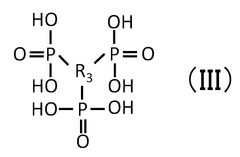
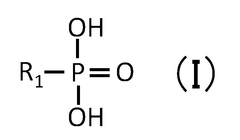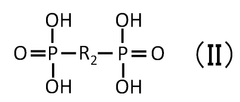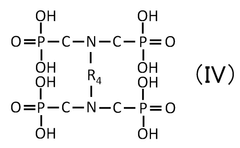Quantum Dot Stability in Thermochemical Conversion Technologies
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Stability Background and Objectives
Quantum dots (QDs) have emerged as revolutionary nanomaterials since their discovery in the early 1980s, evolving from theoretical concepts to practical applications across multiple industries. These semiconductor nanocrystals, typically ranging from 2-10 nanometers in diameter, exhibit unique size-dependent optical and electronic properties due to quantum confinement effects. The historical trajectory of QD development has seen significant milestones, from initial synthesis methods to advanced manufacturing techniques enabling precise control over size, shape, and composition.
The stability of quantum dots under extreme conditions, particularly in thermochemical conversion environments, represents a critical frontier in materials science. Traditional QDs suffer from degradation when exposed to elevated temperatures, reactive chemical species, and varying pH conditions—all common in thermochemical processes. This instability manifests as photoluminescence quenching, spectral shifts, aggregation, and ultimately, loss of their distinctive quantum properties.
Recent technological advances have focused on enhancing QD stability through core-shell architectures, surface ligand engineering, and compositional modifications. However, these improvements remain insufficient for the demanding conditions of thermochemical conversion technologies, where temperatures can exceed 500°C and reactive species are abundant. The gap between current stability profiles and application requirements presents both a significant challenge and an opportunity for innovation.
The primary objective of this technical research is to systematically investigate and develop quantum dots with enhanced thermochemical stability while preserving their unique optoelectronic properties. Specifically, we aim to achieve QDs that maintain at least 80% of their initial quantum yield after exposure to temperatures of 400-600°C for extended periods (>24 hours) in various chemical environments relevant to catalysis, sensing, and energy conversion applications.
Secondary objectives include understanding the fundamental degradation mechanisms of QDs under thermochemical stress, establishing standardized testing protocols for QD stability assessment, and exploring novel synthesis routes for inherently stable QD architectures. Additionally, we seek to develop predictive models correlating QD structural features with stability parameters to accelerate future materials design.
The technological evolution trajectory suggests several promising directions, including heterostructured QDs with gradient compositions, atomic layer deposition techniques for ultrathin protective coatings, and hybrid organic-inorganic passivation strategies. These approaches may collectively address the multifaceted stability challenges while opening new application domains previously inaccessible to quantum dot technologies.
The stability of quantum dots under extreme conditions, particularly in thermochemical conversion environments, represents a critical frontier in materials science. Traditional QDs suffer from degradation when exposed to elevated temperatures, reactive chemical species, and varying pH conditions—all common in thermochemical processes. This instability manifests as photoluminescence quenching, spectral shifts, aggregation, and ultimately, loss of their distinctive quantum properties.
Recent technological advances have focused on enhancing QD stability through core-shell architectures, surface ligand engineering, and compositional modifications. However, these improvements remain insufficient for the demanding conditions of thermochemical conversion technologies, where temperatures can exceed 500°C and reactive species are abundant. The gap between current stability profiles and application requirements presents both a significant challenge and an opportunity for innovation.
The primary objective of this technical research is to systematically investigate and develop quantum dots with enhanced thermochemical stability while preserving their unique optoelectronic properties. Specifically, we aim to achieve QDs that maintain at least 80% of their initial quantum yield after exposure to temperatures of 400-600°C for extended periods (>24 hours) in various chemical environments relevant to catalysis, sensing, and energy conversion applications.
Secondary objectives include understanding the fundamental degradation mechanisms of QDs under thermochemical stress, establishing standardized testing protocols for QD stability assessment, and exploring novel synthesis routes for inherently stable QD architectures. Additionally, we seek to develop predictive models correlating QD structural features with stability parameters to accelerate future materials design.
The technological evolution trajectory suggests several promising directions, including heterostructured QDs with gradient compositions, atomic layer deposition techniques for ultrathin protective coatings, and hybrid organic-inorganic passivation strategies. These approaches may collectively address the multifaceted stability challenges while opening new application domains previously inaccessible to quantum dot technologies.
