhances CO2 sorption efficiency with low pressure drop and high surface area, allowing for effective CO2 capture and regeneration through thermal or electric swing adsorption.
Environmental Impact Assessment
The environmental impact of CO₂ capture sorbents extends far beyond their primary function of reducing greenhouse gas emissions. These materials interact with the environment throughout their lifecycle, from production to disposal, creating a complex web of environmental considerations that must be carefully assessed.
The manufacturing process of CO₂ capture sorbents often requires significant energy input and raw materials, potentially generating substantial carbon footprints that could partially offset their environmental benefits. For instance, amine-based sorbents typically require energy-intensive chemical synthesis processes, while solid sorbents like metal-organic frameworks (MOFs) may involve rare or toxic metals in their production.
Water consumption represents another critical environmental concern, particularly for aqueous amine systems that require substantial water resources for operation. This becomes especially problematic in water-stressed regions where competing demands for water resources already exist. Additionally, the potential for water contamination through chemical leaching must be considered in environmental risk assessments.
Chemical degradation of sorbents during operation can release byproducts with potential environmental toxicity. Amine degradation products, for example, may include nitrosamines and nitramines, compounds with known carcinogenic properties. The environmental fate and transport of these compounds require thorough investigation to prevent unintended ecological consequences.
Land use impacts vary significantly between different capture technologies. Direct air capture facilities utilizing solid sorbents may require substantial land area, potentially competing with agriculture or natural habitats. In contrast, point-source capture systems integrated into existing industrial facilities generally have smaller physical footprints but may present more concentrated environmental risks.
The end-of-life management of spent sorbents presents additional environmental challenges. Disposal options must account for potential leaching of hazardous components, while regeneration processes consume energy and may produce secondary waste streams requiring treatment. Developing circular economy approaches for sorbent materials represents an emerging priority to minimize these impacts.
Comprehensive life cycle assessment (LCA) studies indicate that despite these environmental considerations, properly designed CO₂ capture systems using appropriate sorbents generally deliver net positive environmental benefits when considering their primary function of greenhouse gas reduction. However, these assessments highlight the importance of system optimization to minimize trade-offs between carbon capture efficiency and other environmental impact categories.
The manufacturing process of CO₂ capture sorbents often requires significant energy input and raw materials, potentially generating substantial carbon footprints that could partially offset their environmental benefits. For instance, amine-based sorbents typically require energy-intensive chemical synthesis processes, while solid sorbents like metal-organic frameworks (MOFs) may involve rare or toxic metals in their production.
Water consumption represents another critical environmental concern, particularly for aqueous amine systems that require substantial water resources for operation. This becomes especially problematic in water-stressed regions where competing demands for water resources already exist. Additionally, the potential for water contamination through chemical leaching must be considered in environmental risk assessments.
Chemical degradation of sorbents during operation can release byproducts with potential environmental toxicity. Amine degradation products, for example, may include nitrosamines and nitramines, compounds with known carcinogenic properties. The environmental fate and transport of these compounds require thorough investigation to prevent unintended ecological consequences.
Land use impacts vary significantly between different capture technologies. Direct air capture facilities utilizing solid sorbents may require substantial land area, potentially competing with agriculture or natural habitats. In contrast, point-source capture systems integrated into existing industrial facilities generally have smaller physical footprints but may present more concentrated environmental risks.
The end-of-life management of spent sorbents presents additional environmental challenges. Disposal options must account for potential leaching of hazardous components, while regeneration processes consume energy and may produce secondary waste streams requiring treatment. Developing circular economy approaches for sorbent materials represents an emerging priority to minimize these impacts.
Comprehensive life cycle assessment (LCA) studies indicate that despite these environmental considerations, properly designed CO₂ capture systems using appropriate sorbents generally deliver net positive environmental benefits when considering their primary function of greenhouse gas reduction. However, these assessments highlight the importance of system optimization to minimize trade-offs between carbon capture efficiency and other environmental impact categories.
