Comparative Analysis of CO₂ Capture Sorbent Reactivity
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO₂ Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past several decades, driven by increasing global concerns about climate change and greenhouse gas emissions. The journey began in the 1970s with basic absorption processes using amines, primarily developed for natural gas sweetening rather than climate mitigation. By the 1990s, as climate science advanced, these technologies were adapted specifically for CO₂ capture from power plants and industrial facilities, marking the first generation of purposeful carbon capture systems.
The early 2000s witnessed the emergence of second-generation technologies, including advanced solvents with improved energy efficiency and novel materials like metal-organic frameworks (MOFs) and zeolites. This period also saw the first large-scale demonstration projects, providing valuable operational data while highlighting significant challenges in energy penalties and economic viability.
The current third generation of technologies, developing since approximately 2010, has focused on transformative approaches including direct air capture (DAC), membrane-based systems, and advanced solid sorbents with enhanced CO₂ selectivity and reduced regeneration energy requirements. These innovations represent a paradigm shift from treating carbon capture as an end-of-pipe solution to integrating it into comprehensive carbon management strategies.
The evolution of CO₂ capture sorbent technology specifically has progressed from simple liquid amine scrubbers to sophisticated engineered materials with precisely controlled physical and chemical properties. Modern sorbents are designed at the molecular level to optimize binding energy, selectivity, and regeneration characteristics. This progression reflects broader technological trends toward materials science innovation and process intensification.
The primary objectives of current CO₂ capture technology development center on several key parameters: reducing energy penalties associated with sorbent regeneration, improving sorbent durability through multiple capture-release cycles, enhancing CO₂ selectivity in mixed gas environments, and dramatically reducing overall system costs. These objectives are driven by the recognition that widespread deployment requires economic viability without substantial subsidies.
Additional technical goals include developing sorbents capable of functioning effectively across diverse operating conditions, from high-temperature industrial processes to ambient air capture systems. Researchers also aim to minimize environmental impacts of the sorbents themselves, addressing concerns about toxicity, resource intensity, and end-of-life management.
The field is now moving toward integrated systems thinking, where sorbent reactivity is evaluated not in isolation but as part of complete carbon capture, utilization, and storage (CCUS) value chains. This holistic approach recognizes that optimal sorbent characteristics may vary depending on downstream utilization pathways or storage mechanisms.
The early 2000s witnessed the emergence of second-generation technologies, including advanced solvents with improved energy efficiency and novel materials like metal-organic frameworks (MOFs) and zeolites. This period also saw the first large-scale demonstration projects, providing valuable operational data while highlighting significant challenges in energy penalties and economic viability.
The current third generation of technologies, developing since approximately 2010, has focused on transformative approaches including direct air capture (DAC), membrane-based systems, and advanced solid sorbents with enhanced CO₂ selectivity and reduced regeneration energy requirements. These innovations represent a paradigm shift from treating carbon capture as an end-of-pipe solution to integrating it into comprehensive carbon management strategies.
The evolution of CO₂ capture sorbent technology specifically has progressed from simple liquid amine scrubbers to sophisticated engineered materials with precisely controlled physical and chemical properties. Modern sorbents are designed at the molecular level to optimize binding energy, selectivity, and regeneration characteristics. This progression reflects broader technological trends toward materials science innovation and process intensification.
The primary objectives of current CO₂ capture technology development center on several key parameters: reducing energy penalties associated with sorbent regeneration, improving sorbent durability through multiple capture-release cycles, enhancing CO₂ selectivity in mixed gas environments, and dramatically reducing overall system costs. These objectives are driven by the recognition that widespread deployment requires economic viability without substantial subsidies.
Additional technical goals include developing sorbents capable of functioning effectively across diverse operating conditions, from high-temperature industrial processes to ambient air capture systems. Researchers also aim to minimize environmental impacts of the sorbents themselves, addressing concerns about toxicity, resource intensity, and end-of-life management.
The field is now moving toward integrated systems thinking, where sorbent reactivity is evaluated not in isolation but as part of complete carbon capture, utilization, and storage (CCUS) value chains. This holistic approach recognizes that optimal sorbent characteristics may vary depending on downstream utilization pathways or storage mechanisms.
