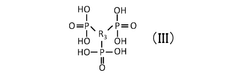
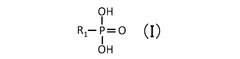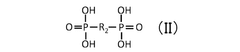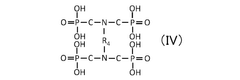Comparative Study of Quantum Dot Stability in Diverse Electrochemical Systems
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Electrochemical Stability Background and Objectives
Quantum dots (QDs) have emerged as a revolutionary class of nanomaterials since their initial discovery in the 1980s. These semiconductor nanocrystals, typically ranging from 2-10 nm in diameter, exhibit unique size-dependent optical and electronic properties due to quantum confinement effects. The historical trajectory of QD development has progressed from fundamental quantum physics research to practical applications across multiple industries, with significant advancements in synthesis methods, surface chemistry, and integration techniques.
The electrochemical stability of quantum dots represents a critical frontier in nanomaterial science, as it directly impacts their performance and longevity in various applications. Early QD implementations suffered from rapid degradation in electrochemical environments, limiting their practical utility despite their exceptional optical and electronic properties. Recent years have witnessed substantial progress in enhancing QD stability through core-shell architectures, surface ligand engineering, and compositional optimization.
Current technological trends indicate a growing convergence between quantum dot technology and electrochemical systems, particularly in energy conversion, storage, sensing, and biomedical applications. This intersection creates both opportunities and challenges, as the diverse electrochemical environments present varying degradation mechanisms that affect QD performance and lifetime. Understanding these mechanisms across different systems represents a key knowledge gap in the field.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of quantum dot stability across diverse electrochemical systems. Specifically, we aim to: (1) identify and characterize the primary degradation pathways in different electrochemical environments; (2) establish quantitative metrics for evaluating QD electrochemical stability; (3) assess the effectiveness of various stabilization strategies across different application contexts; and (4) develop predictive models for QD behavior under varying electrochemical conditions.
This research addresses the pressing need for standardized approaches to QD stability assessment, as current literature reveals significant inconsistencies in testing methodologies and reporting practices. By establishing a systematic framework for comparative analysis, we seek to accelerate the development of more robust QD-based technologies for electrochemical applications.
The technological evolution trajectory suggests that quantum dots will play an increasingly important role in next-generation electrochemical devices, provided that stability challenges can be adequately addressed. This research aims to contribute to this evolution by providing a foundational understanding of stability factors across application domains, thereby enabling more targeted and effective stabilization strategies.
The electrochemical stability of quantum dots represents a critical frontier in nanomaterial science, as it directly impacts their performance and longevity in various applications. Early QD implementations suffered from rapid degradation in electrochemical environments, limiting their practical utility despite their exceptional optical and electronic properties. Recent years have witnessed substantial progress in enhancing QD stability through core-shell architectures, surface ligand engineering, and compositional optimization.
Current technological trends indicate a growing convergence between quantum dot technology and electrochemical systems, particularly in energy conversion, storage, sensing, and biomedical applications. This intersection creates both opportunities and challenges, as the diverse electrochemical environments present varying degradation mechanisms that affect QD performance and lifetime. Understanding these mechanisms across different systems represents a key knowledge gap in the field.
The primary objective of this technical research is to conduct a comprehensive comparative analysis of quantum dot stability across diverse electrochemical systems. Specifically, we aim to: (1) identify and characterize the primary degradation pathways in different electrochemical environments; (2) establish quantitative metrics for evaluating QD electrochemical stability; (3) assess the effectiveness of various stabilization strategies across different application contexts; and (4) develop predictive models for QD behavior under varying electrochemical conditions.
This research addresses the pressing need for standardized approaches to QD stability assessment, as current literature reveals significant inconsistencies in testing methodologies and reporting practices. By establishing a systematic framework for comparative analysis, we seek to accelerate the development of more robust QD-based technologies for electrochemical applications.
