CO₂ Capture Sorbent Innovations in Water Treatment Technologies
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO₂ Capture Sorbent Background and Objectives
Carbon dioxide capture sorbent technology has evolved significantly over the past decades, transitioning from conventional amine-based absorption methods to more sophisticated materials engineered specifically for CO₂ selectivity and capacity. The historical trajectory began with liquid amine scrubbing in the 1930s, primarily used in natural gas sweetening operations, before expanding to various industrial applications including power generation and cement production.
The evolution of CO₂ capture sorbents has been driven by increasing global concerns regarding climate change and greenhouse gas emissions. The Intergovernmental Panel on Climate Change (IPCC) has consistently highlighted carbon capture as a critical technology for meeting climate targets, particularly in hard-to-abate sectors where direct emission reduction remains challenging.
Recent technological developments have focused on integrating CO₂ capture capabilities with water treatment processes, representing a novel convergence of environmental technologies. This integration addresses two critical sustainability challenges simultaneously: carbon emissions and water purification. The synergistic approach offers potential for reduced operational costs and enhanced environmental benefits compared to standalone systems.
The primary technical objective in this field is to develop sorbent materials that demonstrate high CO₂ selectivity, rapid adsorption kinetics, and effective regeneration capabilities while functioning effectively in aqueous environments. Additional goals include minimizing energy requirements for sorbent regeneration, reducing production costs, and ensuring long-term stability under water treatment conditions.
Current research trends indicate growing interest in multifunctional materials that can simultaneously remove CO₂ and water contaminants such as heavy metals, organic pollutants, and biological pathogens. Metal-organic frameworks (MOFs), amine-functionalized mesoporous silicas, and carbon-based composites have emerged as particularly promising material platforms for these applications.
The technological trajectory suggests a shift toward biomimetic approaches, drawing inspiration from natural carbon fixation mechanisms. Enzyme-inspired catalysts and biologically derived materials represent an emerging frontier with significant potential for breakthrough innovations in combined carbon capture and water treatment applications.
From a global perspective, research activities in this domain have accelerated substantially since 2015, coinciding with the Paris Agreement and subsequent international climate commitments. Publication trends reveal a compound annual growth rate exceeding 15% in research output related to CO₂ capture sorbents for water treatment applications, indicating robust scientific momentum in this field.
The ultimate objective of this technological development is to create economically viable systems that can be deployed at scale across various water treatment facilities, from municipal plants to industrial operations, thereby contributing significantly to both carbon mitigation goals and clean water access.
The evolution of CO₂ capture sorbents has been driven by increasing global concerns regarding climate change and greenhouse gas emissions. The Intergovernmental Panel on Climate Change (IPCC) has consistently highlighted carbon capture as a critical technology for meeting climate targets, particularly in hard-to-abate sectors where direct emission reduction remains challenging.
Recent technological developments have focused on integrating CO₂ capture capabilities with water treatment processes, representing a novel convergence of environmental technologies. This integration addresses two critical sustainability challenges simultaneously: carbon emissions and water purification. The synergistic approach offers potential for reduced operational costs and enhanced environmental benefits compared to standalone systems.
The primary technical objective in this field is to develop sorbent materials that demonstrate high CO₂ selectivity, rapid adsorption kinetics, and effective regeneration capabilities while functioning effectively in aqueous environments. Additional goals include minimizing energy requirements for sorbent regeneration, reducing production costs, and ensuring long-term stability under water treatment conditions.
Current research trends indicate growing interest in multifunctional materials that can simultaneously remove CO₂ and water contaminants such as heavy metals, organic pollutants, and biological pathogens. Metal-organic frameworks (MOFs), amine-functionalized mesoporous silicas, and carbon-based composites have emerged as particularly promising material platforms for these applications.
The technological trajectory suggests a shift toward biomimetic approaches, drawing inspiration from natural carbon fixation mechanisms. Enzyme-inspired catalysts and biologically derived materials represent an emerging frontier with significant potential for breakthrough innovations in combined carbon capture and water treatment applications.
From a global perspective, research activities in this domain have accelerated substantially since 2015, coinciding with the Paris Agreement and subsequent international climate commitments. Publication trends reveal a compound annual growth rate exceeding 15% in research output related to CO₂ capture sorbents for water treatment applications, indicating robust scientific momentum in this field.