Market Demand for Carbon Capture Solutions
The global carbon capture market is experiencing unprecedented growth, driven by increasing environmental concerns and stringent regulatory frameworks aimed at reducing greenhouse gas emissions. Current market valuations indicate that the carbon capture, utilization, and storage (CCUS) sector reached approximately 3.5 billion USD in 2022, with projections suggesting a compound annual growth rate exceeding 15% through 2030. This rapid expansion reflects the urgent need for effective CO₂ capture solutions across multiple industries.
Industrial sectors, particularly power generation, cement production, steel manufacturing, and chemical processing, represent the primary demand drivers for carbon capture technologies. These industries collectively account for over 60% of global CO₂ emissions, creating substantial market opportunities for advanced sorbent technologies. The power generation sector alone constitutes nearly 40% of the current carbon capture market, highlighting its significance as a target for CO₂ capture sorbent development.
Regional analysis reveals varying levels of market maturity and adoption. North America and Europe lead in terms of technology deployment and investment, supported by favorable policy environments and carbon pricing mechanisms. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment due to rapid industrialization coupled with emerging climate commitments. Middle Eastern countries are increasingly investing in carbon capture technologies to diversify their economies while maintaining their position in the global energy market.
From a technological demand perspective, there is a clear shift toward more efficient, cost-effective sorbent materials with enhanced CO₂ selectivity and regeneration capabilities. End-users are prioritizing sorbents that demonstrate high reactivity under real-world operating conditions, including tolerance to impurities and stability across multiple capture-release cycles. This market preference is driving research toward novel materials such as metal-organic frameworks, functionalized porous carbons, and advanced amine-modified sorbents.
Economic factors significantly influence market dynamics, with cost per ton of CO₂ captured remaining a critical consideration. Current capture costs range between 40-100 USD per ton depending on the technology and application, substantially higher than many carbon pricing schemes. This cost gap underscores the market demand for more reactive sorbents that can achieve higher capture efficiencies while reducing energy penalties and operational expenses.
The regulatory landscape continues to evolve favorably for carbon capture technologies. Enhanced climate policies, including carbon taxes, cap-and-trade systems, and direct subsidies for carbon capture projects, are creating strong market incentives. Notable examples include the expanded 45Q tax credits in the United States, the EU Emissions Trading System, and similar mechanisms emerging in Asian markets, all of which are stimulating demand for high-performance CO₂ capture solutions.
Industrial sectors, particularly power generation, cement production, steel manufacturing, and chemical processing, represent the primary demand drivers for carbon capture technologies. These industries collectively account for over 60% of global CO₂ emissions, creating substantial market opportunities for advanced sorbent technologies. The power generation sector alone constitutes nearly 40% of the current carbon capture market, highlighting its significance as a target for CO₂ capture sorbent development.
Regional analysis reveals varying levels of market maturity and adoption. North America and Europe lead in terms of technology deployment and investment, supported by favorable policy environments and carbon pricing mechanisms. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment due to rapid industrialization coupled with emerging climate commitments. Middle Eastern countries are increasingly investing in carbon capture technologies to diversify their economies while maintaining their position in the global energy market.
From a technological demand perspective, there is a clear shift toward more efficient, cost-effective sorbent materials with enhanced CO₂ selectivity and regeneration capabilities. End-users are prioritizing sorbents that demonstrate high reactivity under real-world operating conditions, including tolerance to impurities and stability across multiple capture-release cycles. This market preference is driving research toward novel materials such as metal-organic frameworks, functionalized porous carbons, and advanced amine-modified sorbents.
Economic factors significantly influence market dynamics, with cost per ton of CO₂ captured remaining a critical consideration. Current capture costs range between 40-100 USD per ton depending on the technology and application, substantially higher than many carbon pricing schemes. This cost gap underscores the market demand for more reactive sorbents that can achieve higher capture efficiencies while reducing energy penalties and operational expenses.
The regulatory landscape continues to evolve favorably for carbon capture technologies. Enhanced climate policies, including carbon taxes, cap-and-trade systems, and direct subsidies for carbon capture projects, are creating strong market incentives. Notable examples include the expanded 45Q tax credits in the United States, the EU Emissions Trading System, and similar mechanisms emerging in Asian markets, all of which are stimulating demand for high-performance CO₂ capture solutions.
Current Sorbent Technologies and Limitations
Carbon dioxide capture technologies have evolved significantly over the past decades, with various sorbent materials being developed to address the growing need for efficient CO₂ removal. Currently, the market is dominated by several key sorbent technologies, each with distinct advantages and limitations that affect their commercial viability and environmental impact.
