How Material Science Advances Shape CO₂ Capture Sorbent Development
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO₂ Capture Materials Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past several decades, driven by the urgent need to mitigate climate change and reduce greenhouse gas emissions. The journey began in the 1930s with basic amine scrubbing techniques used in natural gas processing, which laid the groundwork for modern CO₂ capture methods. By the 1970s and 1980s, environmental concerns started to shift focus toward developing more efficient capture technologies, particularly for industrial applications and power plants.
The 1990s marked a turning point with the Kyoto Protocol highlighting the importance of carbon management strategies. This period saw increased research into various sorbent materials, including activated carbons, zeolites, and early metal-organic frameworks (MOFs). The early 2000s witnessed significant advancements in material science that revolutionized CO₂ capture approaches, with novel nanoporous materials and functionalized sorbents emerging as promising candidates.
Recent technological evolution has been characterized by interdisciplinary collaboration between material scientists, chemical engineers, and environmental researchers. This has led to breakthroughs in developing advanced sorbents with unprecedented CO₂ selectivity, capacity, and regeneration efficiency. The integration of computational modeling and high-throughput screening methods has accelerated material discovery, allowing researchers to predict and design sorbents with optimal properties before synthesis.
The primary objectives in CO₂ capture sorbent development focus on several key performance indicators. First is increasing CO₂ selectivity in mixed gas environments, particularly in low concentration scenarios like direct air capture. Second is enhancing adsorption capacity to maximize the amount of CO₂ captured per unit of sorbent material. Third is improving regeneration efficiency to reduce the energy penalty associated with sorbent regeneration, which remains one of the most significant barriers to widespread implementation.
Additional objectives include developing materials with long-term stability under real-world operating conditions, reducing manufacturing costs to enable economic viability at scale, and minimizing the environmental footprint of the sorbents themselves. The ultimate goal is to create materials that can be deployed across various sectors, from power generation to industrial processes and transportation.
Looking forward, the field is trending toward multifunctional materials that can simultaneously capture CO₂ and convert it into value-added products, representing a paradigm shift from mere carbon capture to carbon utilization. Biomimetic approaches, inspired by natural carbon fixation processes, are also gaining traction as researchers seek to replicate and enhance nature's efficiency in managing carbon.
The 1990s marked a turning point with the Kyoto Protocol highlighting the importance of carbon management strategies. This period saw increased research into various sorbent materials, including activated carbons, zeolites, and early metal-organic frameworks (MOFs). The early 2000s witnessed significant advancements in material science that revolutionized CO₂ capture approaches, with novel nanoporous materials and functionalized sorbents emerging as promising candidates.
Recent technological evolution has been characterized by interdisciplinary collaboration between material scientists, chemical engineers, and environmental researchers. This has led to breakthroughs in developing advanced sorbents with unprecedented CO₂ selectivity, capacity, and regeneration efficiency. The integration of computational modeling and high-throughput screening methods has accelerated material discovery, allowing researchers to predict and design sorbents with optimal properties before synthesis.
The primary objectives in CO₂ capture sorbent development focus on several key performance indicators. First is increasing CO₂ selectivity in mixed gas environments, particularly in low concentration scenarios like direct air capture. Second is enhancing adsorption capacity to maximize the amount of CO₂ captured per unit of sorbent material. Third is improving regeneration efficiency to reduce the energy penalty associated with sorbent regeneration, which remains one of the most significant barriers to widespread implementation.
Additional objectives include developing materials with long-term stability under real-world operating conditions, reducing manufacturing costs to enable economic viability at scale, and minimizing the environmental footprint of the sorbents themselves. The ultimate goal is to create materials that can be deployed across various sectors, from power generation to industrial processes and transportation.
Looking forward, the field is trending toward multifunctional materials that can simultaneously capture CO₂ and convert it into value-added products, representing a paradigm shift from mere carbon capture to carbon utilization. Biomimetic approaches, inspired by natural carbon fixation processes, are also gaining traction as researchers seek to replicate and enhance nature's efficiency in managing carbon.
Market Demand for Carbon Capture Technologies
The global carbon capture market is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce greenhouse gas emissions. As of 2023, the carbon capture, utilization, and storage (CCUS) market was valued at approximately $4 billion, with projections indicating growth to reach $12 billion by 2030, representing a compound annual growth rate of 17%. This rapid expansion reflects the urgent need for effective carbon management solutions across various industries.
