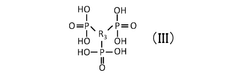
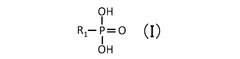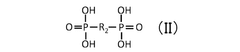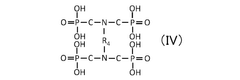Quantum Dot Stability in Solid Electrolytes for Fuel Cells
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Stability Background and Objectives
Quantum dots (QDs) have emerged as a revolutionary nanomaterial with exceptional optical and electronic properties since their discovery in the early 1980s. These semiconductor nanocrystals, typically ranging from 2-10 nanometers in diameter, exhibit quantum confinement effects that enable precise tuning of their bandgap through size manipulation. The evolution of quantum dot technology has progressed from fundamental research to practical applications across multiple industries, including display technologies, biomedical imaging, and more recently, energy conversion systems.
In the context of fuel cell technology, quantum dots represent a promising avenue for enhancing efficiency and durability. Fuel cells have been under development for decades as clean energy conversion devices, with solid oxide fuel cells (SOFCs) and proton exchange membrane fuel cells (PEMFCs) being the most commercially advanced variants. However, these technologies continue to face challenges related to cost, durability, and efficiency that limit widespread adoption.
The integration of quantum dots into solid electrolytes for fuel cells has emerged as a potential breakthrough approach to address these limitations. Historically, solid electrolytes have offered advantages over liquid counterparts in terms of safety and form factor, but have struggled with lower ionic conductivity and interfacial resistance issues. Quantum dots, with their tunable electronic properties and high surface-to-volume ratio, could potentially enhance charge transfer processes and catalytic activity at electrolyte interfaces.
The primary technical challenge lies in the stability of quantum dots within the harsh electrochemical environment of fuel cells. Quantum dots are susceptible to degradation mechanisms including oxidation, agglomeration, and leaching when exposed to high temperatures, varying pH conditions, and electrochemical potentials present in fuel cell operations. This instability compromises both the performance and longevity of quantum dot-enhanced fuel cell systems.
The objective of this research is to comprehensively investigate the fundamental mechanisms affecting quantum dot stability in solid electrolyte environments specific to fuel cell applications. We aim to identify and characterize the primary degradation pathways, develop strategies for enhancing quantum dot resilience through surface modification and compositional engineering, and establish design principles for quantum dot-solid electrolyte interfaces that maintain functionality under operational conditions.
Additionally, this research seeks to establish quantitative metrics for evaluating quantum dot stability over extended operational periods, correlate stability characteristics with fuel cell performance parameters, and ultimately develop predictive models that can accelerate the design of next-generation quantum dot-enhanced solid electrolyte systems for fuel cells with superior durability and efficiency.
In the context of fuel cell technology, quantum dots represent a promising avenue for enhancing efficiency and durability. Fuel cells have been under development for decades as clean energy conversion devices, with solid oxide fuel cells (SOFCs) and proton exchange membrane fuel cells (PEMFCs) being the most commercially advanced variants. However, these technologies continue to face challenges related to cost, durability, and efficiency that limit widespread adoption.
The integration of quantum dots into solid electrolytes for fuel cells has emerged as a potential breakthrough approach to address these limitations. Historically, solid electrolytes have offered advantages over liquid counterparts in terms of safety and form factor, but have struggled with lower ionic conductivity and interfacial resistance issues. Quantum dots, with their tunable electronic properties and high surface-to-volume ratio, could potentially enhance charge transfer processes and catalytic activity at electrolyte interfaces.
The primary technical challenge lies in the stability of quantum dots within the harsh electrochemical environment of fuel cells. Quantum dots are susceptible to degradation mechanisms including oxidation, agglomeration, and leaching when exposed to high temperatures, varying pH conditions, and electrochemical potentials present in fuel cell operations. This instability compromises both the performance and longevity of quantum dot-enhanced fuel cell systems.
The objective of this research is to comprehensively investigate the fundamental mechanisms affecting quantum dot stability in solid electrolyte environments specific to fuel cell applications. We aim to identify and characterize the primary degradation pathways, develop strategies for enhancing quantum dot resilience through surface modification and compositional engineering, and establish design principles for quantum dot-solid electrolyte interfaces that maintain functionality under operational conditions.