Amine-based sorbents remain the most widely deployed technology, particularly monoethanolamine (MEA) solutions which offer high CO₂ absorption capacity and relatively fast kinetics. However, these systems suffer from significant energy penalties during regeneration, typically requiring temperatures of 100-120°C. Additionally, amine degradation through oxidation and thermal stress leads to solvent losses of 1-3 kg per ton of CO₂ captured, increasing operational costs and creating potential environmental concerns from degradation byproducts.
Solid adsorbents such as zeolites, activated carbons, and metal-organic frameworks (MOFs) have emerged as promising alternatives. Zeolites demonstrate excellent selectivity for CO₂ but experience dramatic capacity reduction in humid conditions, limiting their practical application in flue gas environments. MOFs offer unprecedented surface areas (up to 7000 m²/g) and tunable pore structures, yet face challenges in stability under repeated cycling and scale-up manufacturing constraints.
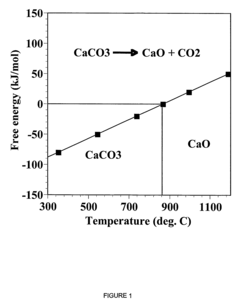
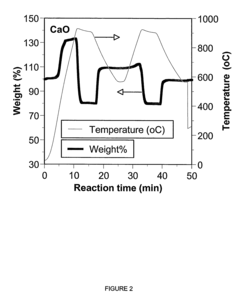
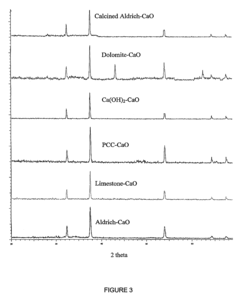
Calcium looping technology, utilizing limestone (CaCO₃) as a sorbent, benefits from abundant, low-cost raw materials and high theoretical CO₂ uptake capacity. However, the rapid capacity decay due to sintering during high-temperature (850°C) calcination cycles remains a significant barrier, with reactivity typically dropping by 70-80% after just 20 cycles.
Novel hybrid materials combining the advantages of different sorbent classes have been developed to overcome individual limitations. Amine-functionalized silica materials, for instance, offer improved stability compared to liquid amines while maintaining high selectivity, but their production costs remain prohibitively high for large-scale deployment.
The energy requirements for sorbent regeneration represent a universal challenge across all technologies. Current systems demand between 2.5-4.0 GJ per ton of CO₂ captured, significantly reducing the net efficiency of power plants and industrial processes. This energy penalty translates to a 20-30% increase in electricity costs when implemented at commercial scale.
Water tolerance presents another critical limitation, particularly for physical adsorbents. Most flue gas streams contain 5-15% water vapor, which can displace CO₂ on many sorbent surfaces, dramatically reducing working capacity under real operating conditions.
Mechanical stability during cycling operations also remains problematic, with attrition rates in fluidized bed systems causing sorbent losses of 1-5% per day for some materials, necessitating continuous replacement and increasing operational expenses.
Amine-based sorbents remain the most widely deployed technology, particularly monoethanolamine (MEA) solutions which offer high CO₂ absorption capacity and relatively fast kinetics. However, these systems suffer from significant energy penalties during regeneration, typically requiring temperatures of 100-120°C. Additionally, amine degradation through oxidation and thermal stress leads to solvent losses of 1-3 kg per ton of CO₂ captured, increasing operational costs and creating potential environmental concerns from degradation byproducts.
Solid adsorbents such as zeolites, activated carbons, and metal-organic frameworks (MOFs) have emerged as promising alternatives. Zeolites demonstrate excellent selectivity for CO₂ but experience dramatic capacity reduction in humid conditions, limiting their practical application in flue gas environments. MOFs offer unprecedented surface areas (up to 7000 m²/g) and tunable pore structures, yet face challenges in stability under repeated cycling and scale-up manufacturing constraints.
Calcium looping technology, utilizing limestone (CaCO₃) as a sorbent, benefits from abundant, low-cost raw materials and high theoretical CO₂ uptake capacity. However, the rapid capacity decay due to sintering during high-temperature (850°C) calcination cycles remains a significant barrier, with reactivity typically dropping by 70-80% after just 20 cycles.
Novel hybrid materials combining the advantages of different sorbent classes have been developed to overcome individual limitations. Amine-functionalized silica materials, for instance, offer improved stability compared to liquid amines while maintaining high selectivity, but their production costs remain prohibitively high for large-scale deployment.
