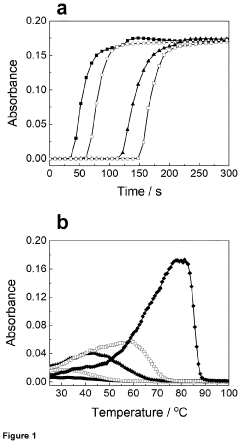
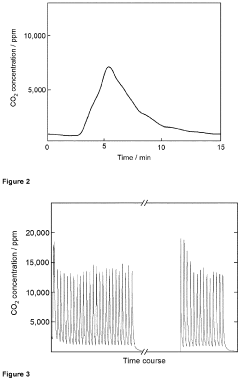
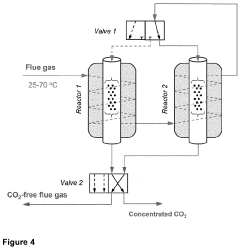
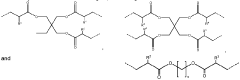
Comparative Study of CO₂ Capture Sorbent Materials
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO₂ Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past several decades, driven by increasing global concerns about climate change and greenhouse gas emissions. The journey began in the 1970s with basic absorption processes using aqueous amines, primarily developed for natural gas sweetening operations. These early technologies were not optimized for climate mitigation but laid important groundwork for future developments.
The 1990s marked a turning point as climate change gained international recognition, catalyzing research into more efficient CO₂ capture methods. This period saw the emergence of the first generation of purpose-designed carbon capture systems, still largely based on liquid solvents but with improved energy efficiency and capture rates.
By the early 2000s, research expanded beyond liquid solvents to include solid sorbent materials, which offered potential advantages in energy requirements and operational flexibility. This diversification of capture approaches represented a critical evolution in the field, opening new pathways for technological advancement.
The past decade has witnessed an acceleration in sorbent material development, with significant breakthroughs in metal-organic frameworks (MOFs), zeolites, activated carbons, and amine-functionalized silicas. These materials have demonstrated increasingly impressive CO₂ selectivity, capacity, and regeneration characteristics, though each with distinct advantages and limitations.
Current technological objectives in CO₂ sorbent development focus on several key parameters. First is increasing CO₂ selectivity, particularly in flue gas environments where nitrogen and other gases predominate. Second is enhancing sorption capacity to maximize the amount of CO₂ captured per unit of sorbent material. Third is improving regeneration efficiency to reduce the energy penalty associated with sorbent regeneration.
Additional objectives include developing materials with long-term stability under industrial conditions, resistance to contaminants like SOx and NOx, and cost-effectiveness at commercial scale. Researchers are also pursuing sorbents capable of functioning across diverse capture scenarios, from post-combustion flue gases to direct air capture applications.
The evolution trajectory suggests movement toward multi-functional sorbent materials that combine high selectivity with rapid kinetics and low regeneration energy. Hybrid approaches that leverage the strengths of different material classes are gaining traction, as are advanced manufacturing techniques that enable precise control over material properties at the nanoscale.
The 1990s marked a turning point as climate change gained international recognition, catalyzing research into more efficient CO₂ capture methods. This period saw the emergence of the first generation of purpose-designed carbon capture systems, still largely based on liquid solvents but with improved energy efficiency and capture rates.
By the early 2000s, research expanded beyond liquid solvents to include solid sorbent materials, which offered potential advantages in energy requirements and operational flexibility. This diversification of capture approaches represented a critical evolution in the field, opening new pathways for technological advancement.
The past decade has witnessed an acceleration in sorbent material development, with significant breakthroughs in metal-organic frameworks (MOFs), zeolites, activated carbons, and amine-functionalized silicas. These materials have demonstrated increasingly impressive CO₂ selectivity, capacity, and regeneration characteristics, though each with distinct advantages and limitations.
Current technological objectives in CO₂ sorbent development focus on several key parameters. First is increasing CO₂ selectivity, particularly in flue gas environments where nitrogen and other gases predominate. Second is enhancing sorption capacity to maximize the amount of CO₂ captured per unit of sorbent material. Third is improving regeneration efficiency to reduce the energy penalty associated with sorbent regeneration.
Additional objectives include developing materials with long-term stability under industrial conditions, resistance to contaminants like SOx and NOx, and cost-effectiveness at commercial scale. Researchers are also pursuing sorbents capable of functioning across diverse capture scenarios, from post-combustion flue gases to direct air capture applications.