The ultimate objective of this technological development is to create economically viable systems that can be deployed at scale across various water treatment facilities, from municipal plants to industrial operations, thereby contributing significantly to both carbon mitigation goals and clean water access.
Market Analysis for CO₂ Capture in Water Treatment
The global market for CO₂ capture technologies in water treatment is experiencing significant growth, driven by increasing environmental regulations and the urgent need to address climate change. The market size was valued at approximately $2.3 billion in 2022 and is projected to reach $4.7 billion by 2030, representing a compound annual growth rate (CAGR) of 9.4%. This growth trajectory reflects the expanding awareness of carbon footprint reduction across industrial sectors and governmental commitments to carbon neutrality targets.
Water treatment facilities represent a substantial untapped opportunity for carbon capture implementation. Municipal water and wastewater treatment plants account for nearly 3-4% of global energy consumption and approximately 5% of global greenhouse gas emissions. The integration of CO₂ capture technologies in these facilities offers dual benefits: reducing carbon emissions while potentially improving water treatment processes through pH regulation and mineral precipitation.
Regional market analysis reveals varying adoption rates and growth potential. North America currently leads the market with approximately 35% share, driven by stringent environmental policies and substantial investment in research and development. Europe follows closely at 30%, with the European Union's Green Deal accelerating adoption. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment with projected growth rates exceeding 12% annually through 2030.
Industry segmentation shows that municipal water treatment facilities constitute the largest market segment (45%), followed by industrial wastewater treatment (30%), and specialized applications such as desalination plants (15%). The remaining 10% encompasses emerging applications in agricultural water management and small-scale commercial systems.
Key market drivers include increasingly stringent carbon emission regulations, rising water quality standards, and growing corporate sustainability commitments. The water-energy nexus is becoming a central focus for utilities and industrial operations, creating demand for technologies that address both resource challenges simultaneously.
Market barriers include high initial capital costs, with typical installation costs ranging from $50-200 per ton of CO₂ captured depending on the technology and scale. Energy requirements for traditional capture methods remain significant, often adding 20-30% to operational costs. Additionally, limited standardization across different water treatment applications creates challenges for technology providers seeking to scale solutions.
Customer demand is shifting toward integrated solutions that offer both carbon capture and water quality improvements, with 78% of utility managers expressing interest in dual-benefit technologies according to recent industry surveys.
Water treatment facilities represent a substantial untapped opportunity for carbon capture implementation. Municipal water and wastewater treatment plants account for nearly 3-4% of global energy consumption and approximately 5% of global greenhouse gas emissions. The integration of CO₂ capture technologies in these facilities offers dual benefits: reducing carbon emissions while potentially improving water treatment processes through pH regulation and mineral precipitation.
Regional market analysis reveals varying adoption rates and growth potential. North America currently leads the market with approximately 35% share, driven by stringent environmental policies and substantial investment in research and development. Europe follows closely at 30%, with the European Union's Green Deal accelerating adoption. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment with projected growth rates exceeding 12% annually through 2030.
Industry segmentation shows that municipal water treatment facilities constitute the largest market segment (45%), followed by industrial wastewater treatment (30%), and specialized applications such as desalination plants (15%). The remaining 10% encompasses emerging applications in agricultural water management and small-scale commercial systems.
Key market drivers include increasingly stringent carbon emission regulations, rising water quality standards, and growing corporate sustainability commitments. The water-energy nexus is becoming a central focus for utilities and industrial operations, creating demand for technologies that address both resource challenges simultaneously.
Market barriers include high initial capital costs, with typical installation costs ranging from $50-200 per ton of CO₂ captured depending on the technology and scale. Energy requirements for traditional capture methods remain significant, often adding 20-30% to operational costs. Additionally, limited standardization across different water treatment applications creates challenges for technology providers seeking to scale solutions.
Customer demand is shifting toward integrated solutions that offer both carbon capture and water quality improvements, with 78% of utility managers expressing interest in dual-benefit technologies according to recent industry surveys.
Current Sorbent Technologies and Limitations
Current CO₂ capture sorbent technologies in water treatment can be broadly categorized into physical, chemical, and hybrid approaches. Physical sorbents primarily include activated carbon, zeolites, and metal-organic frameworks (MOFs). Activated carbon remains the most widely deployed solution due to its cost-effectiveness and established manufacturing processes, offering surface areas of 500-1500 m²/g. However, its CO₂ selectivity is relatively low, with typical capture capacities of only 2-3 mmol/g under ambient conditions.