Additionally, this research seeks to establish quantitative metrics for evaluating quantum dot stability over extended operational periods, correlate stability characteristics with fuel cell performance parameters, and ultimately develop predictive models that can accelerate the design of next-generation quantum dot-enhanced solid electrolyte systems for fuel cells with superior durability and efficiency.
Market Analysis for Quantum Dot-Enhanced Fuel Cells
The quantum dot-enhanced fuel cell market is experiencing significant growth potential as industries seek more efficient and sustainable energy solutions. Current market projections indicate that the global fuel cell market will reach approximately $13.7 billion by 2026, with solid oxide fuel cells (SOFCs) representing a substantial segment of this growth. The integration of quantum dot technology into these systems represents an emerging niche with considerable expansion opportunities.
The primary market drivers for quantum dot-enhanced fuel cells include increasing demand for clean energy solutions, stringent environmental regulations, and the push for higher energy conversion efficiencies. Government initiatives supporting renewable energy research and development across North America, Europe, and Asia-Pacific regions have created favorable conditions for market development. Additionally, the declining costs of quantum dot production technologies have begun to make commercial applications more economically viable.
Market segmentation reveals several key application sectors for this technology. The transportation sector, particularly automotive and aviation industries, shows strong interest in quantum dot-enhanced fuel cells due to their potential for higher power density and efficiency compared to conventional systems. Stationary power generation represents another significant market segment, with applications in residential, commercial, and industrial settings where reliable backup power or off-grid solutions are required.
Regional market analysis indicates that North America currently leads in research and development investments for quantum dot fuel cell technologies, with several prominent research institutions and companies establishing innovation centers. Asia-Pacific, particularly Japan, South Korea, and China, demonstrates the fastest growth rate in terms of patent applications and commercial development initiatives for this technology.
Consumer electronics and portable power applications constitute an emerging market segment with substantial growth potential. The superior energy density and potentially longer operational lifetimes of quantum dot-enhanced fuel cells make them attractive alternatives to conventional battery technologies in certain applications.
Market challenges include high initial production costs, technical barriers related to quantum dot stability in solid electrolytes, and competition from established energy technologies. The cost-performance ratio remains a critical factor influencing market penetration rates. Additionally, the lack of standardized testing protocols and regulatory frameworks specifically addressing quantum dot applications in fuel cells creates market uncertainty.
Industry experts project that early commercial applications will likely emerge in premium market segments where performance advantages outweigh cost considerations, such as military applications, specialized industrial equipment, and high-end consumer electronics. Mass market adoption is expected to follow as manufacturing processes mature and economies of scale reduce production costs.
The primary market drivers for quantum dot-enhanced fuel cells include increasing demand for clean energy solutions, stringent environmental regulations, and the push for higher energy conversion efficiencies. Government initiatives supporting renewable energy research and development across North America, Europe, and Asia-Pacific regions have created favorable conditions for market development. Additionally, the declining costs of quantum dot production technologies have begun to make commercial applications more economically viable.
Market segmentation reveals several key application sectors for this technology. The transportation sector, particularly automotive and aviation industries, shows strong interest in quantum dot-enhanced fuel cells due to their potential for higher power density and efficiency compared to conventional systems. Stationary power generation represents another significant market segment, with applications in residential, commercial, and industrial settings where reliable backup power or off-grid solutions are required.
Regional market analysis indicates that North America currently leads in research and development investments for quantum dot fuel cell technologies, with several prominent research institutions and companies establishing innovation centers. Asia-Pacific, particularly Japan, South Korea, and China, demonstrates the fastest growth rate in terms of patent applications and commercial development initiatives for this technology.
Consumer electronics and portable power applications constitute an emerging market segment with substantial growth potential. The superior energy density and potentially longer operational lifetimes of quantum dot-enhanced fuel cells make them attractive alternatives to conventional battery technologies in certain applications.
Market challenges include high initial production costs, technical barriers related to quantum dot stability in solid electrolytes, and competition from established energy technologies. The cost-performance ratio remains a critical factor influencing market penetration rates. Additionally, the lack of standardized testing protocols and regulatory frameworks specifically addressing quantum dot applications in fuel cells creates market uncertainty.
Industry experts project that early commercial applications will likely emerge in premium market segments where performance advantages outweigh cost considerations, such as military applications, specialized industrial equipment, and high-end consumer electronics. Mass market adoption is expected to follow as manufacturing processes mature and economies of scale reduce production costs.