Industrial sectors, particularly power generation, cement production, steel manufacturing, and chemical processing, represent the primary demand drivers for carbon capture technologies. These industries collectively account for over 60% of global CO2 emissions, creating substantial market potential for advanced sorbent materials. The power generation sector alone contributes nearly 40% of global carbon emissions, making it the largest potential market for carbon capture solutions.
Regional analysis reveals varying levels of market maturity and adoption. North America and Europe currently lead in carbon capture implementation, supported by strong regulatory frameworks and carbon pricing mechanisms. The European Union's commitment to carbon neutrality by 2050 has accelerated investment in carbon capture infrastructure, with planned capacity increases of 300% by 2030. Meanwhile, the Asia-Pacific region, particularly China and India, represents the fastest-growing market segment due to rapid industrialization coupled with increasing environmental commitments.
Government policies and international agreements significantly influence market dynamics. Carbon pricing mechanisms, tax incentives, and direct subsidies for carbon capture projects have emerged as critical market enablers. The implementation of carbon taxes in over 40 countries has created economic incentives for industries to adopt carbon capture technologies, directly impacting the demand for advanced sorbent materials.
End-user industries are increasingly recognizing carbon capture not merely as a compliance requirement but as a strategic business opportunity. The growing market for captured carbon in enhanced oil recovery, synthetic fuel production, and building materials manufacturing has expanded the commercial viability of carbon capture technologies. This trend is reflected in the 45% increase in corporate investments in carbon capture projects between 2020 and 2023.
Material science innovations in CO2 sorbents directly respond to specific market demands for more cost-effective, energy-efficient capture solutions. Current market analysis indicates that reducing the energy penalty of carbon capture remains a primary concern, with industries seeking sorbent materials that can decrease regeneration energy requirements by at least 30% compared to conventional amine scrubbing technologies.
Industrial sectors, particularly power generation, cement production, steel manufacturing, and chemical processing, represent the primary demand drivers for carbon capture technologies. These industries collectively account for over 60% of global CO2 emissions, creating substantial market potential for advanced sorbent materials. The power generation sector alone contributes nearly 40% of global carbon emissions, making it the largest potential market for carbon capture solutions.
Regional analysis reveals varying levels of market maturity and adoption. North America and Europe currently lead in carbon capture implementation, supported by strong regulatory frameworks and carbon pricing mechanisms. The European Union's commitment to carbon neutrality by 2050 has accelerated investment in carbon capture infrastructure, with planned capacity increases of 300% by 2030. Meanwhile, the Asia-Pacific region, particularly China and India, represents the fastest-growing market segment due to rapid industrialization coupled with increasing environmental commitments.
Government policies and international agreements significantly influence market dynamics. Carbon pricing mechanisms, tax incentives, and direct subsidies for carbon capture projects have emerged as critical market enablers. The implementation of carbon taxes in over 40 countries has created economic incentives for industries to adopt carbon capture technologies, directly impacting the demand for advanced sorbent materials.
End-user industries are increasingly recognizing carbon capture not merely as a compliance requirement but as a strategic business opportunity. The growing market for captured carbon in enhanced oil recovery, synthetic fuel production, and building materials manufacturing has expanded the commercial viability of carbon capture technologies. This trend is reflected in the 45% increase in corporate investments in carbon capture projects between 2020 and 2023.
Material science innovations in CO2 sorbents directly respond to specific market demands for more cost-effective, energy-efficient capture solutions. Current market analysis indicates that reducing the energy penalty of carbon capture remains a primary concern, with industries seeking sorbent materials that can decrease regeneration energy requirements by at least 30% compared to conventional amine scrubbing technologies.
Current Sorbent Technologies and Limitations
Carbon dioxide capture technologies currently employ various sorbent materials, each with distinct advantages and limitations. Amine-based sorbents, particularly monoethanolamine (MEA), represent the most mature and widely deployed technology in industrial settings. These liquid sorbents offer high CO₂ selectivity and relatively fast kinetics, achieving capture efficiencies of 85-95%. However, they suffer from significant drawbacks including high regeneration energy requirements (3.5-4.2 GJ/tCO₂), equipment corrosion issues, and degradation through oxidation and thermal stress, necessitating frequent replacement.