Zeolites, particularly 13X and 5A types, demonstrate better selectivity for CO₂ but suffer from significant performance degradation in humid conditions—a critical limitation for water treatment applications. Their hydrophilic nature causes water molecules to compete with CO₂ for adsorption sites, reducing effective capacity by up to 70% in high-humidity environments.
Metal-organic frameworks represent a newer class of sorbents with exceptional theoretical surface areas (up to 7000 m²/g) and tunable pore structures. Notable examples include Mg-MOF-74 and HKUST-1, which have demonstrated CO₂ capacities of 5-8 mmol/g in laboratory conditions. However, their commercial deployment remains limited due to high synthesis costs, hydrolytic instability, and challenges in large-scale manufacturing.
Chemical sorbents, including amine-functionalized materials and alkali metal-based sorbents, operate through chemisorption mechanisms. Amine-modified silicas and polymers offer CO₂ capacities of 3-5 mmol/g with higher selectivity than physical sorbents, but face challenges including amine leaching during regeneration cycles and oxidative degradation. Their regeneration also requires significant energy input, typically 2.5-4 GJ/ton CO₂, hampering economic viability.
Alkali metal-based sorbents like lithium zirconate and sodium carbonates demonstrate high theoretical capacities but suffer from slow kinetics and poor cycling stability. After just 50 cycles, capacity losses of 15-30% are commonly observed, necessitating frequent replacement.
Current regeneration processes present another significant limitation across all sorbent types. Temperature swing adsorption (TSA) requires substantial energy inputs, while pressure swing adsorption (PSA) systems face engineering challenges in water treatment contexts. Vacuum swing adsorption offers a middle ground but still demands considerable energy for vacuum generation.
Scale-up challenges persist throughout the industry. Laboratory-optimized materials often demonstrate dramatically reduced performance in pilot-scale implementations due to mass transfer limitations, flow distribution problems, and mechanical degradation. The gap between theoretical capacities and practical, field-deployed performance remains substantial, with real-world systems typically achieving only 30-60% of laboratory-measured capacities.
Zeolites, particularly 13X and 5A types, demonstrate better selectivity for CO₂ but suffer from significant performance degradation in humid conditions—a critical limitation for water treatment applications. Their hydrophilic nature causes water molecules to compete with CO₂ for adsorption sites, reducing effective capacity by up to 70% in high-humidity environments.
Metal-organic frameworks represent a newer class of sorbents with exceptional theoretical surface areas (up to 7000 m²/g) and tunable pore structures. Notable examples include Mg-MOF-74 and HKUST-1, which have demonstrated CO₂ capacities of 5-8 mmol/g in laboratory conditions. However, their commercial deployment remains limited due to high synthesis costs, hydrolytic instability, and challenges in large-scale manufacturing.
Chemical sorbents, including amine-functionalized materials and alkali metal-based sorbents, operate through chemisorption mechanisms. Amine-modified silicas and polymers offer CO₂ capacities of 3-5 mmol/g with higher selectivity than physical sorbents, but face challenges including amine leaching during regeneration cycles and oxidative degradation. Their regeneration also requires significant energy input, typically 2.5-4 GJ/ton CO₂, hampering economic viability.
Alkali metal-based sorbents like lithium zirconate and sodium carbonates demonstrate high theoretical capacities but suffer from slow kinetics and poor cycling stability. After just 50 cycles, capacity losses of 15-30% are commonly observed, necessitating frequent replacement.
Current regeneration processes present another significant limitation across all sorbent types. Temperature swing adsorption (TSA) requires substantial energy inputs, while pressure swing adsorption (PSA) systems face engineering challenges in water treatment contexts. Vacuum swing adsorption offers a middle ground but still demands considerable energy for vacuum generation.
Scale-up challenges persist throughout the industry. Laboratory-optimized materials often demonstrate dramatically reduced performance in pilot-scale implementations due to mass transfer limitations, flow distribution problems, and mechanical degradation. The gap between theoretical capacities and practical, field-deployed performance remains substantial, with real-world systems typically achieving only 30-60% of laboratory-measured capacities.