The evolution trajectory suggests movement toward multi-functional sorbent materials that combine high selectivity with rapid kinetics and low regeneration energy. Hybrid approaches that leverage the strengths of different material classes are gaining traction, as are advanced manufacturing techniques that enable precise control over material properties at the nanoscale.
Market Analysis for Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.5 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade. This growth trajectory is supported by substantial government investments, with the US Inflation Reduction Act allocating $369 billion toward climate initiatives, including carbon capture technologies.
The demand for CO₂ capture sorbent materials is particularly strong in power generation, cement production, and chemical manufacturing sectors, which collectively account for over 60% of global industrial carbon emissions. Enhanced oil recovery (EOR) currently represents the largest commercial application for captured carbon, though dedicated geological storage is gaining traction as carbon pricing mechanisms mature in various markets.
Regional analysis reveals that North America leads the carbon capture market with approximately 38% market share, followed by Europe at 29% and Asia-Pacific at 24%. China's recent policy shifts toward carbon neutrality by 2060 have accelerated investments in capture technologies, with over 40 large-scale projects announced since 2021.
The economic viability of carbon capture solutions varies significantly by industry and capture method. Current cost ranges for post-combustion capture using various sorbent materials span from $40-120 per ton of CO₂, with amine-based solutions representing the most commercially deployed technology despite their energy penalties. Emerging solid sorbents and membrane technologies are demonstrating potential cost reductions of 30-50% in pilot applications.
Market adoption faces several barriers, including high capital expenditure requirements, uncertain regulatory frameworks, and limited carbon utilization pathways. However, the implementation of carbon pricing mechanisms in 46 countries has begun creating economic incentives for capture technologies. The voluntary carbon market's growth, reaching $2 billion in 2022, provides additional revenue streams for capture projects.
Customer segmentation reveals three primary buyer categories: compliance-driven industries seeking to meet regulatory requirements, strategic adopters positioning for competitive advantage in a carbon-constrained economy, and innovation partners focused on developing next-generation capture technologies. Each segment demonstrates different price sensitivity and performance requirements for sorbent materials, influencing product development priorities.
The demand for CO₂ capture sorbent materials is particularly strong in power generation, cement production, and chemical manufacturing sectors, which collectively account for over 60% of global industrial carbon emissions. Enhanced oil recovery (EOR) currently represents the largest commercial application for captured carbon, though dedicated geological storage is gaining traction as carbon pricing mechanisms mature in various markets.
Regional analysis reveals that North America leads the carbon capture market with approximately 38% market share, followed by Europe at 29% and Asia-Pacific at 24%. China's recent policy shifts toward carbon neutrality by 2060 have accelerated investments in capture technologies, with over 40 large-scale projects announced since 2021.
The economic viability of carbon capture solutions varies significantly by industry and capture method. Current cost ranges for post-combustion capture using various sorbent materials span from $40-120 per ton of CO₂, with amine-based solutions representing the most commercially deployed technology despite their energy penalties. Emerging solid sorbents and membrane technologies are demonstrating potential cost reductions of 30-50% in pilot applications.
Market adoption faces several barriers, including high capital expenditure requirements, uncertain regulatory frameworks, and limited carbon utilization pathways. However, the implementation of carbon pricing mechanisms in 46 countries has begun creating economic incentives for capture technologies. The voluntary carbon market's growth, reaching $2 billion in 2022, provides additional revenue streams for capture projects.
Customer segmentation reveals three primary buyer categories: compliance-driven industries seeking to meet regulatory requirements, strategic adopters positioning for competitive advantage in a carbon-constrained economy, and innovation partners focused on developing next-generation capture technologies. Each segment demonstrates different price sensitivity and performance requirements for sorbent materials, influencing product development priorities.
Current Sorbent Materials Landscape and Challenges
The current landscape of CO₂ capture sorbent materials is characterized by a diverse array of options, each with distinct advantages and limitations. Amine-based sorbents, particularly monoethanolamine (MEA), have dominated industrial applications due to their high CO₂ selectivity and established operational protocols. However, these materials suffer from significant energy penalties during regeneration, corrosion issues, and degradation over multiple capture-release cycles, limiting their long-term economic viability.