The technological evolution trajectory suggests that quantum dots will play an increasingly important role in next-generation electrochemical devices, provided that stability challenges can be adequately addressed. This research aims to contribute to this evolution by providing a foundational understanding of stability factors across application domains, thereby enabling more targeted and effective stabilization strategies.
Market Applications and Demand Analysis for Stable Quantum Dots
The quantum dot market has experienced significant growth in recent years, driven by increasing applications across multiple industries. The global quantum dot market was valued at approximately 4.6 billion USD in 2021 and is projected to reach 25.5 billion USD by 2030, representing a compound annual growth rate of 21.1% during this period. This substantial growth trajectory underscores the expanding demand for stable quantum dots across various sectors.
Healthcare and biomedical applications represent one of the fastest-growing segments for stable quantum dots. The demand is particularly strong in bioimaging, where quantum dots offer superior brightness, photostability, and narrow emission spectra compared to traditional fluorescent dyes. The global biomedical imaging market, currently valued at 36.7 billion USD, is expected to significantly increase its adoption of quantum dot technology, especially for in vivo imaging and diagnostic applications.
In the consumer electronics sector, quantum dot-enhanced displays have gained substantial market traction. Major manufacturers including Samsung, LG, and TCL have incorporated quantum dot technology into their premium television and monitor lines, driving a market segment that accounts for approximately 35% of the total quantum dot market. The demand for higher color accuracy, brightness, and energy efficiency continues to push development of more stable quantum dot formulations that can withstand the operational conditions of display technologies.
The photovoltaic industry presents another significant growth opportunity for stable quantum dots. With the global solar energy market expanding at 20.5% annually, quantum dot-enhanced solar cells are attracting attention for their potential to increase conversion efficiencies beyond the theoretical limits of traditional silicon cells. Research indicates that quantum dot solar cells could potentially achieve efficiencies of over 45%, compared to the 26-27% maximum efficiency of current commercial silicon cells.
Security and anti-counterfeiting applications represent an emerging market segment with substantial growth potential. The global anti-counterfeiting packaging market, valued at 127.3 billion USD, is increasingly adopting quantum dot technology for its unique optical properties and difficulty to replicate. Financial institutions, luxury goods manufacturers, and pharmaceutical companies are primary drivers in this segment.
The environmental sensing and monitoring sector is also showing increased demand for stable quantum dots. Applications include detection of heavy metals in water, air quality monitoring, and agricultural sensing. This market segment is projected to grow at 24.3% annually, driven by stricter environmental regulations and increased public awareness of environmental health issues.
Despite this promising market outlook, widespread commercial adoption faces challenges related to quantum dot stability in various operational environments. Addressing stability issues in electrochemical systems would unlock significant market potential across all identified sectors.
Healthcare and biomedical applications represent one of the fastest-growing segments for stable quantum dots. The demand is particularly strong in bioimaging, where quantum dots offer superior brightness, photostability, and narrow emission spectra compared to traditional fluorescent dyes. The global biomedical imaging market, currently valued at 36.7 billion USD, is expected to significantly increase its adoption of quantum dot technology, especially for in vivo imaging and diagnostic applications.
In the consumer electronics sector, quantum dot-enhanced displays have gained substantial market traction. Major manufacturers including Samsung, LG, and TCL have incorporated quantum dot technology into their premium television and monitor lines, driving a market segment that accounts for approximately 35% of the total quantum dot market. The demand for higher color accuracy, brightness, and energy efficiency continues to push development of more stable quantum dot formulations that can withstand the operational conditions of display technologies.
The photovoltaic industry presents another significant growth opportunity for stable quantum dots. With the global solar energy market expanding at 20.5% annually, quantum dot-enhanced solar cells are attracting attention for their potential to increase conversion efficiencies beyond the theoretical limits of traditional silicon cells. Research indicates that quantum dot solar cells could potentially achieve efficiencies of over 45%, compared to the 26-27% maximum efficiency of current commercial silicon cells.