The energy requirements for sorbent regeneration represent a universal challenge across all technologies. Current systems demand between 2.5-4.0 GJ per ton of CO₂ captured, significantly reducing the net efficiency of power plants and industrial processes. This energy penalty translates to a 20-30% increase in electricity costs when implemented at commercial scale.
Water tolerance presents another critical limitation, particularly for physical adsorbents. Most flue gas streams contain 5-15% water vapor, which can displace CO₂ on many sorbent surfaces, dramatically reducing working capacity under real operating conditions.
Mechanical stability during cycling operations also remains problematic, with attrition rates in fluidized bed systems causing sorbent losses of 1-5% per day for some materials, necessitating continuous replacement and increasing operational expenses.
Benchmark Methodologies for Sorbent Reactivity Assessment
01 Metal-based sorbents for CO₂ capture
Metal-based materials, particularly those containing alkali metals, alkaline earth metals, and transition metals, demonstrate high reactivity for CO₂ capture. These sorbents work through various mechanisms including chemisorption and physisorption processes. The metal components provide active sites for CO₂ binding, with materials like lithium zirconate, calcium oxide, and metal-organic frameworks showing promising capture capacity and selectivity under various operating conditions.- Metal-based sorbents for CO₂ capture: Metal-based materials, particularly those containing alkali metals, alkaline earth metals, and transition metals, demonstrate high reactivity for CO₂ capture. These sorbents work through various mechanisms including chemisorption and physisorption processes. The metal components can form stable carbonates or complex structures that effectively bind CO₂ molecules. These materials often exhibit enhanced selectivity, capacity, and regeneration capabilities compared to conventional sorbents.
- Amine-functionalized sorbents: Amine-functionalized materials represent a significant class of CO₂ capture sorbents with high reactivity. These materials incorporate various amine groups onto supports such as silica, polymers, or metal-organic frameworks. The amine groups react with CO₂ through carbamate formation mechanisms, allowing for efficient and selective capture. The reactivity can be tuned by adjusting the type, density, and accessibility of the amine groups, while maintaining good regeneration properties under mild conditions.
- Porous framework materials for enhanced CO₂ reactivity: Porous framework materials, including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and zeolites, offer exceptional CO₂ capture reactivity due to their high surface area and tunable pore structures. These materials provide numerous active sites for CO₂ adsorption and can be engineered with specific functional groups to enhance selectivity. The controlled porosity allows for rapid diffusion of CO₂ molecules to reactive sites, improving overall capture kinetics and capacity under various operating conditions.
- Temperature-responsive CO₂ sorbent systems: Temperature-responsive sorbent systems exhibit variable reactivity toward CO₂ depending on thermal conditions, enabling efficient capture and release cycles. These materials often incorporate phase-change components or thermally sensitive functional groups that modify their CO₂ affinity with temperature changes. This property allows for energy-efficient regeneration processes, as the sorbents can release captured CO₂ with minimal energy input during temperature swing operations, making them particularly valuable for industrial applications.
- Composite and hybrid sorbent materials: Composite and hybrid sorbent materials combine multiple active components to achieve synergistic effects in CO₂ capture reactivity. These materials integrate different functional elements such as metal oxides, amines, polymers, or carbonaceous materials into unified structures. The resulting composites often demonstrate improved stability, enhanced capture capacity, better selectivity, and superior regeneration characteristics compared to single-component sorbents. The synergistic interactions between components can significantly boost overall reactivity while mitigating individual limitations.