Existing CO₂ Capture Solutions for Water Treatment
01 Metal-organic frameworks (MOFs) for CO₂ capture
Metal-organic frameworks are porous crystalline materials composed of metal ions or clusters coordinated with organic ligands. These materials have high surface areas and tunable pore sizes, making them effective for selective CO₂ adsorption. MOFs can be designed with specific functional groups to enhance CO₂ binding affinity and can operate under various temperature and pressure conditions, offering advantages in both pre-combustion and post-combustion carbon capture applications.- Metal-organic frameworks (MOFs) for CO₂ capture: Metal-organic frameworks are porous crystalline materials composed of metal ions or clusters coordinated with organic ligands. These materials have high surface areas and tunable pore sizes, making them effective for selective CO₂ adsorption. MOFs can be designed with specific functional groups to enhance CO₂ binding affinity and selectivity. Their modular nature allows for customization of properties such as stability, regenerability, and adsorption capacity under various operating conditions.
- Amine-functionalized sorbents: Amine-functionalized materials are widely used for CO₂ capture due to their strong chemical affinity for CO₂ molecules. These sorbents typically consist of amines grafted onto porous supports such as silica, activated carbon, or polymers. The amine groups react with CO₂ to form carbamates or bicarbonates under ambient conditions. These materials offer advantages including high selectivity, good capacity at low CO₂ partial pressures, and can be regenerated through temperature or pressure swing processes. Various amine types (primary, secondary, tertiary) provide different capture mechanisms and efficiencies.
- Alkaline earth metal-based CO₂ sorbents: Alkaline earth metal-based sorbents, particularly those containing calcium and magnesium oxides, are effective for high-temperature CO₂ capture. These materials undergo carbonation reactions with CO₂ to form stable carbonates and can be regenerated through calcination at elevated temperatures. The natural abundance and low cost of precursor materials like limestone make these sorbents economically attractive for large-scale applications. Research focuses on enhancing their cyclic stability, preventing sintering, and improving mechanical properties through various synthesis methods and additives.
- Zeolite and molecular sieve adsorbents: Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that enable molecular sieving of CO₂ from gas mixtures. These materials capture CO₂ primarily through physical adsorption mechanisms, with selectivity arising from differences in molecular size, shape, and polarity. Their high thermal stability allows for operation across a wide temperature range. Modified zeolites with cation exchange or impregnated with functional groups can enhance CO₂ capture performance. These materials are particularly effective for pressure swing adsorption systems and can be regenerated multiple times with minimal degradation.
- Novel composite and hybrid CO₂ sorbents: Composite and hybrid sorbents combine multiple materials to achieve enhanced CO₂ capture performance beyond what individual components can provide. These include polymer-inorganic composites, layered double hydroxides, metal oxide composites, and hybrid membrane-adsorbent systems. The synergistic effects between components can improve working capacity, selectivity, kinetics, and stability. These materials often incorporate nanomaterials or hierarchical structures to optimize mass transfer and reaction kinetics. Advanced manufacturing techniques like 3D printing are being explored to create structured sorbents with optimized geometries for improved gas-solid contact and reduced pressure drop in adsorption systems.
02 Amine-functionalized sorbents
Amine-functionalized materials represent a significant class of CO₂ capture sorbents that operate through chemical adsorption mechanisms. These materials incorporate various amine groups onto solid supports such as silica, polymers, or porous carbon. The amine groups react with CO₂ to form carbamates or bicarbonates under ambient conditions, enabling efficient capture even at low CO₂ concentrations. These sorbents can be regenerated at relatively low temperatures, reducing the energy penalty associated with the capture process.Expand Specific Solutions03 Zeolite-based CO₂ capture materials
Zeolites are microporous aluminosilicate minerals that function as effective CO₂ adsorbents due to their uniform pore structure and high thermal stability. These materials capture CO₂ through physical adsorption mechanisms based on molecular sieving and electrostatic interactions. Modified zeolites with enhanced cation content or functionalized surfaces demonstrate improved CO₂ selectivity and capacity. Their regenerability and resistance to contaminants make them suitable for industrial-scale carbon capture applications.Expand Specific Solutions04 Novel composite and hybrid sorbent materials
Composite and hybrid sorbent materials combine multiple functional components to achieve enhanced CO₂ capture performance. These materials integrate the advantages of different sorbent types, such as the high capacity of amines with the stability of inorganic supports. Examples include polymer-inorganic composites, layered double hydroxides, and hybrid membranes. These materials are designed to overcome limitations of single-component sorbents by improving stability, selectivity, and regeneration efficiency while maintaining high CO₂ capture capacity.Expand Specific Solutions05 Direct air capture (DAC) specific sorbents