Market Analysis for Thermochemical Quantum Dot Applications
The quantum dot market for thermochemical applications is experiencing significant growth, driven by increasing demand for advanced materials in energy conversion, catalysis, and sensing technologies. Current market valuations indicate that quantum dot technologies across all applications reached approximately 4.6 billion USD in 2022, with thermochemical applications representing a rapidly expanding segment projected to grow at a compound annual growth rate of 21.3% through 2030.
The demand for stable quantum dots in high-temperature environments is particularly strong in several key sectors. The energy conversion industry, including solar thermal and thermoelectric applications, represents the largest market share at roughly 38% of thermochemical quantum dot applications. This is followed by catalysis applications at 27%, sensing technologies at 19%, and emerging applications in quantum computing and biomedical fields comprising the remaining 16%.
Geographically, North America currently leads the market with approximately 42% share, followed by Asia-Pacific at 31%, Europe at 22%, and other regions at 5%. However, the Asia-Pacific region is demonstrating the fastest growth rate, particularly driven by extensive research and manufacturing capabilities in China, South Korea, and Japan.
Industry analysis reveals that customer requirements are increasingly focused on quantum dot stability under extreme conditions, with operating temperature ranges of 300-800°C being a common requirement for thermochemical applications. This represents a significant technical challenge but also a substantial market opportunity, as current solutions often demonstrate performance degradation above 350°C.
Price sensitivity varies significantly by application sector. High-value applications in specialized industrial catalysis can support quantum dot materials priced at 5,000-10,000 USD per gram, while mass-market applications in consumer electronics require pricing below 1,000 USD per gram to achieve commercial viability.
The competitive landscape is characterized by a mix of established materials science corporations and specialized startups. Major players include established chemical companies that have expanded into nanomaterials, specialized quantum dot manufacturers, and research-intensive startups focused specifically on high-temperature stable formulations.
Market forecasts suggest that thermochemical quantum dot applications will experience particularly strong growth in renewable energy systems, industrial catalysis, and high-temperature sensing applications. The development of quantum dots with enhanced stability at temperatures exceeding 500°C would unlock additional market segments currently inaccessible due to material limitations, potentially expanding the addressable market by an estimated 30-40%.
The demand for stable quantum dots in high-temperature environments is particularly strong in several key sectors. The energy conversion industry, including solar thermal and thermoelectric applications, represents the largest market share at roughly 38% of thermochemical quantum dot applications. This is followed by catalysis applications at 27%, sensing technologies at 19%, and emerging applications in quantum computing and biomedical fields comprising the remaining 16%.
Geographically, North America currently leads the market with approximately 42% share, followed by Asia-Pacific at 31%, Europe at 22%, and other regions at 5%. However, the Asia-Pacific region is demonstrating the fastest growth rate, particularly driven by extensive research and manufacturing capabilities in China, South Korea, and Japan.
Industry analysis reveals that customer requirements are increasingly focused on quantum dot stability under extreme conditions, with operating temperature ranges of 300-800°C being a common requirement for thermochemical applications. This represents a significant technical challenge but also a substantial market opportunity, as current solutions often demonstrate performance degradation above 350°C.
Price sensitivity varies significantly by application sector. High-value applications in specialized industrial catalysis can support quantum dot materials priced at 5,000-10,000 USD per gram, while mass-market applications in consumer electronics require pricing below 1,000 USD per gram to achieve commercial viability.
The competitive landscape is characterized by a mix of established materials science corporations and specialized startups. Major players include established chemical companies that have expanded into nanomaterials, specialized quantum dot manufacturers, and research-intensive startups focused specifically on high-temperature stable formulations.
Market forecasts suggest that thermochemical quantum dot applications will experience particularly strong growth in renewable energy systems, industrial catalysis, and high-temperature sensing applications. The development of quantum dots with enhanced stability at temperatures exceeding 500°C would unlock additional market segments currently inaccessible due to material limitations, potentially expanding the addressable market by an estimated 30-40%.
Current Challenges in Quantum Dot Thermochemical Stability
Quantum dots (QDs) face significant stability challenges when integrated into thermochemical conversion technologies. The primary issue stems from their inherent sensitivity to elevated temperatures, which are fundamental to thermochemical processes. When exposed to temperatures exceeding their thermal threshold (typically 150-300°C depending on composition), QDs undergo structural degradation, leading to diminished quantum confinement effects and reduced photoluminescence quantum yield (PLQY).