02 Amine-functionalized sorbents
Amine-functionalized materials represent a significant class of CO₂ capture sorbents with enhanced reactivity. These materials incorporate various amine groups onto supports such as silica, polymers, or porous frameworks. The amine functionality provides strong chemical affinity for CO₂ through carbamate formation mechanisms. These sorbents demonstrate high selectivity for CO₂ even in the presence of moisture and can be designed with different amine loadings to optimize capture capacity and regeneration energy requirements.Expand Specific Solutions03 Porous framework materials for CO₂ adsorption
Highly porous framework materials including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and zeolites demonstrate exceptional CO₂ capture reactivity due to their tunable pore structures and high surface areas. These materials can be engineered with specific pore sizes and functionalities to enhance CO₂ selectivity and adsorption kinetics. The controlled architecture of these frameworks allows for rapid CO₂ diffusion and efficient utilization of internal surface area, resulting in high volumetric and gravimetric capture capacities.Expand Specific Solutions04 Regeneration methods for CO₂ capture sorbents
Various regeneration techniques significantly impact the long-term reactivity and performance of CO₂ capture sorbents. Temperature swing adsorption (TSA), pressure swing adsorption (PSA), and vacuum swing adsorption (VSA) methods are commonly employed to release captured CO₂ and restore sorbent activity. Advanced regeneration approaches include microwave-assisted desorption, electrical swing adsorption, and hybrid methods that minimize energy requirements while preserving sorbent structure and reactivity over multiple capture-release cycles.Expand Specific Solutions05 Composite and hybrid sorbent materials
Composite and hybrid sorbent materials combine multiple active components to achieve synergistic effects in CO₂ capture reactivity. These materials integrate different functional elements such as metal oxides with amine groups, or porous supports with reactive nanoparticles. The composite structure often provides improved thermal stability, mechanical strength, and resistance to degradation compared to single-component sorbents. Additionally, these materials can be designed to operate effectively across a wider range of temperatures, pressures, and gas compositions, making them suitable for diverse CO₂ capture applications.Expand Specific Solutions
Leading Organizations in Carbon Capture Research
The CO₂ capture sorbent reactivity market is currently in a growth phase, with increasing global focus on carbon reduction technologies. The competitive landscape features diverse players across the value chain, from energy giants like Saudi Aramco, Sinopec, and ExxonMobil to specialized carbon capture innovators such as Climeworks AG. Academic institutions including King Fahd University, Huazhong University, and California Institute of Technology contribute significant research advancements. The market is characterized by varying technology maturity levels, with traditional sorbents well-established while novel materials remain in development stages. Major energy corporations are investing heavily in scalable solutions, while research institutions focus on breakthrough technologies, creating a dynamic ecosystem where collaboration between industry and academia drives innovation in meeting growing carbon management demands.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced amine-based CO₂ capture technologies with proprietary solvents that demonstrate enhanced absorption capacity and reduced regeneration energy. Their approach combines traditional monoethanolamine (MEA) with novel additives to create hybrid solvents that achieve capture rates exceeding 90% while reducing energy penalties by approximately 15-20% compared to conventional systems[1]. Sinopec has implemented large-scale carbon capture facilities at their refineries and petrochemical plants, with their Qilu-Shengli Carbon Capture Utilization and Storage (CCUS) project capturing over 1 million tons of CO₂ annually[2]. Their sorbent technology focuses on optimizing the balance between absorption kinetics and regeneration energy requirements through careful molecular engineering of the amine functional groups and supporting structures.
Strengths: Extensive industrial implementation experience; integrated CCUS value chain capabilities; proprietary solvent formulations with improved energy efficiency. Weaknesses: Technologies primarily focused on high-concentration CO₂ sources; relatively high capital costs for implementation; potential amine degradation and environmental concerns with some sorbent formulations.
Climeworks AG
Technical Solution: Climeworks has pioneered direct air capture (DAC) technology using solid sorbent materials to extract CO₂ directly from ambient air. Their proprietary technology employs amine-functionalized filter materials that selectively bind with CO₂ molecules even at the low atmospheric concentration of approximately 415 ppm[3]. The company's modular collectors operate in cycles where air passes through the filters, capturing CO₂ which is then released through temperature-swing adsorption when heated to 80-100°C, requiring significantly less energy than liquid-based systems[4]. Climeworks' latest generation sorbents demonstrate improved durability with thousands of adsorption-desorption cycles and enhanced selectivity for CO₂ over water vapor, addressing a key challenge in DAC operations. Their Orca plant in Iceland, operational since 2021, captures 4,000 tons of CO₂ annually and permanently stores it through mineralization in basaltic rock formations, representing the world's first commercial-scale negative emissions facility[5].
Strengths: Purpose-built technology for direct air capture; modular and scalable system design; integration with renewable energy and geothermal heat sources; permanent storage solution through mineralization. Weaknesses: Currently high cost per ton of CO₂ captured (estimated $600-800/ton); relatively low capture capacity compared to point-source technologies; significant land and energy requirements for large-scale deployment.