Metal-organic frameworks (MOFs) represent a promising alternative with exceptional surface areas exceeding 7,000 m²/g and highly tunable pore structures. Notable examples include Mg-MOF-74 and HKUST-1, which demonstrate remarkable CO₂ uptake capacities. Despite these advantages, MOFs face challenges in moisture stability, manufacturing scalability, and production costs that currently restrict their widespread industrial deployment.
Zeolites, with their well-defined microporous structures, offer another viable option for CO₂ capture. Materials such as 13X and 5A zeolites exhibit good selectivity and thermal stability. Their primary limitations include reduced performance in humid conditions and relatively lower CO₂ capacities compared to newer materials, necessitating larger equipment footprints for industrial applications.
Activated carbons present a cost-effective solution with excellent thermal stability and hydrophobicity. While their CO₂ adsorption capacity is moderate, their low regeneration energy requirements and resistance to contaminants make them particularly suitable for flue gas applications. However, their relatively low selectivity for CO₂ over N₂ remains a significant challenge.
Recent developments in hybrid materials and composites aim to overcome the limitations of individual sorbent types. Amine-functionalized silicas combine the high selectivity of amines with the structural stability of inorganic supports. Similarly, amine-grafted MOFs seek to enhance selectivity while maintaining high surface areas. These hybrid approaches show promise but face integration challenges and increased manufacturing complexity.
The geographical distribution of sorbent material development shows concentration in North America, Europe, and East Asia, with the United States, Germany, China, and Japan leading research efforts. This distribution reflects both historical expertise in chemical engineering and current environmental policy priorities.
A critical challenge across all sorbent materials is the trade-off between adsorption capacity, selectivity, and regeneration energy. Materials with high CO₂ affinity typically require more energy for regeneration, creating an optimization challenge that has yet to be fully resolved. Additionally, the stability of materials under real-world conditions—including the presence of SOx, NOx, and water vapor—remains a significant hurdle for widespread implementation.
Metal-organic frameworks (MOFs) represent a promising alternative with exceptional surface areas exceeding 7,000 m²/g and highly tunable pore structures. Notable examples include Mg-MOF-74 and HKUST-1, which demonstrate remarkable CO₂ uptake capacities. Despite these advantages, MOFs face challenges in moisture stability, manufacturing scalability, and production costs that currently restrict their widespread industrial deployment.
Zeolites, with their well-defined microporous structures, offer another viable option for CO₂ capture. Materials such as 13X and 5A zeolites exhibit good selectivity and thermal stability. Their primary limitations include reduced performance in humid conditions and relatively lower CO₂ capacities compared to newer materials, necessitating larger equipment footprints for industrial applications.
Activated carbons present a cost-effective solution with excellent thermal stability and hydrophobicity. While their CO₂ adsorption capacity is moderate, their low regeneration energy requirements and resistance to contaminants make them particularly suitable for flue gas applications. However, their relatively low selectivity for CO₂ over N₂ remains a significant challenge.
Recent developments in hybrid materials and composites aim to overcome the limitations of individual sorbent types. Amine-functionalized silicas combine the high selectivity of amines with the structural stability of inorganic supports. Similarly, amine-grafted MOFs seek to enhance selectivity while maintaining high surface areas. These hybrid approaches show promise but face integration challenges and increased manufacturing complexity.
The geographical distribution of sorbent material development shows concentration in North America, Europe, and East Asia, with the United States, Germany, China, and Japan leading research efforts. This distribution reflects both historical expertise in chemical engineering and current environmental policy priorities.
A critical challenge across all sorbent materials is the trade-off between adsorption capacity, selectivity, and regeneration energy. Materials with high CO₂ affinity typically require more energy for regeneration, creating an optimization challenge that has yet to be fully resolved. Additionally, the stability of materials under real-world conditions—including the presence of SOx, NOx, and water vapor—remains a significant hurdle for widespread implementation.
Comparative Analysis of Current Sorbent Technologies
01 Metal-organic frameworks (MOFs) for CO₂ capture
Metal-organic frameworks are porous crystalline materials composed of metal ions or clusters coordinated with organic ligands. These materials have high surface areas and tunable pore sizes, making them effective for selective CO₂ adsorption. MOFs can be designed with specific functional groups to enhance CO₂ binding affinity and selectivity over other gases. Their modular nature allows for customization of properties such as thermal stability and regeneration capacity, which are crucial for practical carbon capture applications.- Metal-organic frameworks (MOFs) for CO₂ capture: Metal-organic frameworks are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. These materials have high surface areas and tunable pore sizes, making them effective for selective CO₂ adsorption. MOFs can be designed with specific functional groups to enhance CO₂ binding affinity and can operate under various temperature and pressure conditions, offering promising solutions for carbon capture applications.