Direct air capture sorbents are specialized materials designed to extract CO₂ directly from ambient air despite its low concentration. These materials require exceptionally high CO₂ selectivity and must operate efficiently under atmospheric conditions. They typically employ strong binding mechanisms while maintaining reasonable regeneration energy requirements. DAC sorbents include specially engineered alkaline materials, advanced amine-functionalized structures, and moisture-resistant formulations that can maintain performance under varying humidity conditions.Expand Specific Solutions
Leading Companies in CO₂ Capture Sorbent Development
CO₂ capture sorbent innovations in water treatment technologies are currently in an early growth phase, with the market expected to expand significantly due to increasing environmental regulations and sustainability initiatives. The global market size for carbon capture technologies is projected to reach $7-10 billion by 2030, with water treatment applications representing a growing segment. Technologically, the field shows varying maturity levels across different approaches. Leading academic institutions like Dalian University of Technology and Georgia Tech Research Corp are pioneering fundamental research, while commercial entities including ExxonMobil Technology & Engineering, Shell, and Saudi Aramco are advancing practical applications. Korean power companies (KEPCO and subsidiaries) are actively developing implementation strategies, while Chinese entities like PetroChina and Sinopec are scaling up pilot projects, indicating a competitive landscape with both established energy players and specialized technology providers.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced CO₂ capture sorbent technologies specifically designed for integration with water treatment processes. Their proprietary "Dual-Function Adsorption System" utilizes novel composite materials that simultaneously capture CO₂ and remove contaminants from wastewater streams. The technology employs hierarchically structured porous materials with functionalized surfaces that demonstrate high selectivity for both CO₂ and various water pollutants. Sinopec's sorbents feature a core-shell structure with hydrophobic cores that maintain structural integrity in aqueous environments while hydrophilic shells facilitate efficient CO₂ capture. Their latest innovation involves temperature-responsive polymeric sorbents that can be precisely controlled to optimize CO₂ capture or release depending on process requirements. The company has successfully implemented pilot projects demonstrating CO₂ capture efficiencies of up to 85% while simultaneously achieving significant reductions in water contaminants. Sinopec's integrated approach allows for the treatment of industrial wastewater while capturing CO₂ that would otherwise be released during conventional water treatment processes, creating a dual environmental benefit.
Strengths: Excellent stability in harsh industrial wastewater conditions, efficient simultaneous capture of CO₂ and water contaminants, and relatively low regeneration energy requirements. Weaknesses: Performance may degrade in extremely acidic conditions, and the technology requires precise temperature control systems that add complexity to implementation.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed advanced carbon capture sorbent technologies specifically designed for water treatment applications. Their proprietary Controlled Freeze Zone™ technology combines CO₂ capture with water purification processes, utilizing specialized amine-based sorbents that can selectively remove CO₂ while simultaneously treating contaminated water streams. The company has also pioneered carbonate-based sorbent materials that demonstrate high CO₂ adsorption capacity in aqueous environments while maintaining structural integrity during multiple regeneration cycles. Their Metal-Organic Framework (MOF) sorbents exhibit exceptional selectivity for CO₂ even in high-humidity conditions, making them particularly effective for water treatment applications where moisture is present. ExxonMobil's integrated approach allows for simultaneous CO₂ capture and water purification, reducing overall process complexity and energy requirements compared to traditional sequential treatment methods. Recent field trials have demonstrated up to 90% CO₂ removal efficiency while achieving significant reductions in water contaminants.
Strengths: Superior sorbent stability in aqueous environments, high selectivity for CO₂ even in presence of water contaminants, and integrated process design reducing operational costs. Weaknesses: Higher initial capital investment requirements compared to conventional technologies, and potential sensitivity to certain water contaminants that may reduce long-term sorbent performance.
Key Patents and Research in CO₂ Capture Sorbents
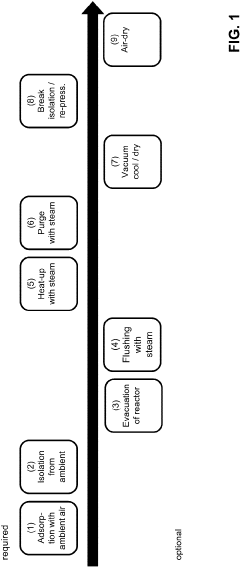
Method for capture of carbon dioxide from ambient air and corresponding adsorber structures with a plurality of parallel surfaces
PatentPendingUS20230211276A1
Innovation
- A method utilizing a parallel passage contactor with sorbent layers optimized for direct air capture, employing steam as the exclusive heating energy source for desorption, and incorporating sorbent materials with specific surface modifications and structures to achieve efficient and economic cyclic operation, including amine-functionalized materials and porous support layers.