Key Innovations in Sorbent Material Science
Separation of carbon dioxide from gas mixtures by calcium based reaction separation
PatentActiveUS8226917B2
Innovation
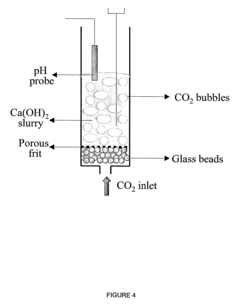
- A method using mesoporous calcium oxide (CaO) sorbents for carbonation and calcination reactions to separate CO2 from gas mixtures, maintaining high reactivity over multiple cycles and optimizing conditions to maximize carbonation extent even in the presence of SO2, integrated with hydrogen production processes for enhanced efficiency.
A carbon dioxide capture structure and a method of making thereof, and a method for removing carbon dioxide from a fluid
PatentWO2023180580A1
Innovation
- A carbon dioxide capture structure using a monolithic three-dimensional porous structure with a carbon-based sorbent material and a potassium silicate binder, which increases CO2 adsorption capacity by up to 500% and provides improved mechanical strength and cycling properties, achieved through a low-temperature manufacturing process.
Environmental Impact Assessment of Sorbent Technologies
The environmental impact of CO₂ capture sorbent technologies extends far beyond their primary function of carbon sequestration. Various sorbent materials, including amine-based compounds, metal-organic frameworks (MOFs), zeolites, and activated carbons, demonstrate significantly different ecological footprints throughout their lifecycle.
Production processes for synthetic sorbents often require substantial energy inputs and chemical precursors, potentially generating considerable greenhouse gas emissions that partially offset their carbon capture benefits. For instance, the synthesis of advanced MOFs typically involves energy-intensive solvent-based processes and high-temperature activation steps that contribute to their environmental burden.
Water consumption represents another critical environmental consideration. Aqueous amine systems, while effective for CO₂ capture, demand substantial water resources for operation and regeneration cycles. This poses particular challenges in water-stressed regions where deployment of such technologies might exacerbate existing resource pressures.
The degradation of sorbent materials during operational cycles introduces additional environmental concerns. Amine-based sorbents can produce harmful byproducts such as nitrosamines and ammonia through oxidative degradation. These compounds may require specialized treatment before release, adding complexity to waste management protocols.
Land use implications vary significantly between sorbent technologies. While solid sorbents generally require smaller physical footprints than liquid systems, the mining operations necessary to obtain precursor materials for certain sorbents can result in habitat disruption and biodiversity loss. This is particularly evident with zeolite-based sorbents that require mineral extraction.
Life cycle assessment (LCA) studies reveal that the environmental performance of different sorbents varies substantially depending on regional energy mixes and operational parameters. Recent comparative analyses indicate that biomass-derived sorbents often demonstrate superior environmental profiles when considering cumulative energy demand and global warming potential metrics.
Regulatory frameworks increasingly incorporate comprehensive environmental impact criteria when evaluating carbon capture technologies. The European Union's taxonomy for sustainable activities, for example, now requires detailed environmental impact assessments that consider not only carbon reduction potential but also broader ecological consequences of sorbent deployment.
Future development of environmentally optimized sorbents will likely focus on bio-derived materials, reduced energy requirements for regeneration, and enhanced durability to minimize replacement frequency and associated environmental costs.
Production processes for synthetic sorbents often require substantial energy inputs and chemical precursors, potentially generating considerable greenhouse gas emissions that partially offset their carbon capture benefits. For instance, the synthesis of advanced MOFs typically involves energy-intensive solvent-based processes and high-temperature activation steps that contribute to their environmental burden.
Water consumption represents another critical environmental consideration. Aqueous amine systems, while effective for CO₂ capture, demand substantial water resources for operation and regeneration cycles. This poses particular challenges in water-stressed regions where deployment of such technologies might exacerbate existing resource pressures.
The degradation of sorbent materials during operational cycles introduces additional environmental concerns. Amine-based sorbents can produce harmful byproducts such as nitrosamines and ammonia through oxidative degradation. These compounds may require specialized treatment before release, adding complexity to waste management protocols.
Land use implications vary significantly between sorbent technologies. While solid sorbents generally require smaller physical footprints than liquid systems, the mining operations necessary to obtain precursor materials for certain sorbents can result in habitat disruption and biodiversity loss. This is particularly evident with zeolite-based sorbents that require mineral extraction.
Life cycle assessment (LCA) studies reveal that the environmental performance of different sorbents varies substantially depending on regional energy mixes and operational parameters. Recent comparative analyses indicate that biomass-derived sorbents often demonstrate superior environmental profiles when considering cumulative energy demand and global warming potential metrics.