- Amine-functionalized sorbents: Amine-functionalized materials represent a significant class of CO₂ capture sorbents that operate through chemical adsorption mechanisms. These materials incorporate various amine groups that react with CO₂ to form carbamates or carbonates. Common supports include silica, polymers, and porous carbon structures that are modified with amines to enhance CO₂ selectivity and capacity. These sorbents typically offer good performance at low CO₂ partial pressures, making them suitable for direct air capture applications.
- Zeolite and molecular sieve-based CO₂ adsorbents: Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that enable molecular sieving effects for CO₂ separation. These materials can be modified with cations to enhance CO₂ adsorption capacity and selectivity. Their thermal stability allows for multiple adsorption-desorption cycles, making them suitable for pressure swing adsorption (PSA) and temperature swing adsorption (TSA) processes in industrial carbon capture applications.
- Carbon-based sorbent materials: Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, serve as effective CO₂ adsorbents due to their high surface area and porous structure. These materials can be functionalized with nitrogen, oxygen, or metal groups to enhance CO₂ selectivity. Carbon-based sorbents offer advantages such as low cost, good stability, and the potential for sustainable production from biomass or waste sources, making them attractive for large-scale carbon capture applications.
- Regenerable alkaline earth metal-based CO₂ sorbents: Alkaline earth metal-based sorbents, particularly those containing calcium and magnesium oxides, capture CO₂ through carbonation reactions to form stable carbonates. These materials can be regenerated through calcination at elevated temperatures, releasing concentrated CO₂ streams. Recent developments focus on enhancing the cyclic stability and mechanical strength of these sorbents by incorporating support materials or dopants. These systems are particularly suitable for high-temperature carbon capture applications such as pre-combustion capture or direct integration with industrial processes.
02 Amine-functionalized sorbents
Amine-functionalized materials represent a significant class of CO₂ capture sorbents that operate through chemical adsorption mechanisms. These materials incorporate various amine groups that react with CO₂ to form carbamates or bicarbonates. Common supports include silica, polymers, and porous carbon materials that are modified with amines to enhance CO₂ capture capacity. These sorbents typically offer high selectivity for CO₂ over other gases and can operate effectively at moderate temperatures, though they may require higher regeneration energy compared to physical adsorbents.Expand Specific Solutions03 Zeolite and molecular sieve-based materials
Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that enable molecular sieving of CO₂ from gas mixtures. These materials can be tailored by adjusting the silicon-to-aluminum ratio and incorporating various cations to optimize CO₂ adsorption properties. Their high thermal stability allows for multiple adsorption-desorption cycles without significant degradation. Zeolites typically operate through physisorption mechanisms and can be effective in pressure swing adsorption systems for carbon capture applications.Expand Specific Solutions04 Carbon-based sorbent materials
Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, offer promising CO₂ capture capabilities due to their high surface areas and tunable pore structures. These materials can be functionalized with nitrogen-containing groups or metal particles to enhance CO₂ adsorption capacity and selectivity. Carbon-based sorbents typically demonstrate good mechanical stability, relatively low regeneration energy requirements, and potential cost advantages compared to other sorbent types. Their production can also incorporate sustainable practices by using biomass or waste materials as precursors.Expand Specific Solutions05 Hybrid and composite sorbent systems
Hybrid and composite sorbent systems combine multiple materials to leverage complementary properties for enhanced CO₂ capture performance. These may include combinations of organic and inorganic components, such as polymer-inorganic composites or mixed matrix materials. By integrating different capture mechanisms (physical and chemical adsorption), these systems can achieve improved adsorption capacity, selectivity, and stability. Composite materials often demonstrate synergistic effects that overcome limitations of individual components, such as enhanced kinetics, reduced regeneration energy, or improved mechanical properties for practical carbon capture applications.Expand Specific Solutions
Leading Organizations in Carbon Capture Research
The CO₂ capture sorbent materials market is currently in a growth phase, with increasing global focus on carbon reduction technologies driving market expansion estimated to reach $2.5 billion by 2025. The competitive landscape features diverse players across academia and industry, with research institutions like King Fahd University, Rice University, and University of California advancing fundamental research, while commercial entities demonstrate varying technology maturity levels. Climeworks leads in direct air capture commercialization with operational plants in Iceland, while established industrial players like Cabot Corp., Corning, and Sinopec leverage their manufacturing expertise to scale carbon capture solutions. Emerging companies like Susteon and Global Thermostat are developing novel sorbent technologies, creating a dynamic ecosystem where collaboration between research institutions and industry partners is accelerating technology development toward commercial viability.