Solid sorbents have emerged as promising alternatives, with activated carbons demonstrating moderate CO₂ uptake capacities (2-3 mmol/g) and excellent stability. Their primary limitations include relatively low selectivity in the presence of water vapor and diminished performance at elevated temperatures. Metal-organic frameworks (MOFs) exhibit exceptional theoretical capacities (up to 8 mmol/g) and tunable pore structures but face challenges in moisture stability, manufacturing scalability, and production costs that currently limit industrial deployment.
Zeolites offer high initial adsorption rates and good selectivity under specific conditions but experience dramatic capacity reduction in humid environments. Their regeneration also requires significant energy input, typically through temperature swing processes that reduce operational efficiency. Hydrotalcites perform well at elevated temperatures (200-400°C) but display limited capacity at ambient conditions, restricting their application range.
Alkaline earth metal oxides, particularly CaO-based sorbents, demonstrate high theoretical capacities (17.8 mmol/g) and operate effectively at high temperatures. However, they suffer from rapid performance degradation over multiple cycles due to sintering and loss of reactive surface area, with capacity often declining by 70-80% after just 100 cycles.
Emerging polymer-based sorbents show promise for their structural versatility but currently lack the combination of capacity, selectivity, and durability required for commercial implementation. Similarly, ionic liquids offer excellent CO₂ solubility but face challenges related to viscosity management and prohibitive production costs.
The fundamental limitations across current sorbent technologies include capacity-stability trade-offs, energy-intensive regeneration processes, sensitivity to contaminants (particularly SOx, NOx, and water vapor), and insufficient durability under industrial cycling conditions. Additionally, most advanced materials with superior theoretical performance face significant manufacturing challenges that impede their transition from laboratory success to industrial application.
Material science innovations are urgently needed to overcome these limitations, particularly in developing hierarchical pore structures, surface functionalization techniques, and composite materials that can maintain performance under real-world conditions while meeting economic constraints for large-scale carbon capture implementation.
Solid sorbents have emerged as promising alternatives, with activated carbons demonstrating moderate CO₂ uptake capacities (2-3 mmol/g) and excellent stability. Their primary limitations include relatively low selectivity in the presence of water vapor and diminished performance at elevated temperatures. Metal-organic frameworks (MOFs) exhibit exceptional theoretical capacities (up to 8 mmol/g) and tunable pore structures but face challenges in moisture stability, manufacturing scalability, and production costs that currently limit industrial deployment.
Zeolites offer high initial adsorption rates and good selectivity under specific conditions but experience dramatic capacity reduction in humid environments. Their regeneration also requires significant energy input, typically through temperature swing processes that reduce operational efficiency. Hydrotalcites perform well at elevated temperatures (200-400°C) but display limited capacity at ambient conditions, restricting their application range.
Alkaline earth metal oxides, particularly CaO-based sorbents, demonstrate high theoretical capacities (17.8 mmol/g) and operate effectively at high temperatures. However, they suffer from rapid performance degradation over multiple cycles due to sintering and loss of reactive surface area, with capacity often declining by 70-80% after just 100 cycles.
Emerging polymer-based sorbents show promise for their structural versatility but currently lack the combination of capacity, selectivity, and durability required for commercial implementation. Similarly, ionic liquids offer excellent CO₂ solubility but face challenges related to viscosity management and prohibitive production costs.
The fundamental limitations across current sorbent technologies include capacity-stability trade-offs, energy-intensive regeneration processes, sensitivity to contaminants (particularly SOx, NOx, and water vapor), and insufficient durability under industrial cycling conditions. Additionally, most advanced materials with superior theoretical performance face significant manufacturing challenges that impede their transition from laboratory success to industrial application.
Material science innovations are urgently needed to overcome these limitations, particularly in developing hierarchical pore structures, surface functionalization techniques, and composite materials that can maintain performance under real-world conditions while meeting economic constraints for large-scale carbon capture implementation.
State-of-the-Art Sorbent Solutions
01 Metal-organic frameworks (MOFs) for CO₂ capture
Metal-organic frameworks are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They exhibit high surface area, tunable pore size, and chemical functionality, making them effective for selective CO₂ adsorption. These materials can be designed with specific metal centers and organic linkers to enhance CO₂ binding affinity and capacity. Their structural flexibility and thermal stability allow for efficient capture and release cycles in various temperature and pressure conditions.- Metal-organic frameworks (MOFs) for CO₂ capture: Metal-organic frameworks are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. These materials exhibit high surface areas, tunable pore sizes, and chemical functionality that can be optimized for CO₂ adsorption. MOFs can be designed with specific binding sites for CO₂ molecules, enhancing selectivity and capacity. Their modular nature allows for systematic modification of metal centers and organic linkers to improve CO₂ capture performance under various conditions.