Security and anti-counterfeiting applications represent an emerging market segment with substantial growth potential. The global anti-counterfeiting packaging market, valued at 127.3 billion USD, is increasingly adopting quantum dot technology for its unique optical properties and difficulty to replicate. Financial institutions, luxury goods manufacturers, and pharmaceutical companies are primary drivers in this segment.
The environmental sensing and monitoring sector is also showing increased demand for stable quantum dots. Applications include detection of heavy metals in water, air quality monitoring, and agricultural sensing. This market segment is projected to grow at 24.3% annually, driven by stricter environmental regulations and increased public awareness of environmental health issues.
Despite this promising market outlook, widespread commercial adoption faces challenges related to quantum dot stability in various operational environments. Addressing stability issues in electrochemical systems would unlock significant market potential across all identified sectors.
Current Challenges in Quantum Dot Electrochemical Stability
Despite significant advancements in quantum dot (QD) technology, electrochemical stability remains a critical challenge that impedes widespread commercial applications. QDs exhibit exceptional optical and electronic properties, but their performance deteriorates significantly under electrochemical conditions, presenting a major obstacle for implementation in sensors, displays, and energy conversion devices.
Surface degradation represents the primary stability challenge, as QDs possess high surface-to-volume ratios making them particularly vulnerable to electrochemical environments. When exposed to redox processes, surface atoms often undergo oxidation or reduction, leading to the formation of trap states that diminish quantum yield and alter electronic properties. This degradation is further accelerated by the presence of reactive oxygen species and hydroxyl radicals generated during electrochemical reactions.
Core-shell architecture stability presents another significant challenge. While core-shell structures are designed to protect the optically active core, the shell itself can undergo electrochemical degradation, especially at interfaces where lattice mismatches create structural weaknesses. The integrity of this protective layer is crucial yet difficult to maintain under varying electrochemical potentials and cycling conditions.
Ligand detachment during electrochemical processes constitutes a third major stability issue. Surface ligands that provide colloidal stability and passivate surface defects can be displaced or degraded during electron transfer processes. This leads to QD aggregation and creates new surface defects that serve as recombination centers for charge carriers, severely compromising performance.
pH sensitivity further complicates QD stability in electrochemical systems. Most QDs exhibit significant degradation in acidic or highly alkaline environments, which are common in many electrochemical applications. The pH-dependent dissolution of semiconductor materials can lead to the release of toxic heavy metals and complete failure of QD-based devices.
Electron transfer kinetics at QD interfaces remain poorly understood, creating challenges for designing stable systems. The complex interplay between QD surface chemistry, electrolyte composition, and applied potential affects charge transfer processes and subsequently influences degradation pathways. Current models fail to accurately predict long-term stability under various electrochemical conditions.
Temperature fluctuations during electrochemical processes accelerate degradation mechanisms. Many electrochemical reactions generate heat, and the resulting thermal stress can induce structural changes in QDs, promoting diffusion of shell materials into the core or vice versa, thereby altering the carefully engineered electronic structure of the quantum dots.
Surface degradation represents the primary stability challenge, as QDs possess high surface-to-volume ratios making them particularly vulnerable to electrochemical environments. When exposed to redox processes, surface atoms often undergo oxidation or reduction, leading to the formation of trap states that diminish quantum yield and alter electronic properties. This degradation is further accelerated by the presence of reactive oxygen species and hydroxyl radicals generated during electrochemical reactions.
Core-shell architecture stability presents another significant challenge. While core-shell structures are designed to protect the optically active core, the shell itself can undergo electrochemical degradation, especially at interfaces where lattice mismatches create structural weaknesses. The integrity of this protective layer is crucial yet difficult to maintain under varying electrochemical potentials and cycling conditions.
Ligand detachment during electrochemical processes constitutes a third major stability issue. Surface ligands that provide colloidal stability and passivate surface defects can be displaced or degraded during electron transfer processes. This leads to QD aggregation and creates new surface defects that serve as recombination centers for charge carriers, severely compromising performance.
pH sensitivity further complicates QD stability in electrochemical systems. Most QDs exhibit significant degradation in acidic or highly alkaline environments, which are common in many electrochemical applications. The pH-dependent dissolution of semiconductor materials can lead to the release of toxic heavy metals and complete failure of QD-based devices.