Climeworks AG
Technical Solution: Climeworks has developed a Direct Air Capture (DAC) technology using solid sorbent materials to capture CO₂ directly from ambient air. Their approach employs amine-functionalized filter materials arranged in modular air collectors. The process works by drawing air into the collector with a fan, where CO₂ binds to the sorbent material. Once saturated, the collector is closed and heated to 80-100°C, releasing concentrated CO₂ that can be collected for storage or utilization. Climeworks' technology operates in a cyclical process where each module alternates between adsorption and regeneration phases. Their commercial plants (like Orca in Iceland) combine this capture technology with geothermal energy for low-carbon operation and permanent storage through mineralization in basaltic rock formations. The company has progressively scaled its technology from laboratory to industrial scale, with multiple plants now operational across Europe.
Strengths: Modular design allows for flexible deployment and scaling; direct integration with geological storage for permanent sequestration; proven commercial implementation with operational plants. Weaknesses: Relatively high energy requirements for the thermal regeneration process; higher cost per ton of CO₂ captured compared to point-source capture; limited by geographical constraints for optimal renewable energy access and storage options.
Cabot Corp.
Technical Solution: Cabot Corporation has developed specialized activated carbon and mesoporous carbon materials as CO₂ capture sorbents. Their technology leverages their expertise in carbon black and specialty carbon materials manufacturing to create tailored porous structures with high surface areas (>1000 m²/g) and controlled pore size distributions. Cabot's approach involves surface modification of carbon materials with nitrogen-containing functional groups and metal oxide nanoparticles to enhance CO₂ selectivity and adsorption capacity. Their DARCO® activated carbon series has been adapted specifically for gas phase applications including CO₂ capture, with reported adsorption capacities of 2-4 mmol/g under ambient conditions. The company has also developed composite materials combining their carbon substrates with amine-containing polymers to create hybrid physical-chemical adsorption mechanisms. These materials operate in pressure swing adsorption (PSA) systems where CO₂ is captured at higher pressures and released during a low-pressure regeneration phase. Cabot has demonstrated the scalability of their materials through pilot projects in industrial settings, focusing particularly on natural gas purification and biogas upgrading applications where their hydrophobic carbon materials offer advantages in humid conditions.
Strengths: Excellent hydrophobicity making the sorbents suitable for humid gas streams; established large-scale manufacturing capabilities; relatively low regeneration energy requirements compared to amine-based systems; good mechanical stability and attrition resistance. Weaknesses: Generally lower CO₂ capacity compared to amine-functionalized materials at ambient conditions; better suited for higher CO₂ concentration streams rather than direct air capture; pressure swing regeneration requires compression energy.
Key Innovations in Sorbent Material Design
Sorbent materials for carbon dioxide separation
PatentInactiveEP4289503A1
Innovation
- The use of cross-linked and substituted branched polyethylenimine sorbent materials with specific linker moieties and substituents, allowing for isothermal CO2 separation at temperatures between ambient and 70°C, reducing energy input and costs.
Sorbent materials for carbon dioxide separation
PatentWO2023237579A1
Innovation
- A sorbent material comprising cross-linked and substituted branched polyethylenimine with specific molecular weight and substitution degrees, allowing for isothermal CO2 separation at temperatures between ambient and 70 °C, reducing energy consumption and costs.
Environmental Impact Assessment of Sorbent Materials
The environmental impact of CO₂ capture sorbent materials extends far beyond their primary function of carbon sequestration. When evaluating these materials, a comprehensive life cycle assessment (LCA) becomes essential to understand their true environmental footprint. Different sorbent classes—including amine-based materials, metal-organic frameworks (MOFs), zeolites, and activated carbons—exhibit varying environmental profiles throughout their production, use, and disposal phases.