Current Challenges in Quantum Dot Solid Electrolyte Integration
The integration of quantum dots (QDs) into solid electrolytes for fuel cell applications presents significant technical challenges that currently impede widespread commercial adoption. One primary obstacle is the inherent instability of quantum dots in the harsh electrochemical environment of fuel cells. When exposed to high temperatures (typically 500-800°C for solid oxide fuel cells), quantum dots tend to undergo sintering and agglomeration, resulting in size distribution changes that compromise their unique quantum confinement properties and catalytic performance.
Chemical degradation represents another critical challenge, as quantum dots can react with electrolyte components or gaseous species present in fuel cells. This is particularly problematic with chalcogenide-based QDs (CdSe, PbS), which may undergo oxidation or sulfidation processes, leading to compositional changes and formation of undesired interfacial phases that increase resistance and reduce overall cell efficiency.
The interface between quantum dots and solid electrolytes presents complex engineering challenges. Poor adhesion and mechanical compatibility often result in delamination during thermal cycling, creating discontinuities in electron transfer pathways. Additionally, the difference in thermal expansion coefficients between QDs and ceramic electrolytes induces mechanical stress during operation, potentially causing microcracks and performance degradation over time.
Charge transfer dynamics at the QD-electrolyte interface remain poorly understood. The presence of surface ligands, necessary for QD synthesis and stabilization, can impede ionic conductivity and create resistive barriers. While complete ligand removal might improve conductivity, it typically leads to QD aggregation and loss of nanoscale properties. This creates a fundamental design paradox that requires innovative solutions.
Long-term operational stability presents perhaps the most significant barrier to commercialization. Current research indicates that QD-enhanced solid electrolytes show promising initial performance but experience rapid degradation under continuous operation. Studies have documented up to 40% reduction in ionic conductivity after just 100 hours of operation at elevated temperatures, highlighting the need for improved stabilization strategies.
Manufacturing scalability also poses significant challenges. Laboratory-scale synthesis methods for QD-electrolyte composites often involve complex, multi-step processes that are difficult to scale industrially. The precise control of QD size, distribution, and interface quality required for optimal performance is challenging to maintain in large-scale production environments, resulting in batch-to-batch variability that impacts device reliability.
Chemical degradation represents another critical challenge, as quantum dots can react with electrolyte components or gaseous species present in fuel cells. This is particularly problematic with chalcogenide-based QDs (CdSe, PbS), which may undergo oxidation or sulfidation processes, leading to compositional changes and formation of undesired interfacial phases that increase resistance and reduce overall cell efficiency.
The interface between quantum dots and solid electrolytes presents complex engineering challenges. Poor adhesion and mechanical compatibility often result in delamination during thermal cycling, creating discontinuities in electron transfer pathways. Additionally, the difference in thermal expansion coefficients between QDs and ceramic electrolytes induces mechanical stress during operation, potentially causing microcracks and performance degradation over time.
Charge transfer dynamics at the QD-electrolyte interface remain poorly understood. The presence of surface ligands, necessary for QD synthesis and stabilization, can impede ionic conductivity and create resistive barriers. While complete ligand removal might improve conductivity, it typically leads to QD aggregation and loss of nanoscale properties. This creates a fundamental design paradox that requires innovative solutions.
Long-term operational stability presents perhaps the most significant barrier to commercialization. Current research indicates that QD-enhanced solid electrolytes show promising initial performance but experience rapid degradation under continuous operation. Studies have documented up to 40% reduction in ionic conductivity after just 100 hours of operation at elevated temperatures, highlighting the need for improved stabilization strategies.
Manufacturing scalability also poses significant challenges. Laboratory-scale synthesis methods for QD-electrolyte composites often involve complex, multi-step processes that are difficult to scale industrially. The precise control of QD size, distribution, and interface quality required for optimal performance is challenging to maintain in large-scale production environments, resulting in batch-to-batch variability that impacts device reliability.