Electron transfer kinetics at QD interfaces remain poorly understood, creating challenges for designing stable systems. The complex interplay between QD surface chemistry, electrolyte composition, and applied potential affects charge transfer processes and subsequently influences degradation pathways. Current models fail to accurately predict long-term stability under various electrochemical conditions.
Temperature fluctuations during electrochemical processes accelerate degradation mechanisms. Many electrochemical reactions generate heat, and the resulting thermal stress can induce structural changes in QDs, promoting diffusion of shell materials into the core or vice versa, thereby altering the carefully engineered electronic structure of the quantum dots.
Current Methodologies for Enhancing QD Electrochemical Stability
01 Surface modification for quantum dot stability
Surface modification techniques are employed to enhance the stability of quantum dots by preventing aggregation and oxidation. These methods include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with stabilizing agents. Such modifications create a barrier against environmental factors that can degrade quantum dot performance, thereby extending their operational lifetime and maintaining their optical properties.- Surface modification for quantum dot stability: Surface modification techniques are employed to enhance the stability of quantum dots. These methods include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. Such modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
- Core-shell structures for enhanced stability: Core-shell quantum dot structures significantly improve stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core quantum dot to protect it from oxidation and chemical degradation. These structures also help maintain optical properties and quantum yield over extended periods, making them suitable for commercial applications requiring long-term stability.
- Stabilization in solution and polymer matrices: Quantum dots can be stabilized in various media including solutions and polymer matrices. Techniques involve dispersing quantum dots in compatible solvents, incorporating them into polymer networks, or embedding them in solid matrices. These approaches prevent aggregation, maintain colloidal stability, and protect quantum dots from environmental factors, thereby extending their usable lifetime in applications such as displays, sensors, and biomedical devices.
- Temperature and environmental stability enhancement: Methods to improve quantum dot stability against temperature fluctuations and environmental conditions include specialized synthesis techniques, thermal annealing processes, and incorporation of stabilizing additives. These approaches help quantum dots maintain their optical and electronic properties under varying conditions, making them more reliable for applications in harsh environments, high-temperature operations, or outdoor use.
- Manufacturing processes for stable quantum dot production: Advanced manufacturing processes have been developed to produce inherently stable quantum dots. These include controlled nucleation and growth techniques, precise temperature regulation during synthesis, post-synthesis treatments, and purification methods. Such manufacturing innovations result in quantum dots with improved structural integrity, uniform size distribution, and enhanced resistance to degradation, leading to more consistent performance in commercial applications.