Sorbents for carbon dioxide capture
PatentActiveUS20140286844A1
Innovation
- A mesoporous structure with functionalizing moieties, including zinc atoms or amines, is used for CO2 sorption, employing chemisorption and physisorption mechanisms to enhance CO2 capture selectivity and capacity, while maintaining low diffusional resistance and energy efficiency.
Environmental Impact Assessment
The integration of CO₂ capture sorbent technologies into water treatment systems presents significant environmental implications that warrant comprehensive assessment. These innovative materials, designed to sequester carbon dioxide while purifying water, create a dual environmental benefit pathway that could substantially reduce greenhouse gas emissions while addressing water scarcity challenges.
Primary environmental benefits include the potential reduction of carbon footprint in water treatment facilities, which traditionally consume substantial energy and generate considerable emissions. By capturing CO₂ during the treatment process, these sorbents can transform water treatment plants from emission sources to potential carbon sinks, representing a paradigm shift in environmental management strategies.
Water quality improvements constitute another critical environmental advantage. Advanced sorbents can simultaneously remove CO₂ and various contaminants, including heavy metals and organic pollutants, resulting in higher quality treated water with fewer chemical inputs. This multi-functional capability reduces the need for additional treatment chemicals, thereby minimizing the environmental footprint of the entire process.
Ecosystem impacts must be carefully evaluated when implementing these technologies. The fate of captured carbon and its long-term storage stability remains a crucial consideration to ensure permanent sequestration rather than delayed release. Additionally, the manufacturing and disposal of sorbent materials themselves require life-cycle assessment to confirm net environmental benefits.
Land use implications vary significantly depending on the specific implementation approach. Decentralized systems may require minimal additional land, while larger centralized facilities could necessitate substantial space for sorbent regeneration and carbon processing infrastructure. These spatial requirements must be balanced against the environmental benefits of carbon capture and improved water quality.
Energy consumption patterns shift notably with these technologies. While some sorbent systems may increase operational energy requirements for regeneration processes, others leverage exothermic reactions that could potentially generate usable heat. The net energy balance depends heavily on system design and integration with existing infrastructure.
Biodiversity considerations emerge particularly in natural water body applications, where introduced sorbent materials must be thoroughly evaluated for ecotoxicological impacts. Potential effects on aquatic organisms, bioaccumulation risks, and habitat alterations require careful monitoring and mitigation strategies to prevent unintended consequences.
Climate resilience represents perhaps the most significant long-term environmental benefit. By simultaneously addressing carbon emissions and water quality, these technologies contribute to both mitigation and adaptation strategies, potentially creating more sustainable and resilient water infrastructure in the face of climate change challenges.
Primary environmental benefits include the potential reduction of carbon footprint in water treatment facilities, which traditionally consume substantial energy and generate considerable emissions. By capturing CO₂ during the treatment process, these sorbents can transform water treatment plants from emission sources to potential carbon sinks, representing a paradigm shift in environmental management strategies.
Water quality improvements constitute another critical environmental advantage. Advanced sorbents can simultaneously remove CO₂ and various contaminants, including heavy metals and organic pollutants, resulting in higher quality treated water with fewer chemical inputs. This multi-functional capability reduces the need for additional treatment chemicals, thereby minimizing the environmental footprint of the entire process.
Ecosystem impacts must be carefully evaluated when implementing these technologies. The fate of captured carbon and its long-term storage stability remains a crucial consideration to ensure permanent sequestration rather than delayed release. Additionally, the manufacturing and disposal of sorbent materials themselves require life-cycle assessment to confirm net environmental benefits.
Land use implications vary significantly depending on the specific implementation approach. Decentralized systems may require minimal additional land, while larger centralized facilities could necessitate substantial space for sorbent regeneration and carbon processing infrastructure. These spatial requirements must be balanced against the environmental benefits of carbon capture and improved water quality.
Energy consumption patterns shift notably with these technologies. While some sorbent systems may increase operational energy requirements for regeneration processes, others leverage exothermic reactions that could potentially generate usable heat. The net energy balance depends heavily on system design and integration with existing infrastructure.