Current Approaches to Enhance Quantum Dot Stability
01 Stabilization methods for quantum dots in solid electrolytes
Various methods can be employed to enhance the stability of quantum dots when incorporated into solid electrolytes. These include surface modification techniques, encapsulation strategies, and the use of protective coatings. Such stabilization approaches prevent degradation of quantum dots due to environmental factors and electrochemical processes, thereby maintaining their optical and electronic properties over extended periods in solid electrolyte matrices.- Stabilization methods for quantum dots in solid electrolytes: Various methods can be employed to enhance the stability of quantum dots in solid electrolyte environments. These include surface passivation techniques, core-shell structures, and the use of stabilizing ligands that prevent degradation when exposed to the ionic environment of solid electrolytes. These approaches help maintain quantum dot optical and electronic properties over extended periods by reducing surface defects and preventing ion migration into the quantum dot structure.
- Interface engineering between quantum dots and solid electrolytes: The interface between quantum dots and solid electrolytes plays a crucial role in determining overall stability. Engineering this interface through the use of buffer layers, gradient compositions, or specialized coupling agents can significantly improve the longevity and performance of quantum dot-based devices. These interface modifications help manage charge transfer processes and prevent unwanted chemical reactions that could degrade either the quantum dots or the electrolyte material.
- Composition optimization for enhanced stability: The chemical composition of both quantum dots and solid electrolytes can be optimized to improve system stability. This includes developing quantum dots with more stable core materials, using dopants to enhance resistance to degradation, and formulating solid electrolytes specifically designed to be compatible with quantum dot systems. Tailored compositions can minimize detrimental interactions while maintaining the desired functional properties of both components.
- Environmental protection strategies for quantum dot-electrolyte systems: Protecting quantum dot-solid electrolyte systems from environmental factors such as moisture, oxygen, and temperature fluctuations is essential for long-term stability. This can be achieved through encapsulation techniques, hermetic sealing methods, and the development of protective barrier layers. These approaches prevent external contaminants from reaching the sensitive quantum dot-electrolyte interface and causing degradation through oxidation or other chemical reactions.
- Characterization and testing methods for stability assessment: Advanced characterization techniques are crucial for evaluating the stability of quantum dots in solid electrolytes. These include accelerated aging tests, in-situ spectroscopic monitoring, electrochemical impedance spectroscopy, and computational modeling approaches. These methods help identify degradation mechanisms, quantify stability parameters, and guide the development of more robust quantum dot-solid electrolyte systems for various applications including energy storage, lighting, and sensing technologies.
02 Interface engineering between quantum dots and solid electrolytes
Engineering the interface between quantum dots and solid electrolytes is crucial for maintaining stability. This involves designing compatible surface chemistries, controlling charge transfer processes, and minimizing interfacial strain. Proper interface engineering prevents unwanted reactions, reduces defect formation, and enhances the overall stability of quantum dot-solid electrolyte systems for applications in energy storage, optoelectronics, and sensing technologies.Expand Specific Solutions03 Composition optimization for enhanced quantum dot stability
The composition of both quantum dots and solid electrolytes can be optimized to improve stability. This includes developing core-shell structures for quantum dots, doping strategies, and tailoring the chemical composition of solid electrolytes to be compatible with quantum dots. Optimized compositions minimize degradation pathways, prevent ion migration issues, and enhance the thermal and electrochemical stability of the integrated system.Expand Specific Solutions04 Environmental protection strategies for quantum dot-solid electrolyte systems
Protecting quantum dot-solid electrolyte systems from environmental factors is essential for long-term stability. This involves developing hermetic sealing techniques, moisture barriers, oxygen scavengers, and UV protection layers. These environmental protection strategies prevent degradation mechanisms triggered by exposure to air, moisture, heat, and light, thereby extending the operational lifetime of devices incorporating quantum dots in solid electrolytes.Expand Specific Solutions05 Advanced characterization techniques for stability assessment
Specialized characterization methods are crucial for evaluating and understanding the stability of quantum dots in solid electrolytes. These include in-situ spectroscopy, advanced microscopy techniques, electrochemical impedance spectroscopy, and accelerated aging tests. Such techniques enable researchers to identify degradation mechanisms, monitor changes in quantum dot properties over time, and develop more effective stabilization strategies for practical applications.Expand Specific Solutions
Leading Research Groups and Industry Stakeholders
The quantum dot stability in solid electrolytes for fuel cells market is currently in an early growth phase, characterized by intensive research and development activities. The global market size remains relatively modest but is expected to expand significantly as fuel cell technologies gain wider adoption in clean energy applications. From a technical maturity perspective, this field is still evolving, with key players demonstrating varying levels of advancement. Leading research institutions like California Institute of Technology and Centre National de la Recherche Scientifique are driving fundamental breakthroughs, while commercial entities including Samsung Electronics, Bloom Energy, and Nissan Motor are focusing on practical applications. Companies such as 3M Innovative Properties and Kyocera are leveraging their materials expertise to address stability challenges, while specialized firms like Honeycomb Battery are developing novel integration approaches for enhanced performance.