Cost-Benefit Analysis of Sorbent Technologies
The economic viability of CO₂ capture sorbent technologies hinges on a comprehensive cost-benefit analysis that considers both direct and indirect financial implications. When evaluating sorbent materials such as amine-functionalized silica, metal-organic frameworks (MOFs), and zeolites, capital expenditure represents a significant initial consideration. The production costs of these materials vary substantially, with traditional zeolites typically costing $1-5/kg, while more advanced MOFs may range from $20-100/kg depending on synthesis complexity and scale.
Operational expenditure constitutes another critical dimension, encompassing energy requirements for regeneration cycles, replacement frequency due to degradation, and auxiliary system maintenance. Amine-based sorbents generally require 2.5-3.5 GJ/ton CO₂ for regeneration, whereas some advanced MOFs demonstrate improved energy efficiency at 1.8-2.2 GJ/ton CO₂. This energy differential translates to substantial cost variations over the operational lifetime of capture systems.
Sorbent durability directly impacts economic performance through replacement costs and system downtime. Materials exhibiting thermal and chemical stability across thousands of adsorption-desorption cycles deliver superior lifetime value despite potentially higher initial investment. For instance, hydrothermally stable zeolites may maintain 90% capacity after 1,000 cycles, while some amine-functionalized materials show significant degradation after just 200-300 cycles.
The capture efficiency of sorbents fundamentally affects the cost per ton of CO₂ sequestered. Higher capacity materials (>2 mmol/g) with rapid kinetics reduce both equipment sizing requirements and energy consumption, yielding improved economics. Recent techno-economic assessments indicate that achieving capture costs below $40/ton requires sorbents with capacities exceeding 3 mmol/g under practical operating conditions.
Scalability considerations introduce additional economic factors, as laboratory-optimized materials often face synthesis challenges at industrial scales. Manufacturing processes that utilize common precursors and avoid exotic catalysts typically demonstrate more favorable cost trajectories as production volumes increase. This scalability advantage has maintained zeolites as economically competitive despite the theoretical performance advantages of newer materials.
Regulatory frameworks increasingly influence the cost-benefit equation through carbon pricing mechanisms, tax incentives, and compliance requirements. Markets with carbon prices exceeding $50/ton create favorable economics for even relatively expensive capture technologies, while regions without such mechanisms require more cost-efficient solutions to achieve economic viability.
Operational expenditure constitutes another critical dimension, encompassing energy requirements for regeneration cycles, replacement frequency due to degradation, and auxiliary system maintenance. Amine-based sorbents generally require 2.5-3.5 GJ/ton CO₂ for regeneration, whereas some advanced MOFs demonstrate improved energy efficiency at 1.8-2.2 GJ/ton CO₂. This energy differential translates to substantial cost variations over the operational lifetime of capture systems.
Sorbent durability directly impacts economic performance through replacement costs and system downtime. Materials exhibiting thermal and chemical stability across thousands of adsorption-desorption cycles deliver superior lifetime value despite potentially higher initial investment. For instance, hydrothermally stable zeolites may maintain 90% capacity after 1,000 cycles, while some amine-functionalized materials show significant degradation after just 200-300 cycles.
The capture efficiency of sorbents fundamentally affects the cost per ton of CO₂ sequestered. Higher capacity materials (>2 mmol/g) with rapid kinetics reduce both equipment sizing requirements and energy consumption, yielding improved economics. Recent techno-economic assessments indicate that achieving capture costs below $40/ton requires sorbents with capacities exceeding 3 mmol/g under practical operating conditions.
Scalability considerations introduce additional economic factors, as laboratory-optimized materials often face synthesis challenges at industrial scales. Manufacturing processes that utilize common precursors and avoid exotic catalysts typically demonstrate more favorable cost trajectories as production volumes increase. This scalability advantage has maintained zeolites as economically competitive despite the theoretical performance advantages of newer materials.
Regulatory frameworks increasingly influence the cost-benefit equation through carbon pricing mechanisms, tax incentives, and compliance requirements. Markets with carbon prices exceeding $50/ton create favorable economics for even relatively expensive capture technologies, while regions without such mechanisms require more cost-efficient solutions to achieve economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!