Regulatory frameworks increasingly incorporate comprehensive environmental impact criteria when evaluating carbon capture technologies. The European Union's taxonomy for sustainable activities, for example, now requires detailed environmental impact assessments that consider not only carbon reduction potential but also broader ecological consequences of sorbent deployment.
Future development of environmentally optimized sorbents will likely focus on bio-derived materials, reduced energy requirements for regeneration, and enhanced durability to minimize replacement frequency and associated environmental costs.
Techno-Economic Analysis of Competing Sorbent Systems
The economic viability of CO₂ capture technologies is fundamentally tied to the performance and cost characteristics of different sorbent systems. This techno-economic analysis examines the comparative financial implications of major competing sorbent technologies in the carbon capture landscape.
Amine-based sorbents, particularly monoethanolamine (MEA), represent the most commercially mature technology with implementation costs ranging from $40-80 per ton of CO₂ captured. While offering high capture efficiency (85-95%), these systems suffer from high regeneration energy requirements (3.5-4.2 GJ/ton CO₂) and equipment corrosion issues that increase maintenance expenses by approximately 15-20% compared to alternative systems.
Solid sorbents such as metal-organic frameworks (MOFs) and activated carbons demonstrate promising economic potential with projected capture costs of $30-60 per ton CO₂. Their lower regeneration energy demands (1.8-2.5 GJ/ton CO₂) translate to operational savings of 25-40% compared to amine systems. However, manufacturing costs remain 30-50% higher than liquid sorbents, creating a significant barrier to widespread adoption.
Membrane-based separation systems present a different economic profile with moderate capital expenditure but lower operational flexibility. Cost analyses indicate capture expenses of $45-75 per ton CO₂, with the primary economic advantage being reduced equipment footprint (40-60% smaller than conventional absorption columns) and corresponding reductions in installation costs.
Emerging cryogenic separation technologies demonstrate the highest capital costs among competing systems but potentially the lowest operational expenses for high-concentration CO₂ streams. The economic breakeven point typically requires 7-9 years of operation compared to 4-6 years for amine systems.
Sensitivity analysis reveals that energy costs represent the most significant variable affecting the economic performance of all sorbent systems. A 10% increase in energy prices translates to a 6-8% increase in capture costs for amine systems but only 3-5% for advanced solid sorbents, highlighting the economic resilience of newer technologies under energy price volatility scenarios.
Scaling considerations further differentiate these technologies, with amine systems demonstrating more favorable economics at large scales (>500,000 tons CO₂/year) while solid sorbents and membranes show competitive advantages at smaller scales due to their modular implementation potential and reduced economy-of-scale requirements.
Amine-based sorbents, particularly monoethanolamine (MEA), represent the most commercially mature technology with implementation costs ranging from $40-80 per ton of CO₂ captured. While offering high capture efficiency (85-95%), these systems suffer from high regeneration energy requirements (3.5-4.2 GJ/ton CO₂) and equipment corrosion issues that increase maintenance expenses by approximately 15-20% compared to alternative systems.
Solid sorbents such as metal-organic frameworks (MOFs) and activated carbons demonstrate promising economic potential with projected capture costs of $30-60 per ton CO₂. Their lower regeneration energy demands (1.8-2.5 GJ/ton CO₂) translate to operational savings of 25-40% compared to amine systems. However, manufacturing costs remain 30-50% higher than liquid sorbents, creating a significant barrier to widespread adoption.
Membrane-based separation systems present a different economic profile with moderate capital expenditure but lower operational flexibility. Cost analyses indicate capture expenses of $45-75 per ton CO₂, with the primary economic advantage being reduced equipment footprint (40-60% smaller than conventional absorption columns) and corresponding reductions in installation costs.
Emerging cryogenic separation technologies demonstrate the highest capital costs among competing systems but potentially the lowest operational expenses for high-concentration CO₂ streams. The economic breakeven point typically requires 7-9 years of operation compared to 4-6 years for amine systems.
Sensitivity analysis reveals that energy costs represent the most significant variable affecting the economic performance of all sorbent systems. A 10% increase in energy prices translates to a 6-8% increase in capture costs for amine systems but only 3-5% for advanced solid sorbents, highlighting the economic resilience of newer technologies under energy price volatility scenarios.
Scaling considerations further differentiate these technologies, with amine systems demonstrating more favorable economics at large scales (>500,000 tons CO₂/year) while solid sorbents and membranes show competitive advantages at smaller scales due to their modular implementation potential and reduced economy-of-scale requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!