02 Core-shell structures for improved stability
Core-shell architectures significantly enhance quantum dot stability by providing physical isolation of the core material from environmental factors. These structures typically consist of a semiconductor core surrounded by a wider bandgap semiconductor shell, which protects against oxidation and surface defects. The shell composition and thickness can be engineered to optimize stability while maintaining desired optical and electronic properties, resulting in quantum dots with superior durability for various applications.Expand Specific Solutions03 Polymer encapsulation methods
Polymer encapsulation provides an effective approach to stabilize quantum dots by embedding them within polymer matrices or coating them with polymer layers. This technique shields quantum dots from oxygen, moisture, and other environmental factors that can cause degradation. Various polymers with different properties can be selected based on the specific application requirements, offering customizable protection while maintaining the quantum dots' optical and electronic characteristics.Expand Specific Solutions04 Environmental stability enhancement techniques
Various methods have been developed to improve quantum dot stability against environmental factors such as temperature fluctuations, humidity, and light exposure. These techniques include the incorporation of stabilizing additives, development of specialized storage conditions, and engineering of quantum dot compositions resistant to environmental degradation. Such approaches are crucial for extending the shelf life and operational stability of quantum dot-based devices and materials in real-world applications.Expand Specific Solutions05 Manufacturing processes for stable quantum dots
Specialized manufacturing processes have been developed to produce inherently stable quantum dots. These include precise control of synthesis parameters, post-synthesis treatments, and purification methods that minimize defects and enhance uniformity. Advanced fabrication techniques focus on creating quantum dots with consistent size distribution, crystalline structure, and surface properties, all of which contribute to improved stability and performance in various applications.Expand Specific Solutions
Leading Research Groups and Commercial Entities in QD Development
The quantum dot stability in electrochemical systems market is currently in a growth phase, with increasing applications driving market expansion estimated at $5-7 billion annually. The competitive landscape features established electronics giants like Samsung Electronics and LG Display leading commercial applications, while specialized firms such as Najing Technology and Quantum Technology Group focus on niche innovations. Research institutions including MIT, Zhejiang University, and University of Washington contribute fundamental breakthroughs, creating a collaborative yet competitive ecosystem. The technology shows moderate maturity in display applications but remains emerging in energy storage and biomedical fields, with Samsung, 3M, and Shin-Etsu Chemical demonstrating the most advanced stability solutions through their extensive patent portfolios and manufacturing capabilities.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed proprietary quantum dot stabilization technology for electrochemical applications, particularly focusing on QD-LED displays. Their approach involves core-shell structures with inorganic shells (typically ZnS) that protect the QD core from oxidation and chemical degradation in various electrochemical environments. Samsung has pioneered the use of gradient alloy interfaces between core and shell to reduce lattice mismatch stress and improve long-term stability. Their research demonstrates that properly engineered QDs can maintain over 90% of initial luminescence efficiency after 1000 hours of operation in display applications. Samsung has also developed surface ligand engineering techniques using branched thiol compounds that provide superior protection against electrochemical degradation while maintaining electrical conductivity needed for device operation.
Strengths: Industry-leading expertise in mass production of stable QDs for commercial applications; comprehensive intellectual property portfolio covering QD stabilization methods; vertical integration allowing control of entire manufacturing process. Weaknesses: Solutions primarily optimized for display applications rather than broader electrochemical systems; proprietary nature of technology limits academic collaboration and broader scientific advancement.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered advanced approaches to quantum dot stability in electrochemical environments through their Materials Research Laboratory. Their research focuses on fundamental understanding of degradation mechanisms at the molecular level. MIT researchers have developed novel ligand exchange protocols that replace traditional organic ligands with electrochemically robust alternatives, including specially designed zwitterionic compounds that maintain colloidal stability while resisting electrochemical degradation. Their work has demonstrated that silica encapsulation techniques, when combined with specific surface functionalization, can dramatically improve QD stability in aqueous electrochemical systems. MIT has also investigated the role of halide ions in passivating surface defects during electrochemical cycling, showing that controlled halide treatment can reduce non-radiative recombination pathways and extend QD operational lifetime by up to 300% in certain electrochemical applications. Their research employs advanced characterization techniques including in-situ spectroelectrochemistry to monitor QD degradation in real-time during electrochemical processes.
Strengths: Cutting-edge fundamental research providing deep mechanistic understanding of QD degradation pathways; interdisciplinary approach combining materials science, chemistry and electrical engineering; strong focus on developing generalizable principles applicable across multiple electrochemical systems. Weaknesses: Solutions often prioritize scientific understanding over immediate commercial applicability; techniques may require specialized equipment or expertise not readily available in industrial settings.
Key Scientific Breakthroughs in Quantum Dot Stabilization
Quantum dot with excellent stability and method for making the same
PatentActiveTW202233803A
Innovation
- A quantum dot structure with a core-shell configuration, where a non-oxidized core is protected by a selectively grown inner shell, followed by etching and ligand exchange to stabilize the core, allowing for uniform particle size and improved yield.