Production of amine-based sorbents often involves energy-intensive chemical synthesis processes that generate significant greenhouse gas emissions. Studies indicate that manufacturing one ton of polyethylenimine (PEI)-based sorbents can produce 2-5 tons of CO₂ equivalent emissions, potentially offsetting initial carbon capture benefits. Additionally, the production of these materials frequently requires toxic solvents and precursors that pose risks to aquatic ecosystems if improperly managed.
MOFs present a different environmental challenge. While their highly ordered crystalline structures offer exceptional CO₂ selectivity, their synthesis typically requires rare metals and organic linkers that involve environmentally problematic mining operations and chemical manufacturing. Recent research has highlighted concerns regarding metal leaching from spent MOFs, potentially introducing heavy metal contamination into disposal environments.
Water consumption represents another critical environmental consideration. Zeolite production demands substantial water resources for hydrothermal synthesis and activation processes, with estimates suggesting 50-100 cubic meters of water per ton of material produced. This raises sustainability concerns in water-stressed regions where large-scale sorbent manufacturing might be implemented.
Land use impacts vary significantly between sorbent types. Activated carbon derived from biomass sources can promote sustainable forestry practices when properly managed, but may contribute to deforestation and habitat loss if sourcing protocols are inadequate. Conversely, synthetic sorbents avoid direct land use impacts but rely on petrochemical feedstocks with their associated environmental burdens.
Regeneration energy requirements constitute perhaps the most significant environmental factor for operational sorbents. Temperature swing adsorption (TSA) processes for amine-based materials typically demand 2.5-4 GJ of energy per ton of CO₂ captured, while pressure swing approaches for physical sorbents like zeolites require 1.5-2.5 GJ per ton. These energy demands directly translate to environmental impacts unless renewable energy sources are employed.
End-of-life considerations reveal that most current sorbent materials lack established recycling pathways. Spent materials often contain contaminants that complicate disposal, potentially requiring specialized treatment as hazardous waste. Emerging research into biodegradable sorbent platforms and circular economy approaches shows promise for reducing these terminal environmental impacts, though commercial implementation remains limited.
Production of amine-based sorbents often involves energy-intensive chemical synthesis processes that generate significant greenhouse gas emissions. Studies indicate that manufacturing one ton of polyethylenimine (PEI)-based sorbents can produce 2-5 tons of CO₂ equivalent emissions, potentially offsetting initial carbon capture benefits. Additionally, the production of these materials frequently requires toxic solvents and precursors that pose risks to aquatic ecosystems if improperly managed.
MOFs present a different environmental challenge. While their highly ordered crystalline structures offer exceptional CO₂ selectivity, their synthesis typically requires rare metals and organic linkers that involve environmentally problematic mining operations and chemical manufacturing. Recent research has highlighted concerns regarding metal leaching from spent MOFs, potentially introducing heavy metal contamination into disposal environments.
Water consumption represents another critical environmental consideration. Zeolite production demands substantial water resources for hydrothermal synthesis and activation processes, with estimates suggesting 50-100 cubic meters of water per ton of material produced. This raises sustainability concerns in water-stressed regions where large-scale sorbent manufacturing might be implemented.
Land use impacts vary significantly between sorbent types. Activated carbon derived from biomass sources can promote sustainable forestry practices when properly managed, but may contribute to deforestation and habitat loss if sourcing protocols are inadequate. Conversely, synthetic sorbents avoid direct land use impacts but rely on petrochemical feedstocks with their associated environmental burdens.
Regeneration energy requirements constitute perhaps the most significant environmental factor for operational sorbents. Temperature swing adsorption (TSA) processes for amine-based materials typically demand 2.5-4 GJ of energy per ton of CO₂ captured, while pressure swing approaches for physical sorbents like zeolites require 1.5-2.5 GJ per ton. These energy demands directly translate to environmental impacts unless renewable energy sources are employed.
End-of-life considerations reveal that most current sorbent materials lack established recycling pathways. Spent materials often contain contaminants that complicate disposal, potentially requiring specialized treatment as hazardous waste. Emerging research into biodegradable sorbent platforms and circular economy approaches shows promise for reducing these terminal environmental impacts, though commercial implementation remains limited.