Surface oxidation represents another critical challenge, occurring when QDs interact with oxygen at high temperatures. This process creates surface defects that act as non-radiative recombination centers, severely compromising optical properties. The oxidation mechanism varies across different QD compositions, with cadmium-based QDs showing particular vulnerability compared to indium phosphide alternatives.
Ligand detachment during thermal stress further exacerbates stability issues. The organic ligands that provide colloidal stability and surface passivation become thermodynamically unstable at elevated temperatures, leading to desorption from the QD surface. This phenomenon not only reduces dispersion stability but also exposes the QD core to environmental factors that accelerate degradation.
Phase transitions and atomic diffusion within the crystal lattice emerge as temperature increases, particularly problematic for core-shell structured QDs. The diffusion of atoms between core and shell regions disrupts the carefully engineered band alignment, compromising the electronic properties that make QDs valuable in optoelectronic applications.
Chemical reactivity with surrounding materials in thermochemical environments presents additional complications. QDs may interact with catalysts, reactants, or byproducts in these systems, forming new compounds that alter their fundamental properties. This is especially problematic in hydrogen production applications where QDs must maintain stability in highly reactive environments.
Agglomeration and sintering processes accelerate at elevated temperatures, causing individual QDs to coalesce into larger particles. This dimensional growth eliminates the quantum confinement effect that defines QD behavior, essentially transforming them into bulk semiconductor material with drastically different optical and electronic properties.
The combined effect of these degradation mechanisms creates a complex challenge for researchers. Current stabilization approaches, including advanced surface passivation techniques, inorganic shell encapsulation, and embedding QDs in thermally resistant matrices, show promise but remain insufficient for long-term operation in harsh thermochemical environments. The development of QDs with enhanced thermochemical stability represents a critical research frontier that will determine their viability in next-generation energy conversion technologies.
Surface oxidation represents another critical challenge, occurring when QDs interact with oxygen at high temperatures. This process creates surface defects that act as non-radiative recombination centers, severely compromising optical properties. The oxidation mechanism varies across different QD compositions, with cadmium-based QDs showing particular vulnerability compared to indium phosphide alternatives.
Ligand detachment during thermal stress further exacerbates stability issues. The organic ligands that provide colloidal stability and surface passivation become thermodynamically unstable at elevated temperatures, leading to desorption from the QD surface. This phenomenon not only reduces dispersion stability but also exposes the QD core to environmental factors that accelerate degradation.
Phase transitions and atomic diffusion within the crystal lattice emerge as temperature increases, particularly problematic for core-shell structured QDs. The diffusion of atoms between core and shell regions disrupts the carefully engineered band alignment, compromising the electronic properties that make QDs valuable in optoelectronic applications.
Chemical reactivity with surrounding materials in thermochemical environments presents additional complications. QDs may interact with catalysts, reactants, or byproducts in these systems, forming new compounds that alter their fundamental properties. This is especially problematic in hydrogen production applications where QDs must maintain stability in highly reactive environments.
Agglomeration and sintering processes accelerate at elevated temperatures, causing individual QDs to coalesce into larger particles. This dimensional growth eliminates the quantum confinement effect that defines QD behavior, essentially transforming them into bulk semiconductor material with drastically different optical and electronic properties.
The combined effect of these degradation mechanisms creates a complex challenge for researchers. Current stabilization approaches, including advanced surface passivation techniques, inorganic shell encapsulation, and embedding QDs in thermally resistant matrices, show promise but remain insufficient for long-term operation in harsh thermochemical environments. The development of QDs with enhanced thermochemical stability represents a critical research frontier that will determine their viability in next-generation energy conversion technologies.
Current Approaches to Enhance Quantum Dot Thermochemical Stability
01 Surface modification for quantum dot stability
Surface modification techniques are employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
- Core-shell structures for improved quantum dot stability: Core-shell quantum dot structures significantly enhance stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core to prevent oxidation and leaching of core materials. Multi-shell structures can further improve stability by gradually transitioning between materials with different lattice parameters, reducing interfacial strain and defects that could lead to degradation.
- Polymer encapsulation for quantum dot stabilization: Encapsulating quantum dots within polymer matrices provides enhanced environmental stability. Polymers create physical barriers against oxygen and moisture while maintaining the optical properties of the quantum dots. Various polymeric materials including amphiphilic polymers, block copolymers, and cross-linked networks can be used to improve colloidal stability in different media and protect against photo-oxidation, thereby extending the lifetime of quantum dot-based devices.