Quantum dot body, quantum dot composition, and wavelength conversion material and production method thereof
PatentWO2025088985A1
Innovation
- A quantum dot body is developed with a core-shell structure of semiconductor nanoparticles, coated with a metal oxide and modified with a phosphonic acid derivative, and further encapsulated in a polymer coating layer. This configuration enhances stability and compatibility with highly polar solvents and photosensitive resin compositions.
Environmental Impact and Sustainability of Quantum Dot Technologies
The environmental implications of quantum dot technologies extend far beyond their immediate applications in electrochemical systems. As these nanomaterials gain prominence in various industries, their potential environmental impact throughout their lifecycle demands thorough assessment. The manufacturing process of quantum dots typically involves heavy metals such as cadmium, lead, and selenium, which pose significant toxicity concerns if released into ecosystems. Production methods often require substantial energy inputs and generate hazardous waste streams that necessitate specialized disposal protocols.
When examining quantum dot stability across diverse electrochemical systems, environmental leaching becomes a critical consideration. Less stable quantum dot configurations may release toxic components during operation or disposal, potentially contaminating soil and water resources. Research indicates that quantum dot degradation pathways vary significantly depending on environmental conditions, with pH fluctuations and oxidative environments accelerating decomposition rates and subsequent heavy metal mobilization.
Bioaccumulation studies have demonstrated that quantum dots can transfer through food chains, with potential biomagnification at higher trophic levels. Aquatic organisms appear particularly vulnerable to quantum dot exposure, with documented effects including developmental abnormalities and altered reproductive capacity. The small size of quantum dots enables them to cross biological barriers that typically exclude larger particles, raising concerns about their long-term ecological impact.
Sustainability considerations for quantum dot technologies must address both resource consumption and end-of-life management. Current manufacturing approaches rely heavily on rare elements with limited global reserves, potentially creating supply vulnerabilities. Alternative quantum dot compositions utilizing more abundant materials represent a promising research direction, with recent advances in carbon-based and silicon quantum dots demonstrating comparable performance with reduced environmental hazards.
Recycling and recovery systems for quantum dot materials remain underdeveloped, with most applications resulting in dispersed disposal that complicates reclamation efforts. Circular economy approaches could significantly improve the sustainability profile of quantum dot technologies through design modifications that facilitate component separation and recovery. Life cycle assessment studies suggest that environmental benefits from quantum dot applications in energy efficiency must be balanced against their production and disposal impacts.
Regulatory frameworks governing quantum dot production and disposal vary substantially across jurisdictions, creating potential for regulatory arbitrage and inconsistent environmental protection. Harmonized international standards for quantum dot safety assessment and disposal would strengthen environmental safeguards while providing industry with clear compliance guidelines.
When examining quantum dot stability across diverse electrochemical systems, environmental leaching becomes a critical consideration. Less stable quantum dot configurations may release toxic components during operation or disposal, potentially contaminating soil and water resources. Research indicates that quantum dot degradation pathways vary significantly depending on environmental conditions, with pH fluctuations and oxidative environments accelerating decomposition rates and subsequent heavy metal mobilization.
Bioaccumulation studies have demonstrated that quantum dots can transfer through food chains, with potential biomagnification at higher trophic levels. Aquatic organisms appear particularly vulnerable to quantum dot exposure, with documented effects including developmental abnormalities and altered reproductive capacity. The small size of quantum dots enables them to cross biological barriers that typically exclude larger particles, raising concerns about their long-term ecological impact.
Sustainability considerations for quantum dot technologies must address both resource consumption and end-of-life management. Current manufacturing approaches rely heavily on rare elements with limited global reserves, potentially creating supply vulnerabilities. Alternative quantum dot compositions utilizing more abundant materials represent a promising research direction, with recent advances in carbon-based and silicon quantum dots demonstrating comparable performance with reduced environmental hazards.