Techno-Economic Analysis of Sorbent Implementation
The implementation of CO₂ capture sorbent materials requires thorough techno-economic analysis to determine viability across different industrial applications. Current economic assessments indicate that amine-based sorbents generally present the lowest initial capital investment but often incur higher operational costs due to energy-intensive regeneration processes and material degradation over time.
Metal-organic frameworks (MOFs), while demonstrating superior CO₂ selectivity and capacity, currently face economic barriers with production costs ranging from $100-1000/kg compared to $2-5/kg for conventional amine sorbents. However, economies of scale and manufacturing innovations could potentially reduce MOF costs by 60-80% within the next decade, making them increasingly competitive.
Energy consumption analysis reveals that zeolites typically require 2.5-3.5 GJ/ton CO₂ captured, while advanced amine-functionalized silica materials can achieve efficiencies of 1.8-2.2 GJ/ton CO₂. This energy requirement directly impacts operational expenditure, with current estimates suggesting carbon capture costs of $40-80/ton CO₂ depending on sorbent selection and process optimization.
Lifecycle assessment of various sorbents indicates significant differences in environmental impact. Activated carbon-based sorbents demonstrate the lowest production footprint but moderate capture efficiency, while engineered nanomaterials offer higher performance at the cost of increased embodied energy. The environmental payback period ranges from 0.5-3 years depending on sorbent durability and regeneration requirements.
Scalability considerations favor traditional sorbents like zeolites and activated carbons due to established production infrastructure. However, hybrid materials combining conventional supports with advanced functionalization present promising economic profiles by balancing performance with cost-effectiveness. Recent pilot implementations suggest hybrid sorbents can reduce capture costs by 15-30% compared to single-technology approaches.
Sensitivity analysis of economic factors reveals that sorbent lifetime and regeneration energy requirements are the most critical parameters affecting long-term economic viability. Materials demonstrating stability over 1000+ cycles show significantly improved lifetime costs despite higher initial investment. For example, hydrothermally stable MOFs with maintained performance over extended cycling could achieve cost parity with conventional technologies within 3-5 years of operation.
Integration costs with existing industrial infrastructure vary substantially between sorbent technologies, with retrofit compatibility being a key economic consideration. Modular systems utilizing structured sorbents typically demonstrate 20-40% lower installation costs compared to traditional packed bed configurations, potentially offsetting higher material expenses.
Metal-organic frameworks (MOFs), while demonstrating superior CO₂ selectivity and capacity, currently face economic barriers with production costs ranging from $100-1000/kg compared to $2-5/kg for conventional amine sorbents. However, economies of scale and manufacturing innovations could potentially reduce MOF costs by 60-80% within the next decade, making them increasingly competitive.
Energy consumption analysis reveals that zeolites typically require 2.5-3.5 GJ/ton CO₂ captured, while advanced amine-functionalized silica materials can achieve efficiencies of 1.8-2.2 GJ/ton CO₂. This energy requirement directly impacts operational expenditure, with current estimates suggesting carbon capture costs of $40-80/ton CO₂ depending on sorbent selection and process optimization.
Lifecycle assessment of various sorbents indicates significant differences in environmental impact. Activated carbon-based sorbents demonstrate the lowest production footprint but moderate capture efficiency, while engineered nanomaterials offer higher performance at the cost of increased embodied energy. The environmental payback period ranges from 0.5-3 years depending on sorbent durability and regeneration requirements.
Scalability considerations favor traditional sorbents like zeolites and activated carbons due to established production infrastructure. However, hybrid materials combining conventional supports with advanced functionalization present promising economic profiles by balancing performance with cost-effectiveness. Recent pilot implementations suggest hybrid sorbents can reduce capture costs by 15-30% compared to single-technology approaches.
Sensitivity analysis of economic factors reveals that sorbent lifetime and regeneration energy requirements are the most critical parameters affecting long-term economic viability. Materials demonstrating stability over 1000+ cycles show significantly improved lifetime costs despite higher initial investment. For example, hydrothermally stable MOFs with maintained performance over extended cycling could achieve cost parity with conventional technologies within 3-5 years of operation.
Integration costs with existing industrial infrastructure vary substantially between sorbent technologies, with retrofit compatibility being a key economic consideration. Modular systems utilizing structured sorbents typically demonstrate 20-40% lower installation costs compared to traditional packed bed configurations, potentially offsetting higher material expenses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!