- Amine-functionalized sorbents: Amine-functionalized materials represent a significant class of CO₂ capture sorbents due to their strong chemical affinity for CO₂. These materials typically consist of amines grafted onto high-surface-area supports such as silica, activated carbon, or polymers. The amine groups react with CO₂ to form carbamates or bicarbonates, enabling efficient capture even at low CO₂ concentrations. Key material properties include amine loading density, accessibility of amine sites, stability during multiple adsorption-desorption cycles, and heat of adsorption, which affects regeneration energy requirements.
- Zeolites and molecular sieves for selective CO₂ adsorption: Zeolites and molecular sieves are aluminosilicate materials with well-defined microporous structures that enable molecular sieving effects for selective CO₂ capture. These materials separate CO₂ based on differences in molecular size, shape, and polarity. Important material properties include pore size distribution, Si/Al ratio (which affects hydrophilicity and cation content), framework topology, and thermal stability. Zeolites can be modified with cations such as lithium, sodium, or calcium to enhance CO₂ adsorption capacity and selectivity over other gases like nitrogen or methane.
- Carbon-based sorbents with enhanced CO₂ capture properties: Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, offer versatile platforms for CO₂ capture. These materials can be engineered with high surface areas, controlled pore structures, and surface functionalities to enhance CO₂ adsorption. Key material properties include specific surface area, pore size distribution, surface chemistry, and heteroatom doping (N, S, or O). Carbon-based sorbents are particularly valued for their low cost, high thermal stability, hydrophobicity, and potential for large-scale production from renewable or waste resources.
- Hybrid and composite sorbents for enhanced CO₂ capture performance: Hybrid and composite sorbents combine multiple materials to achieve synergistic effects in CO₂ capture performance. These materials typically integrate the advantages of different components, such as the high surface area of porous supports with the chemical reactivity of functional groups. Examples include polymer-inorganic composites, mixed matrix materials, and layered structures. Critical material properties include component compatibility, interfacial interactions, mechanical stability, and diffusion characteristics. These hybrid materials can overcome limitations of single-component sorbents by offering improved capacity, selectivity, stability, and regeneration properties.
02 Amine-functionalized sorbents
Amine-functionalized materials are widely used for CO₂ capture due to their strong chemical affinity for carbon dioxide. These sorbents typically consist of a porous support material impregnated or grafted with various amine compounds. The amine groups react with CO₂ to form carbamates or bicarbonates, enabling high selectivity even at low CO₂ concentrations. Key material properties include amine loading capacity, thermal stability, moisture tolerance, and regeneration efficiency. These materials can be designed with different amine types to optimize adsorption kinetics and minimize energy requirements for regeneration.Expand Specific Solutions03 Zeolite and molecular sieve sorbents
Zeolites and molecular sieves are aluminosilicate materials with well-defined microporous structures that enable molecular sieving effects for CO₂ capture. Their crystalline framework contains uniform pore sizes that can selectively adsorb CO₂ over other gases based on molecular dimensions. Important material properties include Si/Al ratio, cation type, pore size distribution, and hydrophobicity/hydrophilicity balance. These materials offer advantages such as high thermal stability, mechanical strength, and resistance to chemical degradation, making them suitable for harsh operating conditions in industrial carbon capture applications.Expand Specific Solutions04 Carbon-based sorbent materials
Carbon-based materials including activated carbon, carbon nanotubes, graphene, and carbon molecular sieves are effective CO₂ sorbents due to their high surface area and pore volume. These materials can be modified through physical or chemical activation processes to enhance their CO₂ capture performance. Key properties include specific surface area, pore size distribution, surface functionality, and heteroatom doping. Carbon-based sorbents offer advantages such as low cost, lightweight structure, good thermal conductivity, and stability in various environmental conditions, making them promising candidates for large-scale carbon capture applications.Expand Specific Solutions05 Composite and hybrid sorbent materials
Composite and hybrid sorbents combine multiple material types to achieve enhanced CO₂ capture performance beyond what individual components can provide. These materials typically integrate the advantages of different sorbent classes, such as combining the high surface area of porous supports with the chemical reactivity of functional groups. Important properties include component compatibility, interfacial interactions, structural stability, and synergistic effects. Examples include polymer-inorganic composites, mixed matrix materials, and layered structures. These hybrid approaches allow for tailored material design with optimized adsorption capacity, selectivity, kinetics, and regeneration properties for specific carbon capture applications.Expand Specific Solutions
Leading Organizations in Carbon Capture Materials
The CO₂ capture sorbent development landscape is currently in a growth phase, with the global carbon capture market expected to reach $7-10 billion by 2030. Material science advances are driving innovation across various technological maturity levels. Leading commercial players like Climeworks AG and Global Thermostat have deployed operational direct air capture facilities, while established chemical companies including BASF, Wacker Chemie, and ExxonMobil are leveraging their materials expertise to develop enhanced sorbents. Academic institutions (Arizona State University, Rice University, Norwegian University of Science & Technology) are pioneering next-generation materials with improved capacity and selectivity. Chinese entities (Sinopec, Zhejiang University) are rapidly advancing in this space, particularly in industrial applications. The competitive landscape features both specialized startups focused solely on carbon capture and diversified corporations integrating capture technologies into broader sustainability portfolios.