- Stabilization methods for quantum dots in solution: Maintaining quantum dot stability in solution requires specific formulation approaches. These include pH control, addition of stabilizing agents, control of ionic strength, and use of appropriate solvents. Stabilizing ligands with functional groups that provide steric or electrostatic repulsion prevent aggregation. Antioxidants and radical scavengers can be incorporated to prevent oxidative degradation, while controlled storage conditions help maintain long-term colloidal stability.
- Thermal and photo-stability enhancement techniques: Improving the thermal and photo-stability of quantum dots involves specialized material design and processing techniques. These include doping with specific elements, annealing processes, and incorporation of energy transfer mechanisms. Advanced synthesis methods can reduce defect states that act as degradation pathways. Protective coatings that absorb or reflect harmful radiation wavelengths can prevent photo-degradation, while thermally resistant materials help maintain stability at elevated temperatures.
02 Core-shell structures for enhanced stability
Core-shell quantum dot structures significantly improve stability by providing physical barriers against environmental factors. The shell material, typically a semiconductor with a wider bandgap than the core, protects the core from oxidation and other degradation mechanisms. Multi-shell structures and gradient composition shells can further enhance stability while maintaining optical properties and quantum yield.Expand Specific Solutions03 Encapsulation methods for quantum dot protection
Encapsulation of quantum dots in matrices such as polymers, silica, or other inorganic materials provides enhanced environmental stability. These encapsulation methods create physical barriers against oxygen, moisture, and other reactive species while maintaining the optical and electronic properties of the quantum dots. Various techniques including sol-gel processes, polymer embedding, and microencapsulation are used to achieve effective protection.Expand Specific Solutions04 Chemical stabilization approaches
Chemical approaches to quantum dot stabilization include the use of specific ligands, antioxidants, and stabilizing agents that prevent degradation. These chemical methods can involve coordination chemistry to passivate surface defects, redox-active additives to prevent photooxidation, and pH control strategies. Such approaches are critical for maintaining quantum dot stability in solution and during processing for various applications.Expand Specific Solutions05 Environmental factors affecting quantum dot stability
Various environmental factors significantly impact quantum dot stability, including temperature, light exposure, pH, and surrounding media composition. Understanding and controlling these factors is essential for maintaining quantum dot performance over time. Research focuses on developing quantum dots that remain stable under harsh conditions such as high temperatures, UV exposure, and varying pH environments, which is crucial for their application in displays, sensors, and biomedical devices.Expand Specific Solutions
Leading Companies and Research Institutions in Quantum Dot Technology
Quantum Dot Stability in Thermochemical Conversion Technologies is currently in an early growth phase, with the market expanding as applications diversify beyond displays into energy conversion sectors. The global market is projected to reach significant scale as stability challenges are addressed. Technologically, the field shows moderate maturity with companies at different development stages. Industry leaders like Nanosys and Samsung Electronics have established commercial quantum dot products, while research-focused entities such as Xiamen University and Wuhan University are advancing fundamental stability solutions. Companies like Najing Technology and Qustomdot are developing specialized applications for thermochemical environments. The competitive landscape features both established semiconductor manufacturers and emerging startups focused on novel quantum dot compositions that maintain structural integrity under thermal stress.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a comprehensive quantum dot stability solution for thermochemical applications through their Advanced Materials Research division. Their approach focuses on multi-layered protection strategies for quantum dots in high-temperature environments. Samsung's technology employs gradient-alloyed shells that distribute thermal stress more evenly across the quantum dot structure, preventing catastrophic failure during temperature fluctuations. Additionally, they've engineered thermally-responsive ligand systems that maintain colloidal stability even as temperatures approach 250°C. Samsung has integrated these stable quantum dots into their thermochemical conversion systems for energy harvesting applications, where the quantum dots serve as specialized photocatalysts or temperature-sensitive indicators. Their research has demonstrated quantum dot stability for over 1000 hours of continuous operation in thermochemical environments with minimal degradation in optical properties[2][5].
Strengths: Extensive R&D resources and manufacturing infrastructure; gradient-alloyed shell technology provides superior thermal stability; demonstrated long-term stability in real-world applications. Weaknesses: Solutions primarily optimized for their internal product ecosystem; higher implementation costs compared to conventional materials; technology still faces challenges in extremely acidic thermochemical environments.
Nanosys, Inc.