California Institute of Technology
Technical Solution: Caltech has developed a pioneering approach to quantum dot stability in solid electrolytes through surface functionalization techniques. Their research focuses on creating core-shell structured quantum dots with specialized ligands that form strong chemical bonds with the solid electrolyte matrix. This approach significantly reduces quantum dot aggregation and oxidation, two primary causes of degradation in fuel cell applications. Caltech researchers have demonstrated that silicon-based quantum dots with carboxylate-terminated ligands can maintain stability in ceramic electrolytes for over 1000 hours of operation at elevated temperatures (600-800°C). Their proprietary encapsulation method creates a protective barrier that prevents leaching of toxic elements while maintaining quantum confinement effects essential for catalytic activity. The team has also pioneered in-situ characterization techniques to monitor quantum dot behavior during actual fuel cell operation, providing crucial insights into degradation mechanisms.
Strengths: Superior high-temperature stability compared to conventional approaches; excellent integration with ceramic electrolytes; comprehensive understanding of degradation mechanisms through advanced characterization. Weaknesses: Higher production costs due to complex synthesis procedures; potential scalability challenges for commercial applications; limited testing in real-world fuel cell conditions beyond laboratory settings.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed a proprietary quantum dot stabilization technology for solid electrolyte applications in fuel cells called "QD-SOFC" (Quantum Dot Solid Oxide Fuel Cell). Their approach incorporates graphene-wrapped quantum dots that are uniformly dispersed within a yttria-stabilized zirconia (YSZ) matrix. The graphene encapsulation prevents quantum dot agglomeration while enhancing electrical conductivity throughout the electrolyte. Samsung's research has demonstrated that their CdSe/ZnS core-shell quantum dots maintain structural integrity for over 2000 hours at operating temperatures of 500-700°C, with less than 5% degradation in photoluminescence efficiency. Their patented surface modification technique employs phosphonic acid ligands that form robust bonds with the ceramic electrolyte, preventing migration and coalescence during thermal cycling. Samsung has also developed a co-sintering process that allows quantum dots to be incorporated during electrolyte fabrication without compromising the mechanical properties of the final composite.
Strengths: Excellent long-term stability at high operating temperatures; enhanced electrical conductivity through graphene integration; well-established manufacturing capabilities for potential commercialization. Weaknesses: Potential environmental concerns with cadmium-based quantum dots; higher cost compared to conventional fuel cell materials; limited public data on performance in full fuel cell assemblies rather than just electrolyte components.
Key Patents and Scientific Breakthroughs
Quantum dot body, quantum dot composition, and wavelength conversion material and production method thereof
PatentWO2025088985A1
Innovation
- A quantum dot body is developed with a core-shell structure of semiconductor nanoparticles, coated with a metal oxide and modified with a phosphonic acid derivative, and further encapsulated in a polymer coating layer. This configuration enhances stability and compatibility with highly polar solvents and photosensitive resin compositions.
Quantum dot composition, quantum-dot-composition-containing liquid, light-emitting element, light-emitting device, and method for producing quantum dot composition
PatentPendingUS20250289997A1
Innovation
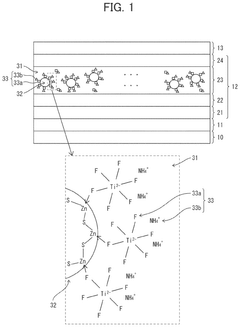
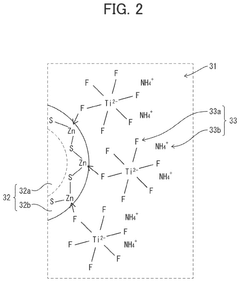
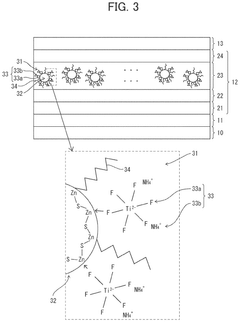
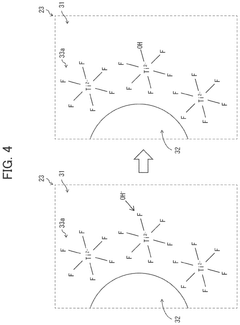
- A quantum dot composition incorporating metal-fluoro complexes, metal-fluoro complexes with hydroxy groups, or metal oxides containing fluorine, with complex stability constants between 0.1 and 20.0, to stabilize the quantum dots against OH groups, enhancing long-term reliability and light emission efficiency.