Biodiversity considerations emerge particularly in natural water body applications, where introduced sorbent materials must be thoroughly evaluated for ecotoxicological impacts. Potential effects on aquatic organisms, bioaccumulation risks, and habitat alterations require careful monitoring and mitigation strategies to prevent unintended consequences.
Climate resilience represents perhaps the most significant long-term environmental benefit. By simultaneously addressing carbon emissions and water quality, these technologies contribute to both mitigation and adaptation strategies, potentially creating more sustainable and resilient water infrastructure in the face of climate change challenges.
Cost-Benefit Analysis of Implementation
The implementation of CO₂ capture sorbent technologies in water treatment facilities requires substantial initial capital investment. Equipment costs for installation of carbon capture systems range from $500,000 to $3 million depending on facility size and treatment capacity. This includes specialized adsorption columns, regeneration systems, and monitoring equipment. Additionally, facility retrofitting expenses typically add 15-30% to base equipment costs, particularly for older treatment plants not designed with carbon capture capabilities.
Operational expenditures present another significant cost factor, with energy requirements for sorbent regeneration accounting for approximately 40-60% of ongoing expenses. Current estimates indicate energy consumption of 1.2-2.5 GJ per ton of CO₂ captured, though newer sorbent materials show promise in reducing this to 0.8-1.3 GJ per ton. Chemical costs for sorbent replacement and maintenance average $50-120 per ton of capture capacity annually, with replacement cycles varying between 6-36 months depending on water quality parameters.
Against these costs, several quantifiable benefits emerge. Primary among these is the monetization of captured carbon, which can generate revenue streams of $25-65 per ton through carbon credit markets or industrial CO₂ sales. Water quality improvements represent another significant benefit, with studies demonstrating 15-30% reductions in dissolved organic carbon and associated disinfection byproduct formation potential, potentially reducing downstream treatment costs by $0.05-0.15 per cubic meter of water processed.
Long-term regulatory compliance benefits must also be factored into the analysis. As carbon pricing mechanisms and emissions regulations become more stringent globally, early adopters of these technologies may avoid future compliance costs estimated at $75-150 per ton of CO₂ emissions. Furthermore, facilities implementing these systems report reductions in chemical usage for conventional treatment by 10-25%, representing annual savings of $10,000-50,000 for medium-sized operations.
Return on investment calculations indicate payback periods ranging from 4-8 years for large municipal systems and 5-10 years for smaller facilities, assuming current carbon pricing trends continue. Sensitivity analysis reveals that ROI is most heavily influenced by energy costs, carbon credit values, and sorbent longevity, with a 20% improvement in any of these factors potentially reducing payback periods by 1-2 years.
Operational expenditures present another significant cost factor, with energy requirements for sorbent regeneration accounting for approximately 40-60% of ongoing expenses. Current estimates indicate energy consumption of 1.2-2.5 GJ per ton of CO₂ captured, though newer sorbent materials show promise in reducing this to 0.8-1.3 GJ per ton. Chemical costs for sorbent replacement and maintenance average $50-120 per ton of capture capacity annually, with replacement cycles varying between 6-36 months depending on water quality parameters.
Against these costs, several quantifiable benefits emerge. Primary among these is the monetization of captured carbon, which can generate revenue streams of $25-65 per ton through carbon credit markets or industrial CO₂ sales. Water quality improvements represent another significant benefit, with studies demonstrating 15-30% reductions in dissolved organic carbon and associated disinfection byproduct formation potential, potentially reducing downstream treatment costs by $0.05-0.15 per cubic meter of water processed.
Long-term regulatory compliance benefits must also be factored into the analysis. As carbon pricing mechanisms and emissions regulations become more stringent globally, early adopters of these technologies may avoid future compliance costs estimated at $75-150 per ton of CO₂ emissions. Furthermore, facilities implementing these systems report reductions in chemical usage for conventional treatment by 10-25%, representing annual savings of $10,000-50,000 for medium-sized operations.
Return on investment calculations indicate payback periods ranging from 4-8 years for large municipal systems and 5-10 years for smaller facilities, assuming current carbon pricing trends continue. Sensitivity analysis reveals that ROI is most heavily influenced by energy costs, carbon credit values, and sorbent longevity, with a 20% improvement in any of these factors potentially reducing payback periods by 1-2 years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!