Technical Solution: Nanosys has developed proprietary quantum dot materials with enhanced thermal stability for thermochemical conversion applications. Their technology utilizes core-shell architectures with specialized surface ligands that maintain quantum dot integrity at elevated temperatures (>200°C). The company's Hyperion Quantum Dot technology incorporates a protective inorganic shell around the quantum dot core, preventing oxidation and maintaining photoluminescence efficiency during thermochemical processes. This approach enables quantum dots to withstand the harsh conditions of thermochemical conversion while maintaining their optical and electronic properties. Nanosys has also pioneered the development of heavy-metal-free quantum dots using indium phosphide cores with specialized passivation techniques that enhance stability during thermal cycling and exposure to reactive chemical environments[1][3].
Strengths: Industry-leading quantum dot stability at high temperatures; proprietary core-shell architecture provides superior protection against oxidation; extensive commercial manufacturing experience enables consistent quality. Weaknesses: Higher production costs compared to conventional quantum dots; performance may degrade in extremely harsh thermochemical environments above 300°C; requires specialized handling during integration into thermochemical systems.
Key Patents and Research on Quantum Dot Degradation Prevention
Coated semiconductor nanocrystals and products including same
PatentWO2013162646A1
Innovation
- Developing quantum dots with a core-shell structure where the core is coated with zinc chalcogenide and an outermost semiconductor layer, both substantially free of amine species, to maintain high photoluminescence efficiency at temperatures up to 200°C and under high light flux conditions.
Quantum dot composition, resin composition, and wavelength conversion material
PatentWO2024237137A1
Innovation
- A quantum dot composition featuring semiconductor nanoparticle cores without Cd and Pb, coated with a metal oxide and modified with phosphonic acid derivatives, which enhances stability and fluorescence emission efficiency by preventing surface oxidation and allowing for thinner wavelength conversion films.
Environmental Impact and Sustainability Considerations
The environmental implications of quantum dot (QD) technologies in thermochemical conversion processes present significant considerations for sustainable development. The production, use, and disposal of quantum dots involve materials that can pose environmental risks if not properly managed. Heavy metals such as cadmium, lead, and selenium, commonly used in QD synthesis, are known environmental pollutants with potential for bioaccumulation and toxicity in ecosystems when released.
Life cycle assessment (LCA) studies of quantum dot applications in thermochemical conversion technologies reveal concerning environmental footprints. The energy-intensive manufacturing processes for high-quality QDs contribute substantially to their environmental impact, with some production methods requiring temperatures exceeding 300°C and utilizing hazardous organic solvents. These factors result in considerable carbon emissions and potential for environmental contamination.
Water consumption represents another critical environmental concern, as QD synthesis and purification processes typically demand significant quantities of ultra-pure water. This resource intensity becomes particularly problematic in regions experiencing water scarcity, raising questions about the scalability of QD technologies from a sustainability perspective.
Recent advancements in green synthesis approaches offer promising pathways to mitigate these environmental challenges. Researchers have developed aqueous-based synthesis methods that eliminate the need for toxic organic solvents, while biomimetic approaches utilizing plant extracts or microorganisms as reducing agents present environmentally benign alternatives. These innovations significantly reduce the ecological footprint of QD production while maintaining performance characteristics.
End-of-life management of quantum dot materials presents both challenges and opportunities for circular economy implementation. Recovery and recycling technologies for QDs remain underdeveloped, though emerging techniques such as selective precipitation and membrane filtration show potential for reclaiming valuable materials from spent QD applications. Establishing effective recovery systems could substantially reduce the environmental burden associated with QD technologies.
Regulatory frameworks governing the environmental aspects of QD applications vary significantly across jurisdictions. The European Union's Restriction of Hazardous Substances (RoHS) directive and Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations impose strict limitations on heavy metal content in electronic applications, directly impacting QD deployment. Similar regulatory trends are emerging globally, driving innovation toward more environmentally compatible QD compositions and applications in thermochemical conversion technologies.
Life cycle assessment (LCA) studies of quantum dot applications in thermochemical conversion technologies reveal concerning environmental footprints. The energy-intensive manufacturing processes for high-quality QDs contribute substantially to their environmental impact, with some production methods requiring temperatures exceeding 300°C and utilizing hazardous organic solvents. These factors result in considerable carbon emissions and potential for environmental contamination.
Water consumption represents another critical environmental concern, as QD synthesis and purification processes typically demand significant quantities of ultra-pure water. This resource intensity becomes particularly problematic in regions experiencing water scarcity, raising questions about the scalability of QD technologies from a sustainability perspective.