Environmental Impact and Sustainability Considerations
The integration of quantum dots in solid electrolytes for fuel cells presents significant environmental and sustainability implications that warrant careful consideration. The manufacturing processes for quantum dots often involve toxic precursors and solvents, raising concerns about potential environmental contamination during production. Heavy metals such as cadmium, lead, or mercury, commonly used in quantum dot synthesis, pose particular risks if released into ecosystems through improper disposal or manufacturing waste.
Life cycle assessment (LCA) studies indicate that quantum dot production can have a substantial carbon footprint, primarily due to energy-intensive synthesis methods requiring high temperatures and controlled environments. However, when evaluating the complete environmental impact, it is essential to consider the efficiency improvements that quantum dot-enhanced fuel cells may deliver, potentially offsetting initial production emissions through operational benefits over the technology's lifespan.
Recycling and end-of-life management represent critical challenges for quantum dot applications in fuel cells. Current recovery methods for nanomaterials from spent fuel cells remain limited in efficiency and economic viability. Research into closed-loop systems that enable the recovery and reuse of quantum dots could significantly enhance the sustainability profile of this technology, reducing dependence on virgin material extraction and minimizing waste generation.
Water consumption during manufacturing processes presents another environmental concern, particularly in regions experiencing water scarcity. Advanced production techniques that minimize water usage or implement water recycling systems could substantially improve the sustainability credentials of quantum dot production for fuel cell applications.
The potential for quantum dots to enhance fuel cell efficiency and durability offers promising sustainability benefits through reduced resource consumption and extended product lifespans. By improving catalytic activity and reducing degradation rates in solid electrolytes, quantum dot integration may decrease the frequency of replacement and maintenance, thereby conserving materials and energy over time.
Regulatory frameworks governing nanomaterials in energy applications continue to evolve globally, with increasing emphasis on environmental protection and sustainable development. Companies developing quantum dot technologies for fuel cells must navigate these regulations while implementing green chemistry principles to minimize environmental impacts throughout the product lifecycle.
Future research directions should prioritize environmentally benign synthesis routes, such as aqueous-based methods and the use of non-toxic precursors. Additionally, developing quantum dots from abundant, non-critical raw materials would enhance supply chain resilience and reduce geopolitical vulnerabilities associated with rare element dependencies.
Life cycle assessment (LCA) studies indicate that quantum dot production can have a substantial carbon footprint, primarily due to energy-intensive synthesis methods requiring high temperatures and controlled environments. However, when evaluating the complete environmental impact, it is essential to consider the efficiency improvements that quantum dot-enhanced fuel cells may deliver, potentially offsetting initial production emissions through operational benefits over the technology's lifespan.
Recycling and end-of-life management represent critical challenges for quantum dot applications in fuel cells. Current recovery methods for nanomaterials from spent fuel cells remain limited in efficiency and economic viability. Research into closed-loop systems that enable the recovery and reuse of quantum dots could significantly enhance the sustainability profile of this technology, reducing dependence on virgin material extraction and minimizing waste generation.
Water consumption during manufacturing processes presents another environmental concern, particularly in regions experiencing water scarcity. Advanced production techniques that minimize water usage or implement water recycling systems could substantially improve the sustainability credentials of quantum dot production for fuel cell applications.
The potential for quantum dots to enhance fuel cell efficiency and durability offers promising sustainability benefits through reduced resource consumption and extended product lifespans. By improving catalytic activity and reducing degradation rates in solid electrolytes, quantum dot integration may decrease the frequency of replacement and maintenance, thereby conserving materials and energy over time.