Climeworks AG
Technical Solution: Climeworks has developed a direct air capture (DAC) technology using solid sorbent materials arranged in modular collectors. Their proprietary amine-functionalized filter material selectively captures CO₂ when air passes through it. The process involves a two-step temperature swing adsorption cycle: first, ambient air is drawn into the collector with a fan where CO₂ binds to the sorbent; then, once saturated, the collector is closed and heated to 80-100°C, releasing concentrated CO₂ for collection or storage. Their latest plants, including Orca in Iceland and the larger Mammoth facility, utilize geothermal energy for the heating process, making the entire carbon capture process more sustainable. Climeworks has continuously improved their sorbent materials to increase CO₂ binding capacity and reduce regeneration energy requirements, achieving approximately 25% efficiency improvements in their latest generation technology[1][2].
Strengths: Modular design allows for scalable deployment; integration with renewable energy sources; proven commercial implementation with operational plants. Weaknesses: Relatively high energy requirements for sorbent regeneration; higher cost per ton of CO₂ captured compared to point-source capture; limited by geographical constraints when seeking renewable energy sources for operation.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed advanced metal-organic framework (MOF) materials for CO₂ capture with exceptional selectivity and capacity. Their proprietary MOF structures feature tailored pore sizes and functionalized binding sites that can selectively adsorb CO₂ from mixed gas streams. The company's approach combines computational material science with high-throughput experimental screening to identify optimal sorbent candidates. Their latest generation of materials demonstrates up to 40% higher CO₂ capacity than conventional amine-based sorbents while requiring significantly less energy for regeneration. ExxonMobil has also pioneered pressure-swing adsorption systems that utilize these advanced materials in industrial settings, particularly for natural gas processing and hydrogen production facilities. Their integrated material-process design approach has yielded systems that can capture CO₂ at costs potentially below $50 per ton when deployed at scale[3][4].
Strengths: Extensive R&D capabilities and resources; integration of material development with process engineering; potential for deployment across existing industrial infrastructure. Weaknesses: Primary focus on point-source rather than direct air capture; technology still largely at demonstration rather than full commercial scale; dependent on favorable policy environment for widespread adoption.
Breakthrough Materials and Patent Analysis
Sorbents, systems including sorbents, and methods using the sorbents
PatentPendingUS20240335784A1
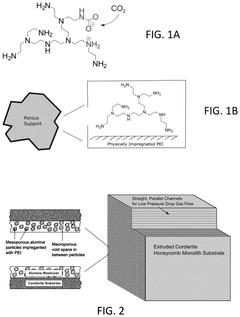
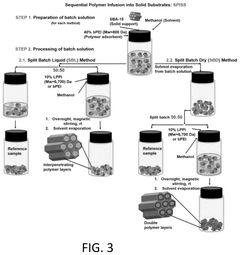
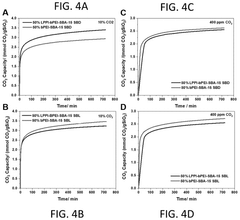
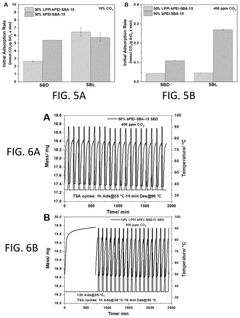
Innovation
- Development of sorbents comprising a CO2-philic phase with a combination of polypropylenimine and polyethylenimine, which provides improved oxidative stability and hydrophilicity, allowing for efficient CO2 capture and regeneration, and are integrated into a structured support for enhanced performance.