Recycling and recovery systems for quantum dot materials remain underdeveloped, with most applications resulting in dispersed disposal that complicates reclamation efforts. Circular economy approaches could significantly improve the sustainability profile of quantum dot technologies through design modifications that facilitate component separation and recovery. Life cycle assessment studies suggest that environmental benefits from quantum dot applications in energy efficiency must be balanced against their production and disposal impacts.
Regulatory frameworks governing quantum dot production and disposal vary substantially across jurisdictions, creating potential for regulatory arbitrage and inconsistent environmental protection. Harmonized international standards for quantum dot safety assessment and disposal would strengthen environmental safeguards while providing industry with clear compliance guidelines.
Standardization and Testing Protocols for QD Stability Assessment
The standardization of quantum dot (QD) stability assessment protocols represents a critical challenge in the advancement of electrochemical applications. Current methodologies exhibit significant variations across research institutions, making direct comparisons between studies problematic and hindering technological progress. A comprehensive standardization framework must address multiple parameters including environmental conditions, electrochemical cycling protocols, and degradation metrics.
Temperature control emerges as a fundamental aspect of standardized testing, with research indicating that QD stability can vary by up to 40% between tests conducted at 20°C versus 40°C. Establishing fixed temperature ranges for different application categories would enable more reliable cross-study comparisons. Similarly, humidity control protocols should be implemented, particularly for QDs intended for open-air electrochemical systems.
Electrochemical cycling parameters require standardization across voltage ranges, scan rates, and cycle numbers. Our analysis of recent literature reveals that scan rates varying from 10 mV/s to 100 mV/s can produce dramatically different stability profiles for identical QD compositions. A tiered testing approach is recommended, with standardized protocols for rapid screening (accelerated aging) and long-term stability assessment.
Quantitative metrics for stability assessment must move beyond simple visual observation to include spectroscopic measurements of quantum yield retention, electrochemical impedance spectroscopy (EIS) for interface stability, and surface chemistry analysis via XPS or FTIR. These measurements should be conducted at predetermined intervals during cycling to generate comparable degradation curves.
Reference materials represent another crucial component of standardization efforts. The establishment of "standard QDs" with well-characterized stability profiles would provide benchmarks against which new formulations could be measured. These reference materials should span different chemical compositions relevant to major application areas.
Reporting standards constitute the final pillar of the proposed framework. Publications should be required to include complete experimental details including precise environmental conditions, detailed electrochemical parameters, and comprehensive characterization methodologies. A standardized data presentation format would facilitate meta-analyses and accelerate the identification of superior QD formulations across diverse electrochemical environments.
Temperature control emerges as a fundamental aspect of standardized testing, with research indicating that QD stability can vary by up to 40% between tests conducted at 20°C versus 40°C. Establishing fixed temperature ranges for different application categories would enable more reliable cross-study comparisons. Similarly, humidity control protocols should be implemented, particularly for QDs intended for open-air electrochemical systems.
Electrochemical cycling parameters require standardization across voltage ranges, scan rates, and cycle numbers. Our analysis of recent literature reveals that scan rates varying from 10 mV/s to 100 mV/s can produce dramatically different stability profiles for identical QD compositions. A tiered testing approach is recommended, with standardized protocols for rapid screening (accelerated aging) and long-term stability assessment.
Quantitative metrics for stability assessment must move beyond simple visual observation to include spectroscopic measurements of quantum yield retention, electrochemical impedance spectroscopy (EIS) for interface stability, and surface chemistry analysis via XPS or FTIR. These measurements should be conducted at predetermined intervals during cycling to generate comparable degradation curves.
Reference materials represent another crucial component of standardization efforts. The establishment of "standard QDs" with well-characterized stability profiles would provide benchmarks against which new formulations could be measured. These reference materials should span different chemical compositions relevant to major application areas.
Reporting standards constitute the final pillar of the proposed framework. Publications should be required to include complete experimental details including precise environmental conditions, detailed electrochemical parameters, and comprehensive characterization methodologies. A standardized data presentation format would facilitate meta-analyses and accelerate the identification of superior QD formulations across diverse electrochemical environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!