Regulatory frameworks governing nanomaterials in energy applications continue to evolve globally, with increasing emphasis on environmental protection and sustainable development. Companies developing quantum dot technologies for fuel cells must navigate these regulations while implementing green chemistry principles to minimize environmental impacts throughout the product lifecycle.
Future research directions should prioritize environmentally benign synthesis routes, such as aqueous-based methods and the use of non-toxic precursors. Additionally, developing quantum dots from abundant, non-critical raw materials would enhance supply chain resilience and reduce geopolitical vulnerabilities associated with rare element dependencies.
Scalability and Manufacturing Challenges
The transition from laboratory-scale quantum dot synthesis to industrial-scale production for fuel cell applications presents significant manufacturing challenges. Current quantum dot production methods, primarily based on colloidal synthesis techniques, demonstrate excellent control over particle size and composition at small scales but face considerable hurdles when scaled to commercial volumes. The precision required for maintaining uniform quantum dot properties—critical for consistent fuel cell performance—becomes increasingly difficult to achieve in large batch processes, resulting in wider property distributions and potential performance inconsistencies.
Integration of quantum dots into solid electrolytes introduces additional complexity to the manufacturing process. The uniform dispersion of quantum dots throughout the electrolyte matrix without agglomeration requires sophisticated mixing and processing techniques that are difficult to standardize at industrial scales. Current methods often result in uneven distribution, creating localized performance variations and potential failure points within fuel cell systems. Furthermore, the interface between quantum dots and solid electrolytes must be carefully engineered to maintain stability during operation, a requirement that becomes more challenging to control in mass production environments.
Cost considerations represent another significant barrier to commercialization. High-purity precursors required for quantum dot synthesis remain expensive, with limited economies of scale currently available. The specialized equipment and controlled environments necessary for production further increase capital expenditure requirements. Economic viability demands significant cost reductions through process optimization and alternative material sourcing strategies, particularly for precious metal components often used in high-performance quantum dots.
Quality control and characterization present additional scalability challenges. Current analytical techniques for quantum dot characterization are predominantly laboratory-focused, time-consuming, and difficult to implement in continuous production environments. Development of rapid, in-line quality control methods capable of monitoring quantum dot properties during manufacturing remains an unresolved challenge, limiting production throughput and increasing costs associated with quality assurance.
Environmental and safety considerations also impact manufacturing scalability. Many quantum dot synthesis methods utilize toxic precursors and solvents that present handling and disposal challenges at industrial scales. Regulatory compliance requirements for large-scale production of nanomaterials continue to evolve, creating uncertainty in manufacturing planning and potential barriers to market entry. Development of greener synthesis routes and safer precursors represents an important direction for improving manufacturing feasibility.
Integration of quantum dots into solid electrolytes introduces additional complexity to the manufacturing process. The uniform dispersion of quantum dots throughout the electrolyte matrix without agglomeration requires sophisticated mixing and processing techniques that are difficult to standardize at industrial scales. Current methods often result in uneven distribution, creating localized performance variations and potential failure points within fuel cell systems. Furthermore, the interface between quantum dots and solid electrolytes must be carefully engineered to maintain stability during operation, a requirement that becomes more challenging to control in mass production environments.
Cost considerations represent another significant barrier to commercialization. High-purity precursors required for quantum dot synthesis remain expensive, with limited economies of scale currently available. The specialized equipment and controlled environments necessary for production further increase capital expenditure requirements. Economic viability demands significant cost reductions through process optimization and alternative material sourcing strategies, particularly for precious metal components often used in high-performance quantum dots.
Quality control and characterization present additional scalability challenges. Current analytical techniques for quantum dot characterization are predominantly laboratory-focused, time-consuming, and difficult to implement in continuous production environments. Development of rapid, in-line quality control methods capable of monitoring quantum dot properties during manufacturing remains an unresolved challenge, limiting production throughput and increasing costs associated with quality assurance.
Environmental and safety considerations also impact manufacturing scalability. Many quantum dot synthesis methods utilize toxic precursors and solvents that present handling and disposal challenges at industrial scales. Regulatory compliance requirements for large-scale production of nanomaterials continue to evolve, creating uncertainty in manufacturing planning and potential barriers to market entry. Development of greener synthesis routes and safer precursors represents an important direction for improving manufacturing feasibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!