Shaped body for reversible chemisorption of co2
PatentWO2024256015A1
Innovation
- A sorbent comprising a shaped body made of amorphous silicon dioxide with dimensions between 0.5 mm to 30 mm, functionalized with a sorbent material, providing a compressive strength of at least 2 N/mm², and having a mesoporous structure with an irregular pore structure, which reduces pressure drop and enhances mechanical stability and sorption efficiency.
Regulatory Framework for Carbon Capture Implementation
The regulatory landscape for carbon capture technologies has evolved significantly over the past decade, creating both opportunities and challenges for material science innovations in CO₂ capture sorbent development. At the international level, the Paris Agreement has established a framework requiring signatory nations to implement carbon reduction strategies, indirectly stimulating research and deployment of advanced capture technologies. This global commitment has cascaded down to regional and national regulatory frameworks that increasingly incorporate specific provisions for carbon capture implementation.
In the United States, the 45Q tax credit has emerged as a pivotal regulatory mechanism, offering up to $50 per metric ton of CO₂ permanently sequestered. This financial incentive has catalyzed private sector investment in developing more efficient sorbent materials that can maximize capture rates while minimizing operational costs. Similarly, the European Union's Emissions Trading System (EU ETS) has created a market-based approach that assigns economic value to carbon reduction, further driving material science research toward commercially viable sorbent solutions.
Regulatory standards for capture efficiency have become increasingly stringent, with many jurisdictions now requiring minimum capture rates of 90% or higher for new installations. These performance requirements directly influence material science research priorities, pushing scientists toward developing sorbents with higher selectivity, capacity, and regeneration efficiency. The regulatory emphasis on operational safety has also shaped material development, necessitating thorough toxicological and environmental impact assessments for novel sorbent materials.
Permitting processes for carbon capture facilities have grown more complex, with regulatory bodies requiring comprehensive data on sorbent performance, degradation characteristics, and waste management protocols. This regulatory scrutiny has accelerated the development of more durable materials with longer operational lifespans and reduced environmental footprints. Additionally, cross-border carbon regulations have created compliance challenges that material scientists must address through standardized performance metrics and testing protocols.
Looking forward, emerging regulatory frameworks are increasingly incorporating lifecycle assessment requirements that evaluate the environmental impact of sorbent materials from production through disposal. This holistic regulatory approach is driving innovation toward bio-based and sustainable sorbent materials that minimize embedded carbon and environmental toxicity. As regulatory frameworks continue to evolve, they will likely place greater emphasis on circular economy principles, potentially mandating recyclability or biodegradability for next-generation carbon capture sorbents.
In the United States, the 45Q tax credit has emerged as a pivotal regulatory mechanism, offering up to $50 per metric ton of CO₂ permanently sequestered. This financial incentive has catalyzed private sector investment in developing more efficient sorbent materials that can maximize capture rates while minimizing operational costs. Similarly, the European Union's Emissions Trading System (EU ETS) has created a market-based approach that assigns economic value to carbon reduction, further driving material science research toward commercially viable sorbent solutions.
Regulatory standards for capture efficiency have become increasingly stringent, with many jurisdictions now requiring minimum capture rates of 90% or higher for new installations. These performance requirements directly influence material science research priorities, pushing scientists toward developing sorbents with higher selectivity, capacity, and regeneration efficiency. The regulatory emphasis on operational safety has also shaped material development, necessitating thorough toxicological and environmental impact assessments for novel sorbent materials.
Permitting processes for carbon capture facilities have grown more complex, with regulatory bodies requiring comprehensive data on sorbent performance, degradation characteristics, and waste management protocols. This regulatory scrutiny has accelerated the development of more durable materials with longer operational lifespans and reduced environmental footprints. Additionally, cross-border carbon regulations have created compliance challenges that material scientists must address through standardized performance metrics and testing protocols.
Looking forward, emerging regulatory frameworks are increasingly incorporating lifecycle assessment requirements that evaluate the environmental impact of sorbent materials from production through disposal. This holistic regulatory approach is driving innovation toward bio-based and sustainable sorbent materials that minimize embedded carbon and environmental toxicity. As regulatory frameworks continue to evolve, they will likely place greater emphasis on circular economy principles, potentially mandating recyclability or biodegradability for next-generation carbon capture sorbents.