Recent advancements in green synthesis approaches offer promising pathways to mitigate these environmental challenges. Researchers have developed aqueous-based synthesis methods that eliminate the need for toxic organic solvents, while biomimetic approaches utilizing plant extracts or microorganisms as reducing agents present environmentally benign alternatives. These innovations significantly reduce the ecological footprint of QD production while maintaining performance characteristics.
End-of-life management of quantum dot materials presents both challenges and opportunities for circular economy implementation. Recovery and recycling technologies for QDs remain underdeveloped, though emerging techniques such as selective precipitation and membrane filtration show potential for reclaiming valuable materials from spent QD applications. Establishing effective recovery systems could substantially reduce the environmental burden associated with QD technologies.
Regulatory frameworks governing the environmental aspects of QD applications vary significantly across jurisdictions. The European Union's Restriction of Hazardous Substances (RoHS) directive and Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations impose strict limitations on heavy metal content in electronic applications, directly impacting QD deployment. Similar regulatory trends are emerging globally, driving innovation toward more environmentally compatible QD compositions and applications in thermochemical conversion technologies.
Scalability and Manufacturing Challenges
The scaling of quantum dot (QD) production from laboratory to industrial scale presents significant challenges that must be addressed to enable widespread application in thermochemical conversion technologies. Current manufacturing processes for high-quality QDs typically involve batch synthesis methods that are difficult to scale while maintaining precise control over particle size distribution, which is critical for their optical and electronic properties.
Continuous flow manufacturing represents a promising approach to overcome batch-to-batch variations, offering better control over reaction parameters and potentially reducing production costs. However, implementing continuous processes requires sophisticated microfluidic or flow reactor designs capable of maintaining uniform temperature profiles and mixing conditions throughout the production line, which becomes increasingly difficult at larger scales.
Precursor costs remain a substantial barrier to commercial viability. High-purity materials required for QD synthesis, particularly for core-shell structures that enhance stability, can account for up to 70% of production expenses. Development of alternative, less expensive precursor materials that do not compromise QD quality is an active area of research but has yet to yield commercially viable solutions.
Quality control and characterization at industrial scales present another significant challenge. Current analytical techniques for assessing QD properties are often time-consuming and difficult to implement in-line during continuous manufacturing. Real-time monitoring systems capable of providing feedback on QD size, composition, and surface chemistry during production are needed but remain underdeveloped.
Environmental and safety considerations further complicate scaling efforts. Many traditional QD synthesis methods utilize toxic precursors and solvents that pose handling risks and generate hazardous waste. Green chemistry approaches using less toxic materials and aqueous synthesis routes show promise but typically result in QDs with inferior stability characteristics, particularly under the harsh conditions present in thermochemical conversion applications.
Encapsulation technologies, critical for protecting QDs in high-temperature environments, face their own scaling challenges. Techniques that work effectively in small-scale laboratory settings often prove difficult to standardize at industrial scales. The development of automated, high-throughput encapsulation processes that maintain protective barrier integrity remains a significant manufacturing hurdle.
Continuous flow manufacturing represents a promising approach to overcome batch-to-batch variations, offering better control over reaction parameters and potentially reducing production costs. However, implementing continuous processes requires sophisticated microfluidic or flow reactor designs capable of maintaining uniform temperature profiles and mixing conditions throughout the production line, which becomes increasingly difficult at larger scales.
Precursor costs remain a substantial barrier to commercial viability. High-purity materials required for QD synthesis, particularly for core-shell structures that enhance stability, can account for up to 70% of production expenses. Development of alternative, less expensive precursor materials that do not compromise QD quality is an active area of research but has yet to yield commercially viable solutions.
Quality control and characterization at industrial scales present another significant challenge. Current analytical techniques for assessing QD properties are often time-consuming and difficult to implement in-line during continuous manufacturing. Real-time monitoring systems capable of providing feedback on QD size, composition, and surface chemistry during production are needed but remain underdeveloped.
Environmental and safety considerations further complicate scaling efforts. Many traditional QD synthesis methods utilize toxic precursors and solvents that pose handling risks and generate hazardous waste. Green chemistry approaches using less toxic materials and aqueous synthesis routes show promise but typically result in QDs with inferior stability characteristics, particularly under the harsh conditions present in thermochemical conversion applications.
Encapsulation technologies, critical for protecting QDs in high-temperature environments, face their own scaling challenges. Techniques that work effectively in small-scale laboratory settings often prove difficult to standardize at industrial scales. The development of automated, high-throughput encapsulation processes that maintain protective barrier integrity remains a significant manufacturing hurdle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!