Sustainability Impact Assessment
The environmental impact of CO₂ capture technologies extends far beyond their immediate carbon reduction capabilities. A comprehensive sustainability assessment reveals that material science innovations in sorbent development are creating cascading positive effects across multiple ecological dimensions.
Carbon capture sorbents, when developed with sustainability principles, demonstrate significant lifecycle benefits. Advanced materials such as metal-organic frameworks (MOFs) and engineered porous carbons show reduced environmental footprints compared to traditional amine-based sorbents. Recent lifecycle assessments indicate that next-generation sorbents can achieve up to 30% lower embodied carbon during manufacturing while maintaining or improving capture efficiency.
Water conservation represents another critical sustainability metric where material science advances are making substantial contributions. Novel hydrophobic sorbents minimize water consumption during the capture process, addressing a major criticism of first-generation technologies that required extensive cooling water. Biomimetic materials inspired by natural water-efficient systems have demonstrated particular promise, with some prototypes reducing operational water requirements by up to 40%.
Energy efficiency improvements directly translate to reduced indirect emissions from capture operations. The development of sorbents with lower regeneration energy requirements—particularly those utilizing phase-change mechanisms rather than thermal swing processes—has dramatically improved the net carbon benefit of capture systems. Materials exhibiting rapid sorption-desorption kinetics further enhance this efficiency by enabling faster processing cycles.
Land use considerations are increasingly incorporated into sorbent development pathways. Bio-derived capture materials sourced from agricultural waste streams represent a circular economy approach that avoids competition with food production while providing value-added applications for what would otherwise be waste material. This approach aligns with sustainable development goals by creating economic opportunities in agricultural communities.
Toxicity profiles of next-generation sorbents show marked improvements over conventional options. Silicon-based alternatives and carbon-derived materials typically exhibit lower ecotoxicity and reduced human health impacts compared to amine compounds. Material scientists are increasingly employing green chemistry principles during development, prioritizing non-toxic precursors and environmentally benign synthesis routes.
The sustainability benefits extend to economic dimensions as well. Longer-lasting, regenerable sorbents with enhanced cycling stability reduce replacement frequency and associated material consumption. Some advanced materials have demonstrated stability over thousands of capture-release cycles, representing a step-change improvement in resource efficiency compared to earlier generations requiring frequent replacement.
Carbon capture sorbents, when developed with sustainability principles, demonstrate significant lifecycle benefits. Advanced materials such as metal-organic frameworks (MOFs) and engineered porous carbons show reduced environmental footprints compared to traditional amine-based sorbents. Recent lifecycle assessments indicate that next-generation sorbents can achieve up to 30% lower embodied carbon during manufacturing while maintaining or improving capture efficiency.
Water conservation represents another critical sustainability metric where material science advances are making substantial contributions. Novel hydrophobic sorbents minimize water consumption during the capture process, addressing a major criticism of first-generation technologies that required extensive cooling water. Biomimetic materials inspired by natural water-efficient systems have demonstrated particular promise, with some prototypes reducing operational water requirements by up to 40%.
Energy efficiency improvements directly translate to reduced indirect emissions from capture operations. The development of sorbents with lower regeneration energy requirements—particularly those utilizing phase-change mechanisms rather than thermal swing processes—has dramatically improved the net carbon benefit of capture systems. Materials exhibiting rapid sorption-desorption kinetics further enhance this efficiency by enabling faster processing cycles.
Land use considerations are increasingly incorporated into sorbent development pathways. Bio-derived capture materials sourced from agricultural waste streams represent a circular economy approach that avoids competition with food production while providing value-added applications for what would otherwise be waste material. This approach aligns with sustainable development goals by creating economic opportunities in agricultural communities.
Toxicity profiles of next-generation sorbents show marked improvements over conventional options. Silicon-based alternatives and carbon-derived materials typically exhibit lower ecotoxicity and reduced human health impacts compared to amine compounds. Material scientists are increasingly employing green chemistry principles during development, prioritizing non-toxic precursors and environmentally benign synthesis routes.
The sustainability benefits extend to economic dimensions as well. Longer-lasting, regenerable sorbents with enhanced cycling stability reduce replacement frequency and associated material consumption. Some advanced materials have demonstrated stability over thousands of capture-release cycles, representing a step-change improvement in resource efficiency compared to earlier generations requiring frequent replacement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!